Abstract
The purpose of this study was to compare neuropsychiatric symptoms (NPS) among people with common dementias and equip interdisciplinary clinicians and health services planners with large-sample data necessary to plan care for patients and families. We analyzed selected variables from baseline assessments of older adults with dementia of one or more etiologies (N = 3,768) from the National Alzheimer's Coordinating Center data repository. Dementias included Alzheimer's disease (AD), Lewy body dementia (DLB), behavioral variant frontotemporal dementia (bvFTD), and vascular dementia (VaD). We compared the prevalence of four NPS clusters (agitation/aggression, depression/dysphoria, anxiety, irritability/lability) across dementia etiologies and stages using logistic regression and AD as the reference group. NPS profiles differed significantly across dementia types and stages. Compared with primary AD, DLB was associated with greater odds of depression/dysphoria (OR = 1.68, 95% confidence interval [CI] 1.28, 2.20) and anxiety (OR = 1.80, 95% CI 1.37, 2.36), with similar findings when DLB was diagnosed in combination with AD (depression/dysphoria: OR = 1.79, 95% CI 1.11, 2.89; anxiety: OR = 1.88, 95% CI 1.17, 3.02). Primary bvFTD was associated with greater odds of agitation/aggression (OR = 1.59, 95% CI 1.17, 2.18). The prevalence of anxiety and irritability/lability was highest in moderate stages of dementia, and agitation/aggression was most prevalent in severe dementia. Differential diagnosis and staging of dementias and inclusion of single and overlapping etiologies is important for planning and implementing appropriate strategies to anticipate, report, and intervene with key NPS that complicate home and health care.
Late-life dementias are pervasive, clinical, cognitive-behavioral syndromes usually caused by neurodegenerative and/or vascular brain disease. Overlapping dementias, due to more than one etiology, are present in approximately 15% of individuals with dementia evaluated using research diagnostic criteria for clinical syndrome (Phelan, Borson, Grothaus, Balch, & Larson, 2012). The ability to distinguish among different types of dementia is important because it allows clinicians to make more accurate prognostic and treatment decisions. While cognitive impairment is the defining diagnostic feature of all dementias, the clinical presentation frequently includes neuropsychiatric symptoms (NPS)—disturbances in mood, thinking processes, and behavior found in up to 80% of patients with dementia (Jalbert, Daiello, & Lapane, 2008). NPS may be associated with reduced quality of life, accelerated cognitive decline, additional costs of care, institutionalization, and caregiver depression and burden (Berger et al., 2005; Kalapatapu & Neugroschl, 2009; Murman & Colenda, 2005; Ornstein et al., 2012). A few NPS are integral to the diagnostic criteria for specific dementias (e.g., disinhibition, apathy, impulsivity in behavioral variant frontotemporal dementia [bvFTD]; visual hallucinations, nighttime behaviors/sleep disturbances in dementia with Lewy bodies [DLB]). However, all forms of NPS can occur across the diagnostic spectrum and can become the focus of clinical intervention.
BACKGROUND
NPS in patients with dementia are more important determinants of caregiver burden and decisions to institutionalize those with dementia than are cognitive symptoms or limitations in everyday function (Berg, Palomäki, Lönnqvist, Lehtihalmes, & Kaste, 2005; Berger et al., 2005; Coen, Swanwick, O'Boyle, & Coakley, 1997; Miyamoto, Ito, Otsuka, & Kurita, 2002; Ornstein et al., 2012; Torti, Gwyther, Reed, Friedman, & Schulman, 2004). Dementia caregivers who experience high burden have high rates of depression (Epstein-Lubow, Davis, Miller, & Tremont, 2008), higher utilization of health services (Draper, Poulos, Cole, Poulos, & Ehrlich, 1992; Kiecolt-Glaser, Dura, Speicher, Trask, & Glaser, 1991; Schubert et al., 2008), higher use of psychotropic medications (Camargos et al., 2012; Pérodeau, Lauzon, Lévesque, & Lachance, 2001), and greater 4-year mortality than noncaregivers (Schulz & Beach, 1999).
