Abstract
The current research explored the neural mechanisms linking social status to perceptions of the social world. Two fMRI studies provide converging evidence that individuals lower in social status are more likely to engage neural circuitry often involved in ‘mentalizing’ or thinking about others' thoughts and feelings. Study 1 found that college students' perception of their social status in the university community was related to neural activity in the mentalizing network (e.g., DMPFC, MPFC, precuneus/PCC) while encoding social information, with lower social status predicting greater neural activity in this network. Study 2 demonstrated that socioeconomic status, an objective indicator of global standing, predicted adolescents' neural activity during the processing of threatening faces, with individuals lower in social status displaying greater activity in the DMPFC, previously associated with mentalizing, and the amygdala, previously associated with emotion/salience processing. These studies demonstrate that social status is fundamentally and neurocognitively linked to how people process and navigate their social worlds.
Keywords: Social status, SES, Mentalizing, fMRI
Social hierarchies are a ubiquitous feature of social groups, from adolescent cliques to the stratification of wealth across societies. Decades of research now suggest that social status, or an individual's place in a social hierarchy, is predictive of a variety of important outcomes, such as physical and mental health (Adler et al., 1994; Gianaros and Manuck, 2010) as well as neurocognitive functioning (e.g., working memory, language processing; Noble et al., 2007). Where one stands in a social hierarchy is a critical determinant of their psychological and biological outcomes.
Social status also seems to affect how people navigate their social worlds. A growing body of research has begun to document status-based differences in social behavior. For example, during interpersonal interactions, individuals who are relatively lower in social status exhibit cues that they are closely attending to an interaction partner (more eye contact, head nodding, laughing; Kraus and Keltner, 2009). By contrast, higher-status individuals' are more likely to behave in ways suggesting less engagement in the interaction (relatively more self-grooming, fidgeting, doodling). Furthermore, relative to higher-status individuals, low-status individuals are more likely to give to others during economic bargaining games, and they donate a greater percentage of their income to charity (Piff et al., 2010; Rucker et al., 2011). Together, these data converge on the idea that social status moderates the extent to which individuals are focused on others.
A related line of research suggests that social status not only guides social behavior, it also influences social cognitive processes. In particular, social status affects performance on tasks that involve thinking about the thoughts and feelings of others. For example, individuals who are lower-status are more accurate at inferring the emotional states of others, relative to their higher-status counterparts (Kraus et al., 2010). When people are experimentally manipulated to feel low status, they are more accurate at reading the emotions of others, compared to when they are made to feel high status (Kraus et al., 2010). Finally, participants who are asked to recall a time in which they had low power (a characteristic similar to status) are more likely to adopt the perspective of another person than individuals who recall a time in which they had high power (Galinsky et al., 2006). Together with the literature on social status-based differences in social behavior, these data provide support for the hypothesis that lower-status individuals are more likely to engage in social cognitive processes that aid in understanding how others think, feel, and behave.
Although past research suggests that social status affects the tendency to try and understand how others think and feel, much of this research has explicitly asked participants to take the perspective of another (e.g., “identify the emotion the person in this photo is feeling”; cf. Galinsky et al., 2006). Thus, it remains relatively unclear whether individuals who are lower in social status spontaneously think about the thoughts and feelings of others when they encounter social situations, or if they are simply better at inferring others' beliefs and emotions when directed to do so. Neuroimaging methods offer an opportunity to examine the extent to which people may be engaging social cognitive processes even when they are not explicitly asked to perform such tasks. Although some neuroimaging studies have examined how people process cues of social status in others (Chiao, 2010; Chiao et al., 2009; Marsh et al., 2009), and the role of social status in the context of performance-based feedback (Zink et al., 2008), no known studies have examined the relationship between an individual's own social status and their neural activity during tasks that involve understanding others.
As it turns out, the neural processes that are engaged when thinking about the thoughts and feelings of others are well documented. Specifically, a network of brain regions, including the dorsomedial prefrontal cortex (DMPFC), medial prefrontal cortex (MPFC), precuneus/posterior cingulate cortex (PCC), temporoparietal junction (TPJ), and posterior superior temporal sulcus (pSTS) is consistently activated during tasks that require understanding the mental states of others, or mentalizing (Frith and Frith, 2006; Lieberman, 2010; Mitchell, 2009).
To examine if social status modulates neural activity in the mentalizing network, we conducted two functional magnetic resonance imaging (fMRI) studies. In Study 1, we examined how a subjective measure of social status related to neural activity when encoding information about another individual. Given that subjective perceptions of social status have been shown to predict certain health outcomes even better than more objective measures of status (i.e., socioeconomic status [SES]; Singh-Manoux et al., 2005), we focused on subjective social status in this study. We employed a task that involved encoding information about another individual, given that this type of task has been shown in prior work to reliably engage the mentalizing network (for a review, see Mitchell, 2009). We predicted that individuals lower in social status would show greater activity in brain regions typically engaged during mentalizing, compared to high status individuals. In a second study, we used a new subject population – adolescents – and examined whether a more objective measure of social status, SES, would modulate neural activity during a threat-processing task. Responses to a threat-processing task may be particularly illuminating of status-based differences in mentalizing, as interpretations of whether another's intentions are threatening – which requires mentalizing – has been shown to be moderated by SES (Chen et al., 2004). We predicted that adolescents from lower SES households would show greater neural activity in brain regions involved in mentalizing during this task compared to high SES adolescents. Across the two studies, we predicted that regardless of subject population and whether the stimuli were threatening or non-threatening, those lower in social status would show greater neural activity in mentalizing-related neural regions.
