Abstract
Objective
BRCA-associated and sporadic ovarian cancers have different pathologic and clinical features. Our goal was to determine if BRCA mutation status is an independent predictor of residual tumor volume following primary surgical cytoreduction.
Methods
We conducted a retrospective analysis of patients with FIGO stage IIIC-IV high-grade serous ovarian cancer classified for the presence or absence of germline BRCA mutations. The primary outcome was tumor-debulking status categorized as complete gross resection (0mm), optimal but visible disease (1-10mm), or suboptimal debulking (>10mm) following primary surgical cytoreduction. Overall survival by residual tumor size and BRCA status was also assessed as a secondary endpoint.
Results
Data from 367 patients (69 BRCA mutated, 298 BRCA wild-type) were analyzed. Rate of optimal tumor debulking (0-10mm) in BRCA wild-type and BRCA-mutated patients were 70.1% and 84.1%, respectively (P=0.02). On univariate analysis, increasing age (10-year OR, 1.33; 95% CI, 1.07–1.65; P=0.01) and wild-type BRCA status (OR, 0.47; 95% CI, 0.23–0.94, P=0.03) were both significantly associated with suboptimal surgical outcome. On multivariate analysis, BRCA mutation status was no longer associated with residual tumor volume (OR, 0.63; 95% CI, 0.31–1.29; P=0.21) while age remained a borderline significant predictor (10-year OR, 1.25; 95% CI,1.01–1.56; P=0.05). Both smaller residual tumor volume and mutant BRCA status were significantly associated with improved overall survival.
Conclusion
BRCA mutation status is not associated with the rate of optimal tumor debulking at primary surgery after accounting for differences in patient age. Improved survival of BRCA carriers is not the result of better surgical outcomes but instead intrinsic tumor biology.
Introduction
Ovarian cancer is the leading cause of death from gynecologic cancer in the United States, accounting for approximately 22,000 new diagnoses and 15,000 deaths annually [1]. The standard of care for patients with advanced (stage III/IV) ovarian cancer is primary surgical cytoreduction followed by platinum-based chemotherapy. Several pooled analyses of clinical trials for newly diagnosed advanced ovarian cancer have found that age, histology, performance status, and residual tumor size after surgical cytoreduction are independent prognostic factors [2-5]. It is now also known that approximately 8-13% of unselected ovarian cancers, and 15-17% of the high-grade serous subtype, are associated with germline BRCA1 or BRCA2 mutations [6,7]. Several studies have found that germline BRCA1/2 status is also a powerful predictor of survival [8-11], independent of traditional prognostic factors such as platinum-sensitivity [12]. BRCA1/2-associated ovarian cancers may also have distinct patterns of recurrence following primary therapy [13]. However, it is unclear if higher rates of optimal surgical cytoreduction may be a contributing factor in the improved overall survival seen in BRCA-associated ovarian cancer.
The underlying biologic differences of BRCA-associated ovarian cancers may also affect the outcome of primary surgical cytoreduction through differences in micro-invasiveness or pattern of spread. Comprehensive pathologic reviews of BRCA1/2-associated ovarian cancers have shown that they are more likely than age-matched sporadic controls to be of serous histology, to be high grade, and to stain strongly for TP53 [14,15]. Review of tumor morphology, necrosis, and mitotic index in sporadic and BRCA-associated ovarian cancers also reveal important differences. In a recent study, tumor-infiltrating lymphocytes, high mitotic index, solid, pseudoendometrioid, and transitional cell carcinoma-like features, pleomorphism, and necrosis were seen more frequently in BRCA1-associated cases than controls [16]. These observations raise the possibly that germline BRCA mutations may affect the results of surgical cytoreduction.
We investigated the association between germline BRCA1/2 mutations in serous ovarian cancers and the outcome of primary surgical cytoreduction. Data from a single center and The Cancer Genome Atlas (TCGA) were pooled. Our goal was to determine if BRCA-associated ovarian cancers have different rates of complete and optimal debulking compared to sporadic ovarian cancers.
Methods
Patients
After Institutional Review Board/Privacy Board (IRB/PB) approval, two cohorts of patients were ascertained.