These findings reinforce the emerging view that health care for patients with dementia must be dyadic in nature, and health service planning must integrate caregivers’ needs into a comprehensive intervention program that includes systematic assessment and clinical monitoring of patient behavior with special attention to those NPS that are most frequently associated with caregiver burden. These NPS include agitation/aggression (Berger et al., 2005; Matsumoto et al., 2007; Shaji, Bose, & Kuriakose, 2009), depression/dysphoria (Neundorfer et al., 2001; Onishi et al., 2005), anxiety (Berger et al., 2005), and irritability/lability (Matsumoto et al., 2007). Despite these compelling realities, health systems have not yet widely embraced either clinical or administrative initiatives to address the burden of NPS for both patients with dementia and family caregivers and the increased time and complex care coordination that must be allocated by clinicians.
The goal of this study was to expand knowledge of the prevalence and diagnostic correlates of these NPS clusters. The majority of dementia NPS studies have focused on Alzheimer's disease (AD) as a single diagnosis. Much less is known about NPS profiles of other dementias, especially those with overlapping or complex etiologies. Studies with modest sample sizes and methodological differences report some variation in NPS for different dementia diagnoses (Binetti, Locascio, Corkin, Vonsattel, & Growdon, 2000; Sultzer, Levin, Mahler, High, & Cummings, 1993) and indicate that, in general, bvFTD (Grochmal-Bach et al., 2009) and DLB (Simard, van Reekum, & Cohen, 2000) are associated with a higher prevalence of overall NPS than AD and vascular dementia (VaD). In this study, we analyzed data from the largest U.S. sample of uniformly diagnosed people to define differences in selected NPS profiles across dementia diagnoses and stages. This information will better equip clinicians and health systems to develop monitoring and management programs to care for patients with dementia and their family caregivers.
METHOD
Design
This study used baseline assessments from the National Alzheimer's Coordinating Center (NACC) repository, which includes data on all participants in the National Institute on Aging-funded Alzheimer's Disease Research Centers (ADRCs) nationwide (Beekly et al., 2007). NACC data are collected using a standard set of tools, the Uniform Data Set (UDS, Morris et al., 2006). ADRCs conduct clinical and biomedical research with healthy volunteers and patients who have AD and related disorders. Centers enroll their study participants in various ways, including referral from clinicians, self-referral by patients themselves or their family members, and recruitment through community organizations (Morris et al., 2006).
The NACC adopted and began implementing data collection for the UDS in 2005. Data are collected prospectively by clinicians, neuropsychologists, and other ADRC research personnel, using up to 18 standardized forms at each visit. Sources of data include patient and family caregiver self-reports, review of medical records, and clinical evaluations (i.e., physical, neurological, psychiatric). Dementia diagnoses are established based on published criteria for AD (McKhann et al., 1984), DLB (McKeith et al., 2005), bvFTD (Neary et al., 1998), and VaD (Román et al., 1993), are characterized as primary and secondary/ contributing, and then sub-characterized as probable (fully meets diagnostic criteria) or possible (partially meets diagnostic criteria). Overlapping dementias are considered to be present when more than one etiol ogy is identified through this process. Dementia stage is classified by the Clinical Dementia Rating scale (CDR), a standard, reliable measure that assigns a stage based on functional categories across six domains—memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care—and along five levels of impairment (Hughes, Berg, Danziger, Coben, & Martin, 1982, Rikkert et al., 2011). NPS were selected from the Neuropsychiatric Inventory Questionnaire (NPI-Q), a brief reliable version of the Neuropsychiatric Inventory (Cummings, 2005; Morris, 1997). Four NPS were chosen for their strong association with caregiver burden and, to avoid confounding with dementia diagnosis, because they are not integral to the diagnostic criteria for any one of the dementias. NPS included agitation/aggression, depression/dysphoria, anxiety, and irritability/lability, scored as present or absent. Access to selected variables from the UDS for this study was sought in 2011, and only certified clean data (2005-2011) were used for analysis.
The NACC protocols and procedures are approved by institutional review boards governing each ADRC and require informed consent from all participants and/or care-givers for inclusion in the repository (Beekly et al., 2007; Morris et al., 2006). The current study was determined by the University's Human Subjects Division to require no additional consent, as data obtained for analysis were fully de-identified.