Study 1
Method
Participants
Participants were 16 undergraduate students (8 males) who were between the ages of 18 and 24 (M age = 19.81 years, SD = 1.8). All participants were Caucasian, right-handed, and reported no history of neurological disorder.
Procedure
Participants underwent an fMRI scan while they viewed pictures and read social and non-social passages describing people and objects. Following the scan, participants completed a measure of subjective social status.
Measures
Social status measure
To measure social status, participants completed a modified version of the MacArthur Subjective Social Status Scale (Adler et al., 2000). Participants were shown a picture of a ladder with ten rungs: at the top of the ladder are the people who are the best off (most money, most education, best jobs); at the bottom of the ladder are the people who are the worst off (least money, least education, worst jobs). They were asked to indicate on which rung they thought they were, in reference to the rest of the UCLA community, given that this was likely to be the group in which status was most salient for a college-student sample. Scores ranged from 2 to 8 (out of a possible 10; M = 6.16, SD = 1.91), indicating that participants varied in their perceptions of their social status within the UCLA community.
Neuroimaging task
Participants were scanned using BOLD fMRI while they were presented with a series of images (described below), each accompanied by a descriptive text passage (see Supplementary Fig. 1; designed by S. Morelli). First, participants viewed a fixation crosshair for 4 s. Then, they completed a self-paced task. Participants were asked to look at a photo, read a passage, and press a button to advance to the next screen when they were finished reading, which yielded a measure of reaction time. Participants completed a total of four trials, alternating between two social-information trials, and two object-information trials (presented in counterbalanced order). After making a button press to indicate they had finished reading the passage, the passage disappeared, and a fixation crosshair was presented for 15 s.
During the “social information” trials of the task, participants viewed an image of a UCLA student that matched the participant's gender and ethnicity, and read two passages the person supposedly wrote. The passages were written from a first-person perspective, using the pronoun “I”. Importantly, participants were not explicitly instructed to take the perspective of the person in the photo. One passage described the pictured individual's thoughts and feelings at the beginning of a new quarter of school; the other described his/her thoughts and feelings about going to lunch with a friend.
During the “object information” trials of the task, participants viewed an image of an inanimate object (e.g., pedometer, flash drive) and read a passage describing the object in an objective, unemotional way. These trials were designed as a comparison task for the social task: They did not involve any social information, but still required participants to view an image and read a description of that image.
fMRI data acquisition
Images were collected using a Siemens Trio 3-T MRI scanner. Participants were instructed to hold as still as possible during the scan; foam padding around the head was provided to restrict motion. A high-resolution structural scan coplanar with the functional scans was obtained for functional image registration during pre-processing (echo planar fast T2-weighted segmented spin echo, TR = 5000 ms, TE = 34 ms, FOV = 220 mm, 33 slices, 4.0 mm slice thickness). Task stimuli were presented on a computer screen through MR-compatible goggles. Both the social-information and object-information tasks were presented during a functional scan lasting approximately 5 min (parameters for functional scan: echo-planar T2*weighted gradient-echo, TR = 2000 ms, TE = 30 ms, flip angle = 75, 33 slices, FOV = 220 mm, 4.0 mm slice thickness).
fMRI data analysis
Data analysis was performed using SPM5 (Wellcome Department of Imaging Neuroscience, London). Images were realigned, coregistered, normalized into Montreal Neurological Institute (MNI) space, resliced into voxels of 3 mm cubed and smoothed with an 8 mm Gaussian kernel. First-level effects were estimated using the general linear model and employed a canonical hemodynamic response function convolved with the experimental design. Low-frequency noise was removed using a high-pass filter (128 s). The task was modeled at the first (subject) level as a block design with two conditions (object information, social information). Linear contrasts between the two conditions were computed for each participant. Random effect analyses of the group were computed using the contrast images generated for each participant.