The first cohort (“MSKCC Cohort”) was composed of patients identified from a prospectively maintained institutional database. Patients had International Federation of Gynecology and Obstetrics (FIGO) stage IIIC–IV epithelial ovarian, fallopian tube, or primary peritoneal carcinoma and had primary surgical cytoreduction at Memorial Sloan-Kettering Cancer Center (MSKCC) between January 1, 2001 and January 31, 2010. All patients in the current analysis also had BRCA mutation testing on one of two IRB-approved prospective studies conducted by the Clinical Genetics Service to investigate the clinical significance of germline BRCA mutations. Details of these two follow-up studies have been published previously.17 During the study period, patients presenting for treatment of newly diagnosed pelvic serous cancer were not required to undergo genetic counseling or testing. Between 2001 and 2008 patients were typically referred based on at least one of the following: 1) family history of breast cancer prior to age 50 or ovarian cancer at any age in a first or second degree relative, 2) Eastern European (Ashkenazi) Jewish heritage 3) patient request, or 4) physician request. Since July 2008, genetic counseling has been offered to (but not required of) all patients diagnosed with high-grade serous ovarian cancer irrespective of family history. Patients tested for BRCA germline mutations beyond 24 months of diagnosis were also excluded from analysis to minimize the selection bias that could result from including patients referred for genetic testing because of prolonged survival. All BRCA1 and BRCA2 mutations were predicted to be deleterious. Patients with variants of unknown significance were considered to be BRCA-negative. Patients were excluded if they received neoadjuvant chemotherapy prior to attempted cytoreductive surgery, had non-serous histology, or had low-grade disease (low-malignant potential or grade 1). Demographics including age at diagnosis, FIGO stage, and histologic grade. Operative notes were reviewed to determine the volume of residual disease. All patients received platinum-based cytotoxic chemotherapy as per appropriate institutional protocol at time of diagnosis. Overall survival was calculated from date of diagnosis to date of death or last follow-up.
The second cohort (“TCGA Cohort”) was obtained from The Cancer Genome Atlas (TCGA) publication on ovarian cancer and through the TCGA Data Portal (http://cancergenome.nih.gov/). TCGA is a clinically annotated collection of untreated high-grade serous ovarian cancer specimens selected to have greater than 70% tumor cell nuclei and less than 20% necrosis. All patients received a platinum agent, and 94% received a taxane after cytoreduction. Only samples from patients with FIGO stage IIIC–IV disease, those with recorded volume of residual disease, and those sequenced by TCGA were included in this analysis. Because MSKCC contributed tumor samples to TCGA, patients included in the MSKCC cohort were excluded from the TCGA cohort to prevent duplicates. The sequencing methods used in TCGA have been described previously [18]. For the purposes of this analysis, only germline BRCA1/2 mutations were considered. Tumors with somatic BRCA1/2 mutations were analyzed as BRCA wild type because the clinical significance of somatic mutations is unclear and to be consistent with the MSKCC cohort for which only germline mutation testing was performed. Data was current as of August 25, 2010.
Statistical Methods
This was a pooled retrospective analysis with the primary objective of determining the rate of optimal debulking in patients following primary cytoreduction by BRCA status (BRCA1/2+, BRCA−). The associations between clinical factors and BRCA status were tested by either Pearson chi-square, Fisher's-exact test for categorical variables, or ANOVA for continuous variables. All P-values reported are two-sided. Univariate and multivariate analyses for age, stage, grade, and BRCA mutation status were performed using binary logistic regression, with optimal debulking (0-10 mm) and suboptimal debulking (>10 mm) as the dependent variables. For these analyses, suboptimal debulking was used as the reference outcome. Overall survival (OS) was calculated from the date of primary cytoreduction surgery to death or last follow-up. Univariate OS analyses were performed, stratifying for debulking and BRCA status. OS rate was estimated using Kaplan–Meier method. P-values were obtained by log-rank test. Variables were regarded as significant at P<0.05. To build the multivariate model a forward selection technique was employed using a significance level of 0.10 for the variable to remain in the model [19]. Analyses were conducted using SPSS version 19 (SPSS, Chicago, IL).