Sample
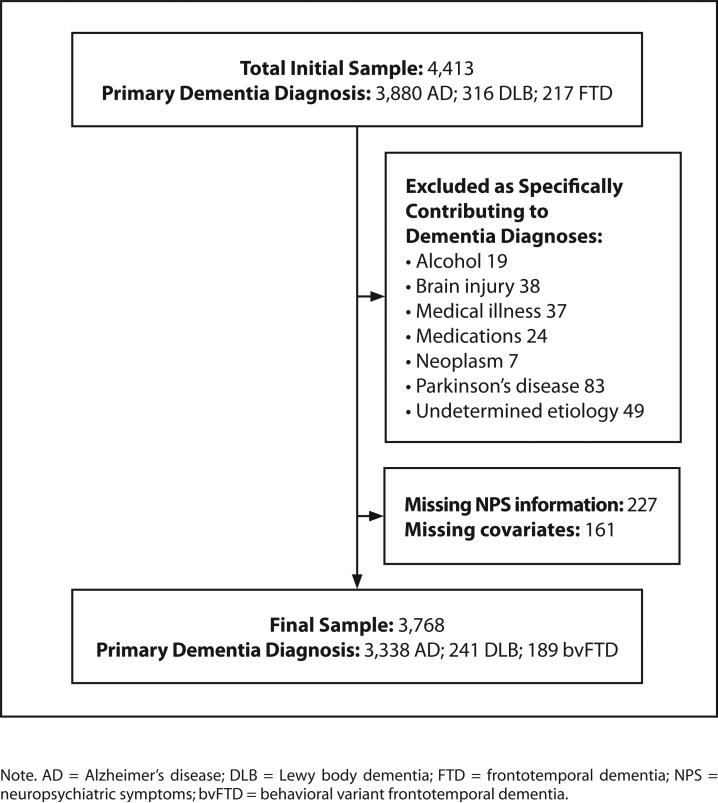
Participants were at least 65 years old; diagnosed with at least one “probable” dementia, indicating a relatively high degree of certainty and conformity with research criteria; and a CDR summary score of 1, 2, or 3+ indicating mild, moderate, or severe stages of impairment, respectively. Participants had a primary diagnosis of AD, DLB, or bvFTD. Primary VaD was rare in the NACC sample overall, so only secondary VaD co-occurring with primary AD was included in this analysis. We also constructed a subsample of individuals with primary AD with and without a second etiology (AD/DLB and AD/VaD). Other dementia types were excluded due to small numbers or non-neurodegenerative, non-vascular etiology, as were participants with missing information about dementia stage, NPS, or demographic data (Figure).
Figure.
Study sample.
Demographic data and sample characteristics are presented for the total sample and by primary dementia diagnosis. Prevalence of target NPS are reported by primary dementia diagnosis and severity and were compared using chi-square tests with two degrees of freedom. Logistic regression with robust standard errors (Huber, 1967) was used to analyze the association of primary dementia diagnosis with each target NPS. All models used AD as the reference group and were adjusted for covariates (age, sex, race/ethnicity, educational level, living situation, marital status, dementia severity, and overlapping etiology) that were considered a priori to be differentially associated with the primary dementia diagnosis and to be risk factors for the target NPS. To determine the impact of dementia severity on the association between primary dementia type and the target NPS, we introduced interaction terms between the primary dementia diagnosis and severity, assessing the statistical significance of all interaction terms with the Wald test (Huber, 1967). Logistic regression was used to assess the association of secondary dementia type and target NPS among patients with primary AD. All statistical tests were performed using STATA version 9 (StataCorp L.P., College Station, TX) with a two-sided alpha level of 0.05.