We first examined the main effect of social information processing by comparing neural activity during the social-information trials with activity during the object-information trials in a whole-brain analysis. Then, to examine how social status related to neural activity in mentalizing regions during social (vs. object) information processing, participants' ratings of their social status were entered as a regressor in the contrast of social information >object information. Given our strong a-priori hypothesis regarding the relation of social status to neural activity in mentalizing brain regions, we restricted our analysis to only search for significantly active clusters within anatomically defined regions-of-interest (ROIs) based on the Automated Anatomical Labeling atlas for regions known to be involved in mentalizing. These included DMPFC (−20<x<20, 30<y<66, 26<z<64), MPFC (−20<x<20, 46<y<76, −10<z<26), bilateral TPJ (for left: −70<x<−38, −64<y<−40, 22<z<38; for right: 38<x<70, −64<y<−40, 22<z<38), bilateral pSTS (for left: −70<x<−46, −58<y<−30, −4<z<16; for right: 46<x<70,−58<y<−30,−4<z<16), and precuneus/PCC (−20<x<20, −82<y<−30, 10<z<84). All ROIs were combined to create one ‘mentalizing mask’. Statistical significance was based on both a peak threshold and a spatial extent threshold that corrects for multiple comparisons to a level of p < .05. Spatial extent threshold was determined by 10,000 Monte Carlo simulations conducted using the AlphaSim program in AFNI. The criteria input to AlphaSim included uncorrected p-value (.005), voxel size (3 × 3 × 3), spatial smoothing kernel (8 mm), and the number of voxels in the mask (5276). Based on these parameters, a cluster extent of 21 voxels was necessary in order to achieve a corrected threshold of p < .05. All coordinates are reported in Montreal Neurological Institute (MNI) format.
Results and discussion
To confirm that the social information trials were associated with greater activity in mentalizing regions, we examined neural activity for the social compared to the object trials. Results indicated that, during social vs. object information trials, participants displayed heightened activity in regions known to comprise the mentalizing neural network, including DMPFC, MPFC, precuneus/PCC, and left pSTS (see Supplementary Table 1 for complete information).
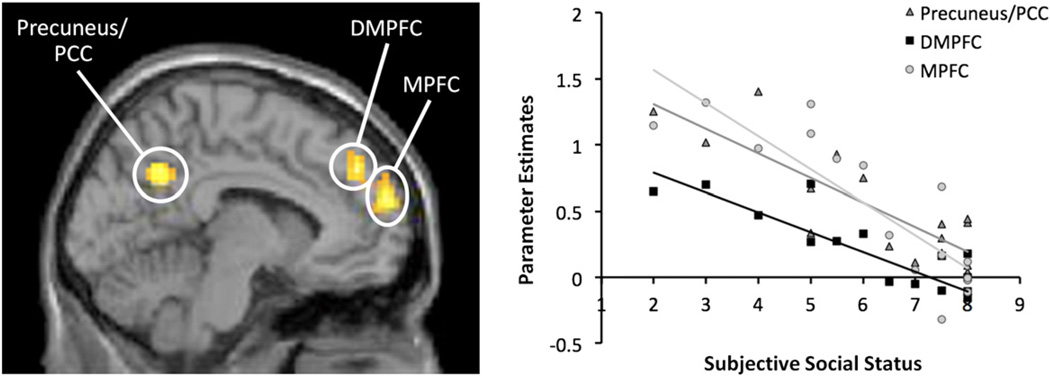
Next, to examine how social status related to neural activity during the social compared to object trials, we examined correlations between social status and activity in mentalizing ROIs. Consistent with hypotheses, results revealed a negative correlation between social status and neural activity in clusters within DMPFC, MPFC, and precuneus/PCC (see Table 1/Fig. 1), such that lower status was associated with greater activity in these regions. There were no significantly active clusters in TPJ or pSTS. When we expanded our search space to include all voxels of the brain (not just the mentalizing ROI mask), there were no additional neural regions that showed a negative correlation with social status. Status did not correlate positively with neural activity in any region.1
Table 1.
Clusters within the mentalizing network that were negatively correlated with social status, in the contrast social information >object information (p < .05, corrected).
| Region | Hemisphere | x | y | z | t | k | BA |
|---|---|---|---|---|---|---|---|
| DMPFC | L | −6 | 45 | 36 | 5.27 | 203 | 9 |
| MPFC | R | 3 | 60 | 12 | 6.33 | 174 | 10 |
| Precuneus/PCC | L | −9 | −60 | 36 | 3.2 | 83 | 7 |
Note. Coordinates are reported in MNI space. Hemisphere refers to the hemisphere of the peak voxel in the cluster; all activations extend bilaterally. BA refers to the putative Brodmann's Area. The following abbreviations are used for the names of specific regions: medial prefrontal cortex (MPFC), dorsomedial prefrontal cortex (DMPFC), posterior cingulate cortex (PCC).
Fig. 1.
Regions that correlated negatively with social status during social information vs. object information trials in Study 1. Clusters within ROIs in DMPFC, MPFC, and Precuneus/PCC that were significantly associated with social status are displayed at left. Scatter plot showing the correlation between activation in each region and social status is displayed at right.
To rule out the possibility that any status-based differences in neural activity were due to differences in the amount of time spent processing the passages, we subtracted reaction times to reading the object information from reaction times to reading the social information, and correlated these difference scores with our measure of social status. There was no correlation between reaction time differences and social status (r = −.05, ns), suggesting that the patterns of neural activity observed as a function of status were not simply due to the amount of time spent processing the stimuli.
Results from Study 1 indicate a reliable, negative association between perceptions of social status and neural activity in regions of the mentalizing network during the encoding of social information. These results suggest that when presented with social information, low-status individuals may spontaneously focus more on the mental states of others.