Results
Surgical Outcomes
The baseline demographics of the two cohorts are shown in Tables 1 and 2. In both cohorts, patients with and without deleterious BRCA mutations were well balanced with regards to stage and grade. As has been described previously, patients with BRCA1/2 mutations were on average approximately 6 years younger at the age of diagnosis than patients without mutations.9 The overall debulking rate (0-10 mm) was 93.7% in the MSKCC cohort and 75.7% in the TCGA cohort. When surgical outcome was broken out in three groups (0mm, 1-10mm, >10mm), there were significant differences in surgical outcomes in the MSKCC, but not the TCGA, cohort. Surgical outcomes were also explored by type of BRCA mutation (BRCA−, BRCA1, and BRCA2) as shown in Table 3. No differences in surgical outcomes were observed regardless of whether completely resected patients (0 mm) were included in the optimal debulking category or analyzed separately.
Table 1.
MSKCC Patient Baseline Demographics
| Variables | All | BRCA (-) | BRCA1/2 (+) | P-value* |
|---|---|---|---|---|
| Whole Cohort | 101 | 69 | 32 | |
| Age | ||||
| Median (mean) | 59.6 (58.5) | 62.1 (60.1) | 56.5 (55.1) | 0.01 |
| Range | 32.2 - 78.2 | 40.3 -78.2 | 32.2 - 74.0 | |
| Diagnosis | ||||
| Ovarian | 78 (77.2%) | 50 (72.5%) | 28 (87.5%) | 0.23 |
| Fallopian | 15 (14.9%) | 12 (17.4%) | 3 (9.4%) | |
| Peritoneum | 8 (7.9%) | 7 (10.1%) | 1 (3.1%) | |
| Pathologic Stage | ||||
| III | 86 (85.1%) | 59 (85.5%) | 27 (84.4%) | 1.00 |
| IV | 15 (15.6%) | 10 (14.5%) | 5 (15.6%) | |
| Grade | ||||
| G2/3 | 101 (100%) | 69 (100%) | 32 (100%) | -- |
| Surgical outcome | ||||
| 0 | 45 (44.6%) | 33 (47.8%) | 12 (37.5%) | 0.04 |
| 1-10mm | 40 (39.6%) | 22 (31.9%) | 18 (56.3%) | |
| >10mm | 16 (15.8%) | 14 (20.3%) | 2 (6.3%) | |
p-values for Diagnosis, Grade, OR Tumor Index by Pearson Chi-Square, Pathologic Stage, Surgical outcome by Fisher's Exact, age by ANOVA (all p-values are double-sided)
Table 2.
TCGA Patients Baseline Demographics
| Variables | All | BRCA (-) | BRCA1/2 (+) | P-value* |
|---|---|---|---|---|
| Whole Cohort | 266 | 229 | 37 | |
| Age (6 missing) | ||||
| Median (mean) | 60.9 (61.0) | 61.4 (61.9) | 53.7 (55.7%) | <0.01 |
| Range | 35.0 - 84.9 | 35.0 - 84.7 | 38.9 - 76.0 | |
| Stage | ||||
| III | 227 (85.3%) | 194 (84.9%) | 33 (89.2%) | 0.62 |
| IV | 39 (14.7%) | 35 (15.3%) | 4 (10.8%) | |
| Grade (4 missing) | ||||
| G2/3 | 262 (100%) | 225 (100%) | 37 (100%) | -- |
| Surgical outcome | ||||
| 1-10mm | 143 (53.8%) | 124 (54.1%) | 19 (51.4%) | |
| >10mm | 84 (31.6%) | 75 (32.8%) | 9 (24.3%) | |
p-value for Stage, Grade by Fisher's Exact test, Surgical outcome by Pearson Chi-Square (all p-values two-sided), age by ANOVA
Table 3.