RESULTS
A total of 3,768 individual participants in the NACC database met inclusion criteria for this study (Figure). Of this group: 3,338 had AD, 241 had DLB, and 189 had bvFTD. Among participants with primary AD, the most common secondary dementia diagnoses were DLB (n = 71, 2.1%) and VaD (n = 54, 1.6%). Table 1 shows demographic characteristics for the total sample and by primary dementia type. The average age of the study sample was 79 (SD = 6.98 years); the majority were women, of White race/ ethnicity and diagnosed with mild dementia. Compared with individuals with AD, those with DLB and bvFTD were younger and more likely to be men, have more than a high school education, be of non-White race/ethnicity, and have dementia with overlapping etiology. Compared with individuals with AD, those with DLB and bvFTD were less likely to be married.
TABLE 1.
Demographic Characteristics by Primary Dementia Diagnosis
| Characteristic | Total (N = 3,768) | AD (n = 3,338) | DLB (n = 241) | bvFTD (n = 189) |
|---|---|---|---|---|
| Mean age (SD) | 79 (6.98) | 80 (6.86) | 77 (6.50) | 73 (5.98) |
| Women, n (%) | 2,121 (56) | 1,984 (59) | 69 (29) | 68 (36) |
| Race/ethnicity, n (%) | ||||
| White | 2,801 (74) | 2,415 (72) | 210 (87) | 176 (93) |
| Black | 518 (14) | 498 (15) | 15 (6) | 5 (3) |
| Hispanic | 354 (9) | 337 (10) | 10 (4) | 7 (4) |
| Asian/native Hawaiian/Pacific Islander | 57 (2) | 55 (2) | 2 (1) | 0 (0) |
| Other | 38 (1) | 33 (1) | 4 (2) | 1 (1) |
| Educational level, n (%) | ||||
| Less than high school | 626 (17) | 586 (18) | 31 (13) | 9 (5) |
| High school graduate | 1,037 (28) | 928 (28) | 62 (26) | 47 (25) |
| More than high school | 2,105 (56) | 1,824 (55) | 148 (61) | 133 (70) |
| Living situation, n (%) | ||||
| Lives with someone | 2,859 (76) | 2,514 (75) | 199 (83) | 146 (77) |
| Lives alone | 492 (13) | 468 (14) | 9 (4) | 15 (8) |
| Other | 417 (11) | 356 (11) | 33 (14) | 28 (15) |
| Currently married, n (%) | 1,427 (38) | 1,344 (40) | 51 (21) | 32 (17) |
| Dementia severity (CDR global), n (%) | ||||
| Mild | 2,386 (63) | 2,147 (64) | 140 (58) | 99 (52) |
| Moderate | 951 (25) | 827 (25) | 69 (29) | 55 (29) |
| Severe | 431 (11) | 364 (11) | 32 (13) | 35 (19) |
| Multiple etiology | 192 (5) | 150 (5) | 23 (10) | 19 (10) |
Note. AD = Alzheimer's disease; DLB = Lewy body dementia; bvFTD = behavioral variant frontotemporal dementia; CDR= Clinical Dementia Rating scale.
Percentages may not total 100 due to rounding.
Table 2 shows the prevalence of NPS by primary dementia type and by dementia severity. Overall, the prevalence of each target NPS was approximately 40%. The prevalence of agitation/aggression, depression/dysphoria, and anxiety varied by primary dementia diagnosis. Participants with primary AD had the lowest prevalence of all selected NPS. Participants with DLB had the highest prevalence of depression/dysphoria and anxiety, and those with bvFTD had the highest prevalence of agitation/aggression. The prevalence of agitation/aggression, anxiety, and irritability/lability varied by dementia severity. Agitation/aggression was most common among those with severe dementia, but anxiety and irritability/lability were most common among those with moderate dementia.
TABLE 2.