Study 2
Study 1 focused on university students' subjective perceptions of their social status. In Study 2, we wanted to examine whether a more objective indicator of social status, SES, related to neural activity in the mentalizing network. We also wanted to extend these findings to a sample of adolescents. Given that SES in childhood and adolescence is a better predictor of health outcomes than adult SES (Kittleson et al., 2006), understanding the neurocognitive correlates of social information processing in children and adolescents is of paramount importance. However, it remains unclear if lower-status adolescents are more likely to engage neural circuitry involved in thinking about others during social processing tasks.
In addition, because threat responses are hypothesized to be one mechanism by which social status influences health, we designed Study 2 to look at neural responses to social threat. Previous research suggests that adolescents who are lower in SES are more likely to interpret social situations as threatening (Chen et al., 2004), and to respond to threatening social cues with more neural activity in the amygdala, a brain structure engaged during the processing of emotion and salience (Gianaros et al., 2007). As a result, Study 2 focused on neural responses to socially threatening images. Based on results from Study 1, we hypothesized that lower SES would be associated with greater mentalizing-related neural activity during the viewing of threatening facial expressions.
Method
Participants
Participants were 22 adolescents (14 females) between the ages of 12 and 13 at the time of the fMRI scan (M = 13.02 years, SD = .29), and were taking part in a large, longitudinal study of neural development during adolescence (e.g., Pfeifer et al., 2011).
Procedure
Participants were invited, with their parents, to UCLA, where they underwent an fMRI scan while viewing pictures of threatening facial expressions (see below for more detail on the MRI task). The participant's parent who brought him/her to the scan was asked to report their SES (see below).
Measures
Social status measure
The parent of each participant was asked to indicate which of 22 categories best represented their household income (range: “under $15,000/year” to “greater than $400,000/year”). Parents also reported on the highest level of education the child's mother completed (e.g., B.A./B.S. degree). These two measures were z-scored and combined to form a composite index of SES (Kraus et al., 2009). Overall, adolescents in the present sample had parents who were well educated (median education level = college degree), and lived in households that were relatively high income (median = $ 100,000–120,000/year). However, there was substantial variability across the sample (range for maternal education = high school diploma–graduate degree; range for household income = $15,000/year to >$320,000/year).
Neuroimaging task
Participants were scanned using BOLD fMRI while they viewed threatening emotional expressions, specifically expressions of anger. Images were taken from the NimStim database of emotional faces (Tottenham et al., 2009). Participants were simply instructed to passively view the facial expression for 2 s. We were particularly interested in the neural response to expressions of anger, as previous research has demonstrated that anger is an emotion expression relevant to social status, particularly for those with low status (Allan and Gilbert, 2002; Wilkinson, 1999).
As a baseline, participants also viewed a fixation crosshair in the center of the screen for a variable interval between the emotional expressions (ranging from 0.5–1.5 s; see Supplementary Fig. 2). Participants viewed 16 angry-face trials and 16 fixation-trials.
fMRI data acquisition
Data were acquired using a Siemens Allegra 3.0 T MRI scanner. A 2D spin-echo scout (TR = 4000 ms, TE = 40 ms, matrix size 256 × 246, 4-mm thick, 1-mm gap) was acquired in the sagittal plane to allow prescription of the slices to be obtained in the remaining scans. For each participant, a high-resolution structural T2-weighted echo-planar imaging volume (spin-echo, TR = 5000 ms, TE = 33 ms, matrix size 128 × 128, FOV = 20 cm, 36 slices, 1.56-mm in-plane resolution, 3-mm thick) was acquired coplanar with the functional scan, for registration during pre-processing. Task stimuli were presented on a computer screen through MR-compatible goggles. Both the angry facial expressions and fixation baseline conditions were presented during a functional scan (parameters for functional scan: gradient-echo, TR = 3000 ms, TE = 25 ms, flip angle = 90, matrix size 64 × 64, FOV = 200 mm, 36 slices, 3.0 mm slice thickness).
fMRI
Using Automated Image Registration (Woods et al., 1998a, 1998b) implemented in the LONI Pipeline Processing Environment (http://www.pipeline.loni.ucla.edu; Rex et al., 2003) all functional images were realigned to correct for head motion, co-registered to their respective high-resolution structural images using a 6-parameter rigid body transformation model, spatially normalized into a Talairach-compatible MR atlas (Woods et al., 1999) using polynomial nonlinear warping, and smoothed using a 6-mm Gaussian kernel.
Statistical analyses were implemented in SPM2 (Wellcome Department of Cognitive Neurology, London, UK). First-level effects were estimated using the general linear model and employed a canonical hemodynamic response function convolved with the experimental design. Low-frequency noise was removed using a high-pass filter. The task was modeled at the first (subject) level as an event-related design, with two conditions (angry, fixation). Linear contrasts comparing these conditions were computed for each participant. Random effects analyses of the group were computed using the contrast images generated for each participant.