BRCA1/2 Subset Analysis (MSKCC and TCGA Patients Combined)
| Variables | All | BRCA (-) | BRCA1 (+) | BRCA2 (+) | P-value |
|---|---|---|---|---|---|
| Whole Cohort | 367 | 298 | 38 | 31 | |
| Surgical outcome | |||||
| 0-10mm | 267 (72.8%) | 209 (70.1%) | 33 (86.8%) | 25 (80.6%) | 0.55 |
| >10mm | 100 (27.2%) | 89 (29.9%) | 5 (13.2%) | 6 (19.4%) | |
| Surgical outcome | |||||
| 0mm | 84 (22.9%) | 63 (21.1%) | 12 (31.6%) | 9 (29.0%) | 0.15 |
| 1-10mm | 183 (49.9%) | 146 (49.0%) | 21 (55.3%) | 16 (51.6%) | |
| >10mm | 100 (27.2%) | 89 (29.9%) | 5 (13.2%) | 6 (19.4%) | |
*p-value's obtained by two-sided Pearson Chi-Square Test
A univariate analysis of surgical outcomes of the combined MSKCC and TCGA cohorts is shown in Table 4. In this analysis, stage was not associated with debulking status. Both age (10-year OR, 1.33; 95% CI, 1.07-1.65, P = 0.01) and BRCA mutations (OR, 0.47, 95% CI, 0.23-0.94, P = 0.03) were significantly associated with surgical outcomes. Finally, patient cohort significantly predicted surgical outcome (OR, 0.41; 95% CI, 0.23-0.74; P = 0.01). A multivariate analysis of surgical outcomes incorporating age at diagnosis, patient cohort, and BRCA mutation status is shown in Table 5. In this multivariate model, age (10-year OR, 1.25; 95% CI, 1.01-1.56; P=0.05) and patient cohort (OR, 0.47; 95% CI, 0.25–0.85; P=0.01) maintained at least borderline significance. BRCA mutation status, however, was not associated with surgical outcome (OR, 0.63; 95% CI, 0.31-1.29; P=0.21).
Table 4.
Surgical outcome Univariate Analysis (MSKCC and TCGA Patients Combined)*
| Variables | No. of Patients | Odds Ratio | 95% Confidence Interval | Log-Rank P |
|---|---|---|---|---|
| Age (10-years) | 361 | 1.33 | 1.07 - 1.65 | 0.01 |
| Stage | ||||
| III | 309 | 0.81 | 0.43 - 1.54 | 0.53 |
| IV | 52 | Ref. | ||
| Patient Cohort | ||||
| MSKCC | 101 | 0.41 | 0.23 - 0.74 | <0.01 |
| TCGA | 260 | Ref. | ||
| BRCA Status | ||||
| BRCA (+) | 67 | 0.47 | 0.23 - 0.94 | 0.03 |
| BRCA (-) | 294 | Ref. | ||
Table 5.
Surgical outcome Multivariate Analysis (MSKCC and TCGA Patients Combined)*
| Variables | No. of Patients | Odds Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|---|
| Age (10 years) | 361 | 1.25 | 1.01 - 1.56 | 0.05 |
| Cohort | ||||
| MSKCC | 101 | 0.47 | 0.25 - 0.85 | 0.01 |
| TCGA | 260 | Ref. | ||
| BRCA | ||||
| BRCA (+) | 67 | 0.63 | 0.31 -1.29 | 0.21 |
| BRCA (-) | 294 | Ref. | ||
All estimates reflect binary logistic model predicting for optimal versus suboptimal debulking (optimal include 0mm and 0-10mm), reference outcome sub-optimal debulking
Survival
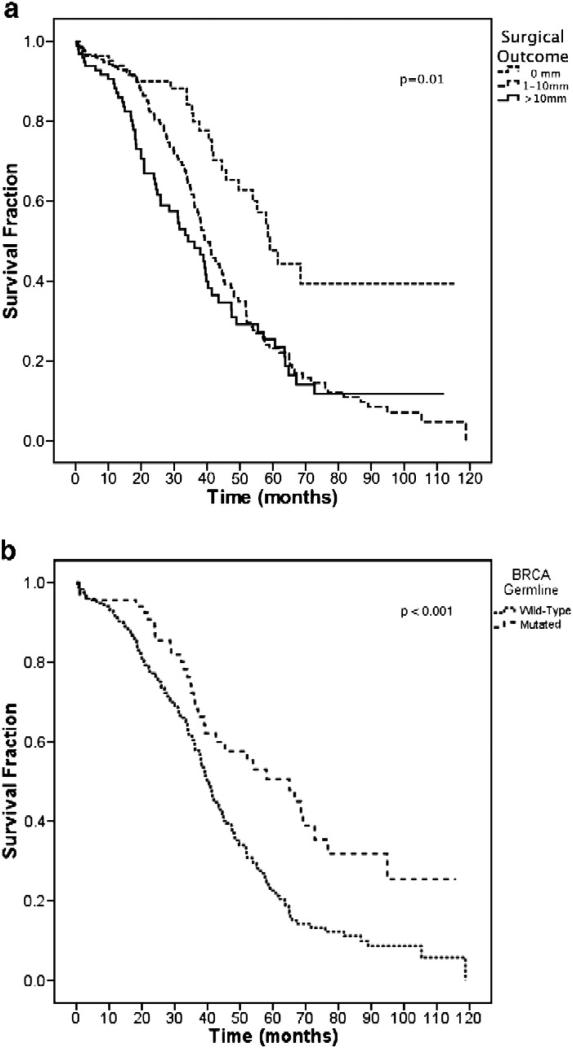