Prevalence of NPS by Primary Dementia Diagnosis and Overall Dementia Severity
| Primary Dementia Diagnosis | Dementia Severity (All Diagnoses Combined) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NPS, n (%) | Total (N = 3,768) | AD (n = 3,338) | DLB (n = 241) | bvFTD (n = 189) | p Value | Mild (n = 2,386) | Moderate (n = 951) | Severe (n = 431) | p Value |
| Agitation/aggression | 1,519 (40) | 1,320 (40) | 99 (41) | 100 (53) | 0.001 | 826 (35) | 451 (47) | 242 (56) | <0.001 |
| Depression/dysphoria | 1,427 (38) | 1,242 (37) | 117 (49) | 68 (36) | 0.002 | 914 (38) | 366 (39) | 147 (34) | 0.23 |
| Anxiety | 1,553 (41) | 1,323 (40) | 134 (56) | 96 (51) | <0.001 | 939 (39) | 434 (46) | 180 (42) | 0.004 |
| Irritability/lability | 1,522 (40) | 1,329 (40) | 103 (43) | 90 (48) | 0.08 | 929 (39) | 426 (45) | 167 (39) | 0.006 |
Note. Neuropsychiatric symptoms (NPS) were reported by participants' caregivers/study partners using the Neuropsychiatric Inventory Questionnaire. For this analysis, NPS were scored as present or absent. Numbers in parentheses are percentages of participants in each diagnostic subgroup whose caregivers indicated the presence of the corresponding NPS.
p values were determined by a chi-square test, df = 2, for the associations of NPS with diagnosis type or severity
AD = Alzheimer's disease; DLB = Lewy body dementia; bvFTD = behavioral variant frontotemporal dementia.
The odds ratios for the associations of primary dementia type with target NPS are displayed in Table 3. After adjusting for age, sex, race/ethnicity, educational level, living situation, marital status, dementia severity, and overlapping etiology, primary dementia type was associated with depression/dysphoria and anxiety. After adjustment, those with DLB had 68% higher odds of depression/dysphoria (95% CI 1.28, 2.20) and 80% higher odds of anxiety (95% CI 1.37, 2.36) compared with those with AD. After adjustment, compared with those with AD, those with bvFTD had 59% higher odds of agitation/aggression (95% CI 1.17, 2.18) and 32% higher odds of anxiety (95% CI 0.97, 1.80).
TABLE 3.
Association of Dementia Type with Specific NPS
| Agitation/Aggression | Depression/Dysphoria | Anxiety | Irritability/Lability | |||||
|---|---|---|---|---|---|---|---|---|
| Primary Dementia Diagnosis | ORunadj [95% CI] | ORadj [95% CI]a | ORunadj [95% CI] | ORadj [95% CI]a | ORunadj [95% CI] | ORadj [95% CI]a | ORunadj [95% CI] | ORadj [95% CI]a |
| AD (ref) | ||||||||
| DLB | 1.07 [0.82, 1.39] | 1.02 [0.77, 1.35] | 1.59 [1.23, 2.07] | 1.68 [1.28, 2.20] | 1.91 [1.47, 2.48] | 1.80 [1.37, 2.36] | 1.13 0.87, 1.47] | 1.05 [0.80, 1.38] |
| bvFTD | 1.72 [1.28, 2.31] | 1.59 [1.17, 2.18] | 0.95 [0.70, 1.29] | 0.92 [0.67, 1.28] | 1.57 [1.17, 2.11] | 1.32 [0.97, 1.80] | 1.37 [1.02, 1.84] | 1.25 [0.93, 1.70] |
| p Value | 0.001 | 0.002 | 0.002 | <0.001 | <0.001 | <0.001 | 0.08 | 0.34 |
Note. p value for the Wald test of the coefficient for association of primary dementia diagnosis and specific neuropsychiatric symptoms (NPS).
OR = odds ratio; unadj = unadjusted; adj = adjusted; CI = confidence interval; AD = Alzheimer's disease; ref = reference group; DLB = Lewy body dementia; bvFTD = behavioral variant frontotemporal dementia.
Adjusted for age, sex, race/ethnicity, educational level, living situation, marital status, dementia severity, and overlapping etiology.
After adjustment, no statistically significant interaction was detected between dementia type and severity for any NPS (Wald test: agitation/aggression p = 0.17; depression/ dysphoria p = 0.60; anxiety p = 0.54; irritability/lability p = 0.82).
Finally, Table 4 shows the odds ratios for the associations of secondary dementia type with specific NPS among those with overlapping dementia/primary AD. After adjusting for age, sex, race/ethnicity, educational level, living situation, marital status, and dementia severity, secondary dementia type was only associated with depression/dysphoria and anxiety. After adjustment, compared with those with primary AD only, those with AD/DLB had 79% higher odds of depression/dysphoria (95% CI 1.11, 2.89) and 88% higher odds of anxiety (95% CI 1.17, 3.02).