We first examined the main effect of viewing angry expressions by comparing neural activity during the viewing of angry expressions vs. fixation in a whole-brain analysis (p < .005, 10 voxels). In addition, because the amygdala is known to respond to viewing negative facial expressions, we conducted ROI analyses of the left and right amygdala. Amygdala ROIs were structurally defined a priori based on the Automated Anatomical Labeling atlas, and we searched for significantly active clusters within these anatomically defined regions (for left amygdala: −32<x<−12, −12<y<4, −24<z<−8; for right amygdala: 12<x<32, −21<y<4, −24<z<−8). All ROIs were normalized into Talairach space, given that functional data were normalized to a standard Talairach template during pre-processing.
Then, to examine how social status related to neural responses in mentalizing regions during the processing of threatening facial expressions, parental SES was entered as a regressor in the contrast of threatening faces >fixation. Based on the results from Study 1, we restricted our analysis to only search for significantly active clusters within anatomically defined regions-of-interest (ROIs) based on the Automated Anatomical Labeling atlas for regions that were significantly correlated with social status in Study 1 (DMPFC, MPFC, precuneus/PCC). We also examined the relation of SES to neural activation in the amygdala, given its known role in SES and threat processing (Gianaros et al., 2007). As in Study 1, statistical significance was based on both a peak threshold and a spatial extent threshold that corrects for multiple comparisons to a level of p < .05. Spatial extent threshold was determined by 10,000 Monte Carlo simulations conducted using the AlphaSim program in AFNI. The criteria input to AlphaSim included uncorrected p-value (.005), voxel size (2 × 2 × 2) spatial smoothing kernel (6 mm), and number of voxels in each ROI (2560 in DMPFC, 4091 in MPFC, 7467 in precuneus/PCC, 716 in bilateral amygdala). Given that this is the first study to investigate how SES influences mentalizing in a younger population, we calculated a separate extent-threshold to achieve a corrected p value of .05 for each ROI in order to more fully probe the neural regions associated with SES in adolescents. Furthermore, because Study 2 uses a more exploratory task that we wouldn't necessarily expect to yield activation in mentalizing regions, correcting across a mask of all ROIs may limit our ability to explore activations that are smaller (i.e., amygdala) or more circumscribed given the task (Lieberman and Cunningham, 2009). This approach resulted in a minimum cluster size of 24 voxels in DMPFC, 28 voxels in MPFC, 33 voxels in precuneus/PCC, and 18 voxels in the amygdala. Although the functional scans were normalized to a Talairach template, for ease of comparison across studies, all coordinates have been converted to MNI space.
Results and discussion
To examine neural activity while viewing angry faces (regardless of SES), we compared neural activity during the viewing of the angry facial expressions to neural activity during fixation. Results showed greater activity in visual and fusiform regions (BA 17/18) during the processing of faces, compared to fixation baseline (x = −22, y = −84, z = −12, t = 7.42, k = 3980). Results from ROI analyses of the amygdala revealed a significantly active cluster in left amygdala (x = −18, y = −8, z = −17, t = 3.01, k = 11) during the processing of angry faces compared to fixation (p < .05). There were no significantly active clusters within the right amygdala.
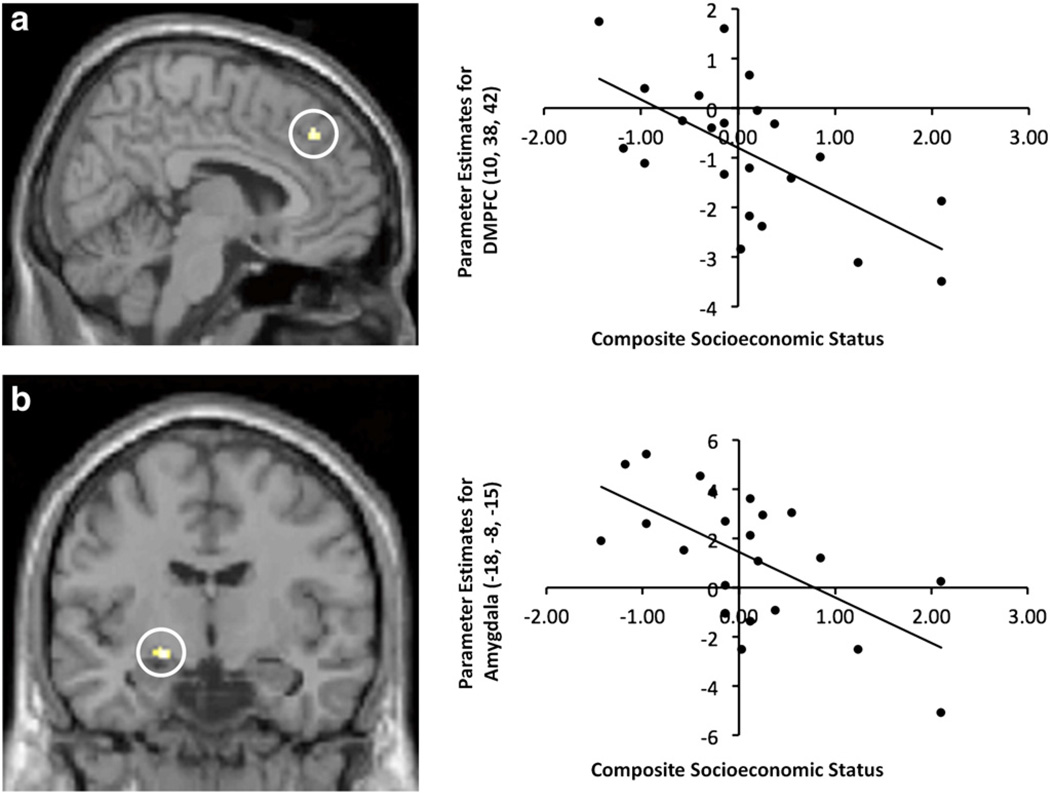
Next, we examined whether SES related to neural activity in the mentalizing network during the processing of angry facial expressions (relative to fixation). Results revealed a negative correlation between SES and neural activity in clusters within the DMPFC, as well as the left amygdala (see Table 2/Fig. 2). There were no significantly active voxels in other regions of the mentalizing network. Thus, lower SES was associated with greater activity in one region often engaged during mentalizing (i.e., DMPFC) as well as the amygdala, often associated with emotion/threat processing. When we expanded our search space to include all voxels of the brain (not just the mentalizing ROIs), there were no additional neural regions that showed a negative correlation with SES. Finally, SES did not correlate positively with neural activity in any regions.2
Table 2.