During the period of follow-up, 208 deaths were observed in the total cohort of 365 patients. Median follow-up time for the entire cohort was 31.2 months, and 27.4 months for patients still alive. Figure 1A depicts the Kaplan-Meier overall survival curves by surgical outcome. Median overall survival was 59.1 months (0 mm), 39.3 months (1-10 mm), and 34.2 months (>10 mm). These differences were significant (P value for group 0.01). Figure 1B depicts the Kaplan-Meier overall survival curves by BRCA status. Median overall survival was 60.0 months for patients with BRCA1/2 mutations, and 40.3 months for patients without BRCA1/2 mutations (P <0.001).
Figure 1.
Overall survival stratified by surgical outcome (A) and BRCA germline mutation (B).
Discussion
Our data suggest that women with BRCA1/2-associated and sporadic ovarian cancer have similar rates of optimal debulking (0-10mm) following primary surgical cytoreduction. Although our univariate analysis suggested BRCA1/2-mutated patients had smaller residual tumor volumes, this effect was no longer observed after controlling for differences in surgical outcome by age. These data suggest that any improvement in surgical outcome observed in BRCA1/2-mutated patients may be the result of the younger age at which these patients develop ovarian cancer.
Our analysis grouped patients with complete gross resection (0 mm) and optimal but visible disease (1-10 mm) in order to improve the power of the multivariate analysis to detect differences in surgical outcome. However, BRCA status remained a non-significant predictor of residual tumor volume when patients with complete gross resection were compared to patients with any visible disease (data not shown).
Age is proven to be a reliable predictor of poorer outcome in advanced ovarian cancer [4], but it is unclear if there is a direct association between older patients and inferior surgical outcomes. It is possible that surgeons are willing to undertake a more aggressive operative approach in younger patients in order to achieve optimal cytoreduction. However, when comparing the older and younger half of the 85 patients in the MSKCC cohort with residual tumor volumes of 0-10 mm, no differences in the rate of bowel resection, ostomy creation, splenectomy, distal pancreatectomy, or diaphragm resection were observed. This suggests that younger patients did not, on average, require more procedures in order to achieve an optimal debulking. If younger patients have better operative outcomes because their surgeons are willing to be more aggressive, one might expect this to be reflected in the number or type of procedure undertaken in these patients. Detailed surgical reports on patients in the TCGA cohort were not available. Few previous studies have specifically examined the effect of increasing age on the outcome of primary surgical cytoreduction. In one series of 360 consecutive patients who underwent primary surgery for ovarian cancer, age was a significant predictor of residual tumor size on univariate, but not multivariate, analysis [20]. In another multicenter study examining the ability of preoperative computed tomography to predict optimal tumor debulking, no age-dependent differences were seen [21].