TABLE 4.
Association of Specific NPS with Complex Dementia Diagnoses: AD Plus a Secondary Diagnosis
| Agitation/Aggression | Depression/Dysphoria | Anxiety | Irritability/ Lability | |
|---|---|---|---|---|
| Secondary Dementia Type | ORadj [95%CI]a | ORadj [95%CI]a | ORadj [95%CI]a | ORadj [95%CI]a |
| No secondary diagnosis (ref) | ||||
| DLB | 1.26 [0.76, 2.07] | 1.79 [1.11, 2.89] | 1.88 [1.17, 3.02] | 1.10 [0.67, 1.79] |
| VaD | 0.64 [0.36, 1.15] | 1.46 [0.84, 2.53] | 0.75 [0.42, 1.33] | 0.94 [0.53, 1.66] |
| p Value | 0.21 | 0.03 | 0.02 | 0.91 |
Note. p value for the Wald test of the coefficient for association of secondary dementia diagnosis and specific neuropsychiatric symptoms (NPS).
AD = Alzheimer's disease; OR = odds ratio; adj = adjusted; CI = confidence interval; ref = reference group; DLB = Lewy body dementia; VaD = vascular dementia.
Adjusted for age, sex, race/ethnicity, educational level, living situation, marital status, and dementia severity.
DISCUSSION
This large-sample study compared NPS among people with common late-life dementias of both single and overlapping diagnoses, ascertained using uniform criteria and consensus diagnoses based on the data derived from medical records, patient/family caregiver reports, a structured clinical examination, and when available, neuroimaging. We analyzed NPS that are commonly associated with high caregiver burden and patient/caregiver health service utilization. Our study confirmed the high prevalence of key NPS among those with dementia and the influence of both diagnosis type and stage on their distribution. Results revealed that DLB dominates the NPS profile in individuals with overlapping AD/DLB. No similar effect was discovered for VaD in overlapping AD/VaD, even though the prevalence of both dementias (secondary DLB and secondary VaD) was similar. Consistent with findings from earlier studies (Lopez et al., 2003; Lyketsos et al., 2000, 2002;), the prevalence of target NPS varied by dementia severity. Contrary to previous findings by Lyketsos et al. (2002), in our sample, NPS varied significantly by dementia diagnosis. Those with primary AD had the lowest prevalence of all selected NPS. Depression/dysphoria and anxiety were most prevalent in DLB and AD/DLB, and agitation/aggression was more prevalent in bvFTD, pointing to the importance of differential diagnosis of dementia subtypes to anticipate and proactively manage NPS in individuals with dementia. Our study findings equip clinicians with data that can be used for discussing NPS with family caregivers as part of the dementia syndrome, and for making prognostic and treatment decisions for early intervention. The ability to anticipate onset of NPS and initiate early management may reduce overall NPS burden for patients, family caregivers, and health systems.
While previous studies have described the prevalence of NPS for selected dementia diagnoses and/or stages, well-conducted reviews of these studies cite relatively small sample sizes, low power, and non-uniform methodologies as significant limitations to generalizability (Boyd et al., 2007; Boyd, Ritchie, Tipton, Studenski, & Wieland, 2008; Marengoni, Rizzuto, Wang, Winblad, & Fratiglioni, 2009). In addition, discrepant findings may occur because of differences in the population sampled: For example, Lyketsos et al. (2002), analyzing data from the Cardiovascular Health Study (CHS), may not have detected significant differences in NPS among different dementia diagnoses due to sample size and combining all non-AD dementias into a category of “other dementias,” which, as expected from CHS’ focus on vascular disease, were predominantly vascular. NACC focuses on AD predominantly, and although sampling is non-random by design, the sample is much larger and allows comparisons among distinct dementia subtypes. The current study and one other (Johnson, Watts, Chapin, Anderson, & Burns, 2011), also based on NACC data, are the first to use a large national sample of individuals diagnosed with dementia by uniform research criteria and assessment of NPS and stage.