Clusters within the mentalizing network, and amygdala that were negatively correlated with socioeconomic status, in the contrast angry faces >baseline (p < .05, FDR corrected).
| Region | Hemisphere | x | y | z | t | k | BA |
|---|---|---|---|---|---|---|---|
| DMPFC | R | 10 | 38 | 42 | 3.18 | 25 | 9 |
| Amygdala | L | −18 | −8 | −15 | 3.84 | 19 |
Note. Coordinates are reported in MNI space. BA refers to the putative Brodmann's Area. DMPFC = dorsomedial prefrontal cortex.
Fig. 2.
Regions that correlated negatively with SES during the viewing of angry faces vs. fixation in Study 2. Panel a (left) depicts the cluster within a DMPFC ROI that was significantly associated with SES, and a scatter plot showing the correlation between activation in this DMPFC cluster and SES is depicted at right. Panel b (left) depicts the cluster within the amygdala ROI that was significantly associated with SES, and a scatter plot showing the correlation between activation in the amygdala cluster and SES is depicted at right.
Consistent with the results from Study 1, Study 2 demonstrated a negative correlation between an objective indicator of status – SES – and neural activity in a brain region involved in thinking about the minds of others (i.e., DMPFC). In addition, Study 2, which used threatening social images, also highlighted a negative relationship between SES and amygdala activity.
General discussion
The present studies investigated how social status relates to neural activity during tasks that may be related to the tendency to think about the thoughts and feelings of others. Across two studies, social status was associated with neural activity in a region of the mentalizing network (DMPFC), such that individuals who were lower in social status showed greater activity in this brain region. In Study 1, college students who reported having lower status in their university showed greater neural activity in mentalizing regions, including DMPFC, MPFC, and precuneus/PCC, while they viewed images and read descriptions of other students. In Study 2, adolescents who came from lower SES backgrounds exhibited greater neural activity in a core node of the mentalizing network (DMPFC), as well as the amygdala, during the processing of angry facial expressions. Together, these studies provide converging evidence that social status is related to neural activity in a region of the mentalizing network, across a variety of age groups, measures of social status, and tasks.
The current results provide the first evidence for a neural mechanism by which being lower in social status may be related to a greater ability to identify how others are thinking and feeling. Activity in mentalizing regions has been shown to lead to greater accuracy in identifying how others are feeling (Zaki et al., 2009). Thus, the fact that lower status individuals are more likely to engage these brain structures during the encoding of social information, even when not explicitly instructed to do so, may be one neural mechanism that leads them to make more accurate judgments about the thoughts and feelings of others (Kraus et al., 2010).
Why might social status relate to neural activity in the mentalizing network? One possible answer emerges when we consider the extent to which individuals of differential social status are dependent upon others to achieve their desired outcomes (Magee and Galinsky, 2008). By virtue of having relatively fewer material and social resources, lower-status individuals must rely more on other people to meet their needs (Kraus et al., 2009). This greater level of dependency likely leads lower-status individuals to be particularly motivated to understand others' thoughts, feelings, and behaviors, thus leading to greater neural activity associated with these types of cognitions.
In addition to finding that social status influences neural activity in a brain region involved in mentalizing, in Study 2 we also observed a negative relationship between SES and amygdala activity during the processing of threatening faces. This result is consistent with prior research showing that lower social status is associated with greater amygdala activity during the processing of angry faces (Gianaros et al., 2007). We did not find a correlation between social status and amygdala activity in Study 1, but this is not surprising given that the task employed involved viewing smiling faces and reading descriptions, which is non-threatening and focused more on linguistic processing.