Our study also did not find differences in surgical outcome in patients with FIGO stage III or stage IV disease. Although it is possible this is due to the small number of stage IV patients (14.8%) in the combined cohort, it is more likely that our exclusion of patients who had neoadjuvant chemotherapy explains this finding. Neoadjuvant chemotherapy followed by interval surgical cytoreduction is an accepted standard of care in patients with clearly unresectable stage IV disease [22]. We chose, however, to exclude patients who received neoadjuvant chemotherapy for several reasons. First, there are many unmeasurable factors that influence a gynecologic oncologist's decision to pursue this treatment strategy. Second, patients who have a suboptimal response to neoadjuvant chemotherapy are often never taken for interval cyctoreduction. Finally, the TCGA cohort excluded patients who received neoadjuvant chemotherapy and including these patients in the MSKCC cohort would introduce potential biases. As a result, the stage IV patients in this study were heavily enriched for patients with lower volume, or more resectable, disease.
Our analysis has several important strengths. Our combined cohort of 367 patients, including 69 with deleterious BRCA1/2 mutations, is fairly large given the relative rarity of BRCA1/2 mutations. Moreover, all patients had high-grade (FIGO Grade 2 or 3) serous cancer. Because histology and possibly grade may influence cytoreducibility and are also related to BRCA status, this restriction minimizes another source of potential confounding [23]. Our analysis includes patients from 15 different high-volume surgical centers staffed by experienced gynecologic oncology surgeons. Surgeon experience and subspecialty training are both factors that are well known to influence the outcome of primary cytoreduction and, in fact, were observed in this study as well [24]. Combined with the fact that the rates of complete and optimal tumor debulking seen here are consistent with previously published rates, it is unlikely these finding are the result a particular set of surgical practices at a single institution or group of institutions.
The cohorts in this analysis were also crafted to limit ascertainment bias with respect to BRCA mutation status. The MSKCC cohort was limited to patients tested for BRCA mutations within 2 years of the initial diagnosis of ovarian cancer. This restriction, which caused us to remove 34 patients from the analysis, eliminated patients who may have been BRCA tested due to unexpected longevity. This is especially important as overall survival is also associated with residual tumor volume. The TCGA cohort was composed of entirely incident ovarian cancer cases selected on the basis of tissue availability that met TCGA requirements. Finally, the control patients in our analysis were all confirmed non-carriers rather than untested matched controls. Our decision to include MSKCC patients with variants of unknown significance (VUS) in the wild-type cohort could potentially bias our findings in favor of the null-hypothesis (ie-concluding there is no difference in optimal cytoreduction between BRCA mutant and wild-type patients) if these mutations were later determined to be deleterious. However, only one of the 69 wild-type MSKCC patients had a VUS and a repeat analysis excluding this patient did not change our results or their significance levels (data not shown).
The clinical outcomes of patients included in our analysis provide further confidence that the cohort is representative of the larger ovarian cancer population. The significant differences in the median survival of patients who achieved complete, optimal, and suboptimal resection are consistent with previously published reports [5,25]. The median survival of patients with BRCA-associated ovarian cancer was better than that for sporadic ovarian cancers, consistent with prior studies [10,11]. There were significant differences in surgical outcomes in the MSKCC and TCGA cohorts. It is possible that these differences are the result of the tissue selection criteria use by the TCGA, which required banking of relatively large tumor specimens. This may have biased the TCGA cohort towards patients with greater disease burdens that were, therefore, less likely to be optimally debulked.
We found that despite previously established differences in the pathologic appearance and biologic behavior of BRCA-associated and sporadic ovarian cancers, the rate of primary optimal tumor debulking of these two entities was not significantly different. Our finding does not rule out the possibility that a much larger study might detect a small, but statistically significant, difference in surgical outcomes of these two groups. We conclude that the improved survival seen in ovarian cancer patients with germline BRCA mutations is unlikely explained by differences in surgical outcome.