STRENGTHS AND LIMITATIONS
The strengths of our study include the features of the NACC data repository itself: a very large, well-characterized sample of people with dementias who are in many ways demographically similar to the overall U.S. older adult population; good representation of ethnic minorities (26% of total sample); uniformity of assessment; and strong validity and reliability of dementia diagnoses (Steenland, Macneil, Bartell, & Lah, 2010). Strengths that are unique to this investigation are its careful definition of study subgroups (restriction to “probable” dementia diagnoses) and the inclusion of clearly defined single and overlapping dementia diagnoses.
This study also has limitations. Although the NACC sample is national in scope, results may not be fully generalizable to all older adults with dementia, because not all dementias are represented in epidemiological proportions; also, participants are volunteers, must have a caregiver to participate, tend to be better educated than the older population in general, and are living in the community. Whereas caregivers provide data about those with dementia, caregiver stress, burden, and depression are not measured. In addition, NPS severity and the use of anti-dementia, psychotropic, and other centrally active medications that might influence NPS profiles were not considered due to small subset sizes.
CONCLUSION AND RECOMMENDATIONS
Our investigation offers insights important for clinicians and health care systems seeking to provide comprehensive dementia care. Since different dementia diagnoses (single and overlapping) vary in their associated NPS profiles, clinicians should receive advanced training in the differential diagnosis of dementias, as well as NPS assessment and management. The new Medicare Annual Wellness Visit benefit, which promotes early detection of cognitive impairment, provides impetus for improving the diagnostic and assessment skills of clinicians relevant to providing high-quality dementia care. Results from this work highlight the NPS clinicians should monitor and manage in tandem with addressing caregiver burden. For example, clinicians who establish a diagnosis of DLB or AD/DLB should be alert to increased risks of depression/dysphoria and anxiety, not just the visual hallucinations and sleep disturbances/nighttime behaviors that are primary or supporting features of the diagnosis. Similarly, a diagnosis of bvFTD should alert clinicians to higher risks of agitation/aggression, not just disinhibition and apathy. Longitudinal multimodal interventions (e.g., Gaugler, Reese, & Mittelman, 2013; Mittelman, Haley, Clay, & Roth, 2006) can provide the structure for detecting burdensome NPS and simultaneously addressing the needs of patients and caregivers, in the process delaying transitions to costly long-term care. Data from our study should help clinicians anticipate and plan for such emergence based on diagnostic subtypes, complexity, and stage. Future research should focus on developing appropriate management strategies that incorporate targeted interventions for the anticipation, prevention, and early detection of NPS, especially NPS that are highly associated with caregiver burden.
Footnotes
The authors have disclosed no potential conflicts of interest, financial or otherwise.
Contributor Information
Tatiana I. Sadak, Department of Psychosocial and Community Health, University of Washington School of Nursing, Seattle, Washington..
Jodie Katon, Health Services Research and Development, Veterans Affairs (VA) Puget Sound Health Care System, Department of Health Services, University of Washington School of Public Health, VA Medical Center, Seattle, Washington..
Cornelia Beck, Department of Geriatrics, College of Medicine, University of Arkansas for Medical Sciences, Little Rock, Arkansas..
Barbara B. Cochrane, Department of Family and Child Nursing, University of Washington School of Nursing, Seattle, Washington..
Soo Borson, Department of Psychiatry and Behavioral Sciences/Geriatric Psychiatry, University of Washington School of Medicine, and Department of Psychosocial and Community Health, University of Washington School of Nursing, Seattle, Washington..