Although the ability to understand others' thoughts and feelings is related to a variety of positive outcomes, including lower levels of aggression (Miller and Eisenberg, 1988), and decreased stereotyping and in-group favoritism (Galinsky and Moskowitz, 2000), a few studies suggest that activity in neural regions associated with thinking about others' thoughts and feelings may have a more negative side as well. For example, neural activity in DMPFC during social rejection or social stress is associated with greater cortisol responses to stress (Dedovic et al., 2009; Eisenberger et al., 2007). Furthermore, activity in DMPFC and precuneus/PCC is associated with greater blood pressure during stress (Gianaros et al., 2005). Together, these results suggest that mentalizing may not be a universally positive phenomenon; rather, thinking about others thoughts and feelings, particularly during conditions of stress or threat, may have negative consequences as well.
While there was some overlap in the neural regions that were correlated with social status in both studies (i.e., DMPFC), there were differences between activations observed in the two studies. Specifically, activity in MPFC and precuneus/PCC was correlated with social status in Study 1, but these regions did not emerge as correlating with status in Study 2. One possible reason for this discrepancy is related to the tasks employed. For example, some research has suggested that MPFC activation during mentalizing is more common when thinking about similar others (compared to dissimilar others; Mitchell et al., 2006), and the stimuli used in Study 1 (photos of gender, age, and ethnicity-matched students) may have been viewed as more similar to subjects than the stimuli presented in Study 2 (photos of an ethnically-diverse sample of adults, where the subjects were adolescents). Furthermore, although the precuneus/PCC is considered part of the mentalizing network, a quantitative review of mentalizing studies suggests that this parietal region is only found in 39% of mentalizing studies, compared to 91% of studies finding DMPFC (Lieberman, 2010). Thus, DMPFC may represent the core node of the mentalizing network, with other regions recruited differentially depending on the exact task demands and subject population. Additional research is needed to disentangle how individual regions contribute to the overall mentalizing network.
The present studies represent an important step in understanding how social status influences neurocognitive processes related to navigating the social world. However, the studies are not without limitations. For example, because we employed two different tasks in the current studies, we cannot examine how social status may influence neural responses to tasks involving understanding others across different periods of development. Future research could address this interesting question by examining longitudinally how neural activity may be modulated by social status. Furthermore, in Study 2 we asked adolescents' parents to report their level of income and education, which may or may not relate to the subjects' own perceptions of their family's socioeconomic status. It will be important for future work to examine if adolescents' subjective perceptions of their family's social status relates to activity in mentalizing regions, or if objective reports of SES have more utility in a younger population. Finally, given that we employed a passive task in Study 2, we cannot rule out the possibility that our results were influenced by some degree of greater attention in the lower SES adolescents. However, even if the lower SES subjects are paying closer attention to the threatening facial expressions, this is still consistent with the interpretation that lower status individuals exert more neurocognitive resources toward others. Future research should focus on disentangling what specific neural processes are modulated by social status.
In sum, the current studies suggest that individuals who are lower in social status are more likely to engage neural circuitry involved in thinking about the minds of others. The consistency of results across two studies is notable, especially given that we investigated different measures of social status (subjective vs. objective), different tasks (social information encoding vs. threat processing), and different samples (adults vs. adolescents). Understanding the neural mechanisms through which social status influences social cognition and social behavior may provide crucial insights regarding both how social status is linked to health and well-being, and ways to improve the psychological and physical well-being of those who stand at the bottom of the ladder looking up.
Supplementary Material
Acknowledgments
We would like to thank Larissa Borofsky, Natalie Colich, Austin Grinberg, Kristin McNealy, and Meghan Meyer for their help with data collection, Will Moore and the University of Oregon Developmental Social Neuroscience Laboratory for providing some of the ROIs used in the analyses, Bob Spunt for help with data analysis, and members of the UCLA Social and Affective Neuroscience Lab, and George Slavich, for comments on a previous version. For generous support, we thank the Santa Fe Institute Consortium, Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, and Northstar Fund. The project was also supported by grants RR12169, RR13642, and RR00865 from the National Center for Research Resources (NCRR), a NARSAD Young Investigator Award (to B.M.W), a National Science Foundation Graduate Research Fellowship (to K.A.M), and a Pre-Doctoral Fellowship in Biobehavioral Training in Mental and Physical Health T32 MH15760 (to K.A.M.).
Footnotes
There was no effect of gender on neural activity in the contrast of social information >object information, nor did gender moderate the correlation between social status and neural activity in mentalizing regions (all p's > .3). There were also no gender differences in reaction times to reading the passages (p > .2).
Once again, there was no effect of gender on neural activity in the contrast of angry faces >fixation, nor did gender moderate the correlation between SES and neural activity in DMPFC or amygdala (all p's > .3).
Appendix A. Supplementary data
Supplementary data to this article can be found online at doi:10.1016/j.neuroimage.2012.01.080.