Acknowledgments
Funding: Project Hope for Ovarian Cancer Research and Education; Kaleidoscope of Hope Foundation; Eileen Genet Fund, Entertainment Industry Foundation Revlon Run/Walk for Women, Chandler Cox Fund for Ovarian Cancer Research
Footnotes
Conflict of Interest/Disclosure Statement:
NK has received consulting fees and has been an expert witness for Pfizer. No other authors have financial disclosures to report.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of Surgical Outcome as Prognostic Factor in Advanced Epithelial Ovarian Cancer: A Combined Exploratory Analysis of 3 Prospectively Randomized Phase 3 Multicenter Trials By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 3.Winter WE, Maxwell GL, Tian CQ, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: A gynecologic oncology group study. Journal of Clinical Oncology. 2008;26:83–89. doi: 10.1200/JCO.2007.13.1953. [DOI] [PubMed] [Google Scholar]
- 4.Winter WE, 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 5.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. Journal of Clinical Oncology. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 6.Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–2816. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 7.Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001 Mar;68(3):700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chetrit A, Hirsh-Yechezkel G, Ben-David Y, Lubin F, Friedman E, Sadetzki S. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: the national Israeli study of ovarian cancer. J Clin Oncol. 2008;26:20–25. doi: 10.1200/JCO.2007.11.6905. [DOI] [PubMed] [Google Scholar]
- 9.Boyd J, Sonoda Y, Federici MG, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA. 2000;283:2260–2265. doi: 10.1001/jama.283.17.2260. [DOI] [PubMed] [Google Scholar]
- 10.Hyman DM, Zhou Q, Iasonos A, et al. Improved survival for BRCA2-associated serous ovarian cancer compared with both BRCA-negative and BRCA1-associated serous ovarian cancer. Cancer. 2011 Dec 2; doi: 10.1002/cncr.26655. Published online ahead of print. doi: 10.1002/cncr.26655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–1565. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127–32. doi: 10.1093/annonc/mdq577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gourley C, Michie CO, Roxburgh P, et al. Increased incidence of visceral metastases in scottish patients with BRCA1/2-defective ovarian cancer: an extension of the ovarian BRCAness phenotype. J Clin Oncol. 2010;28:2505–2511. doi: 10.1200/JCO.2009.25.1082. [DOI] [PubMed] [Google Scholar]
- 14.Shaw PA, McLaughlin JR, Zweemer RP, et al. Histopathologic features of genetically determined ovarian cancer. Int J Gynecol Pathol. 2002;21:407–411. doi: 10.1097/00004347-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin. Cancer Res. 2004;10:2473–2481. doi: 10.1158/1078-0432.ccr-1029-3. [DOI] [PubMed] [Google Scholar]
- 16.Soslow RA, Han G, Park KJ, et al. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod Pathol. 2011 Dec 23; doi: 10.1038/modpathol.2011.183. 2011 Dec 23 Epub ahead of print. doi: 10.1038/modpathol.2011.183. [DOI] [PubMed] [Google Scholar]
- 17.Scheuer L, Kauff N, Robson M, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20:1260–1268. doi: 10.1200/JCO.2002.20.5.1260. [DOI] [PubMed] [Google Scholar]
- 18.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutner MH, Nachtsheim C, Neter J. Applied linear regression models. 4th ed. McGraw-Hill/Irwin; Boston ; New York: 2004. [Google Scholar]
- 20.Fotopoulou C, Richter R, Braicu EI, Schmidt SC, Lichtenegger W, Sehouli J. Can complete tumor resection be predicted in advanced primary epithelial ovarian cancer? A systematic evaluation of 360 consecutive patients. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2010;36:1202–1210. doi: 10.1016/j.ejso.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Axtell AE, Lee MH, Bristow RE, et al. Multi-institutional reciprocal validation study of computed tomography predictors of suboptimal primary cytoreduction in patients with advanced ovarian cancer. J Clin Oncol. 2007;25:384–389. doi: 10.1200/JCO.2006.07.7800. [DOI] [PubMed] [Google Scholar]
- 22.Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 23.Eltabbakh GH, Mount SL, Beatty B, Simmons-Arnold L, Cooper K, Morgan A. Factors associated with cytoreducibility among women with ovarian carcinoma. Gynecologic Oncology. 2004;95:377–383. doi: 10.1016/j.ygyno.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 24.Vernooij F, Heintz P, Witteveen E, van der Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: a systematic review. Gynecol Oncol. 2007;105:801–812. doi: 10.1016/j.ygyno.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]