References
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health: the challenge of the gradient. Am. Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19:586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Allan S, Gilbert P. Anger and anger expression in relation to perceptions of social rank, entrapment, and depressive symptoms. Pers. Individ. Dif. 2002;32:551–565. [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, Matthews KA. Socioeconomic status and health in adolescents: the role of stress interpretations. Child Dev. 2004;75:1039–1052. doi: 10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- Chiao JY. Neural basis of social status hierarchy across species. Curr. Opin. Neurobiol. 2010;20:1–7. doi: 10.1016/j.conb.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Oby ER, Zhang L, Parrish T, Bridge DJ. Neural representations of social status hierarchy in human inferior parietal cortex. Neuropsychologia. 2009;47:354–363. doi: 10.1016/j.neuropsychologia.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Rexroth M, Wolff E, Duchesne A, Scherling C, Beaudry T, Pruessner JC. Neural correlates of processing stressful information: an event-related fMRI study. Brain Res. 2009;1293:49–60. doi: 10.1016/j.brainres.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Galinsky AD, Moskowitz GB. Perspective-taking: decreasing stereotype expression, stereotype accessibility, and in-group favoritism. J. Pers. Soc. Psychol. 2000;78:708–724. doi: 10.1037//0022-3514.78.4.708. [DOI] [PubMed] [Google Scholar]
- Galinsky AD, Magee JC, Inesi ME, Gruenfeld DH. Power and perspectives not taken. Psychol. Sci. 2006;17:1068–1074. doi: 10.1111/j.1467-9280.2006.01824.x. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Manuck SB. Neurobiological pathways linking socioeconomic position and health. Psychosom. Med. 2010;72:450–461. doi: 10.1097/PSY.0b013e3181e1a23c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Derbtshire SWG, May JC, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42:627–635. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Hornstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, Cohen S. Potential neural embedding of parental social standing. Soc. Cogn. Affect. Neurosci. 2007;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittleson MM, Meoni LA, Wang N, Chu AY, Ford DE, Klag MR. Association of childhood socioeconomic status with subsequent coronary heart disease in physicians. Arch. Intern. Med. 2006;166:2356–2361. doi: 10.1001/archinte.166.21.2356. [DOI] [PubMed] [Google Scholar]
- Kraus MW, Keltner D. Signs of socioeconomic status: a thin-slicing approach. Psychol. Sci. 2009;20:99–106. doi: 10.1111/j.1467-9280.2008.02251.x. [DOI] [PubMed] [Google Scholar]
- Kraus MW, Piff PK, Keltner D. Social class, sense of control, and social explanation. J. Pers. Soc. Psychol. 2009;97:992–1004. doi: 10.1037/a0016357. [DOI] [PubMed] [Google Scholar]
- Kraus MW, Cote S, Keltner D. Social class, contextualism, and empathic accuracy. Psychol. Sci. 2010;21:1716–1723. doi: 10.1177/0956797610387613. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of Social Psychology. 5th ed. New York, NY: McGraw-Hill; 2010. pp. 143–193. [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Galinsky AD. Social hierarchy: the self-reinforcing nature of power and status. Acad. Manag. Ann. 2008;2:351–398. [Google Scholar]
- Marsh AA, Blair KS, Jones MM, Soliman N, Blair RJR. Dominance and submission: the ventrolateral prefrontal cortex and responses to status cues. J. Cogn. Neurosci. 2009;21:713–724. doi: 10.1162/jocn.2009.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PA, Eisenberg N. The relation of empathy to aggressive and externalizing/antisocial behavior. Psychol. Bull. 1988;103:324–344. doi: 10.1037/0033-2909.103.3.324. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Inferences about mental states. Philos. Trans. R. Soc. B. 2009;364:1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piff PK, Kraus MW, Cote S, Cheng BH, Keltner D. Having less, giving more: the influence of social class on prosocial behavior. J. Pers. Soc. Psychol. 2010;99:771–784. doi: 10.1037/a0020092. [DOI] [PubMed] [Google Scholar]
- Rex DE, Ma JQ, Toga AW. The LONI pipeline processing environment. NeuroImage. 2003;19:1033–1048. doi: 10.1016/s1053-8119(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Rucker DD, Dubois D, Galinsky AD. Generous paupers and stingy princes: power drives consumers' spending on self versus others. J Consum Res. 2011;37:1015–1029. [Google Scholar]
- Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosom. Med. 2005;67:855–861. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey B, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson RG. Health, hierarchy, and social anxiety. Ann. N.Y. Acad. Sci. 1999;896:48–63. doi: 10.1111/j.1749-6632.1999.tb08104.x. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J. Comput. Assist. Tomogr. 1998a;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J. Comput. Assist. Tomogr. 1998b;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woods RP, Dapretto M, Sicotte NL, Toga AW, Mazziotta JC. Creation and use of a Talairach-compatible atlas for accurate, automated, nonliner intersubject registration, and analysis of functional imaging data. Hum. Brain Mapp. 1999;8:73–79. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<73::AID-HBM1>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proc. Natl. Acad. Sci. U.S.A. 2009;106:11382–11387. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58:273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.