REFERENCES
- Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Kukull WA. The National Alzheimer's Coordinating Center (NACC) database: The uniform data set. Alzheimer's Disease and Associated Disorders. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- Berg A, Palomäki H, Lönnqvist J, Lehtihalmes M, Kaste M. Depression among caregivers of stroke survivors. Stroke. 2005;36:639–643. doi: 10.1161/01.STR.0000155690.04697.c0. [DOI] [PubMed] [Google Scholar]
- Berger G, Bernhardt T, Weimer E, Peters J, Kratzsch T, Frolich L. Longitudinal study on the relationship between symptomatology of dementia and levels of subjective burden and depression among family caregivers in memory clinic patients. Journal of Geriatric Psychiatry and Neurology. 2005;18:119–128. doi: 10.1177/0891988704273375. [DOI] [PubMed] [Google Scholar]
- Binetti G, Locascio JJ, Corkin S, Vonsattel JP, Growdon JH. Differences between Pick disease and Alzheimer disease in clinical appearance and rate of cognitive decline. Archives of Neurology. 2000;57:225–232. doi: 10.1001/archneur.57.2.225. [DOI] [PubMed] [Google Scholar]
- Boyd CM, Boult C, Shadmi E, Leff B, Brager R, Dunbar L, Wegener S. Guided care for multimorbid older adults. The Gerontologist. 2007;47:697–704. doi: 10.1093/geront/47.5.697. [DOI] [PubMed] [Google Scholar]
- Boyd CM, Ritchie CS, Tipton EF, Studenski SA, Wieland D. From bedside to bench: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Comorbidity and Multiple Morbidity in Older Adults. Aging Clinical and Experimental Research. 2008;20:181–188. doi: 10.1007/bf03324775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargos EF, Souza AB, Nascimento AS, Morais-E-Silva AC, Quintas JL, Louzada LL, Medeiros-Souza P. Use of psychotropic medications by caregivers of elderly patients with dementia: Is this a sign of caregiver burden? Arquivos De Neuropsiquiatria. 2012;70:169–174. doi: 10.1590/s0004-282x2012000300003. [DOI] [PubMed] [Google Scholar]
- Coen RF, Swanwick GR, O'Boyle CA, Coakley D. Behaviour disturbance and other predictors of carer burden in Alzheimer's disease. International Journal of Geriatric Psychiatry. 1997;12:331–336. [PubMed] [Google Scholar]
- Cummings JL. Behavioral and neuropsychiatric outcomes in Alzheimer's disease. CNS Spectrums. 2005;10(11 Suppl. 18):22–25. doi: 10.1017/s1092852900014206. [DOI] [PubMed] [Google Scholar]
- Draper BM, Poulos CJ, Cole AM, Poulos RG, Ehrlich F. A comparison of caregivers for elderly stroke and dementia victims. Journal of the American Geriatrics Society. 1992;40:896–901. doi: 10.1111/j.1532-5415.1992.tb01986.x. [DOI] [PubMed] [Google Scholar]
- Epstein-Lubow G, Davis JD, Miller IW, Tremont G. Persisting burden predicts depressive symptoms in dementia caregivers. Journal of Geriatric Psychiatry and Neurology. 2008;21:198–203. doi: 10.1177/0891988708320972. doi:10.1177/0891988708320972. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Reese M, Mittelman MS. Effects of the NYU caregiver intervention-adult child on residential care placement. The Gerontologist. Advance online publication. 2013 doi: 10.1093/geront/gns193. doi:10.1093/geront/gns193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grochmal-Bach B, Bidzan L, Pachalska M, Bidzan M, Lukaszewska B, Pufal A. Aggressive and impulsive behaviors in frontotemporal dementia and Alzheimer's disease. Medical Science Monitor. 2009;15(5):CR248–CR254. [PubMed] [Google Scholar]
- Huber P. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. Vol. 1. University of California Press; Berkeley, CA: 1967. The behavior of maximum likelihood estimates under nonstandard conditions. pp. 221–233. [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Jalbert JJ, Daiello LA, Lapane KL. Dementia of the Alzheimer's type. Epidemiologic Reviews. 2008;30:15–34. doi: 10.1093/epirev/mxn008. doi:10.1093/epirev/mxn008. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Watts AS, Chapin BA, Anderson R, Burns JM. Neuropsychiatric profiles in dementia. Alzheimer's Disease and Associated Disorders. 2011;25:326–332. doi: 10.1097/WAD.0b013e31820d89b6. doi:10.1097/WAD.0b013e31820d89b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalapatapu RK, Neugroschl JA. Update on neuropsychiatric symptoms of dementia: Evaluation and management. Geriatrics. 2009;64(4):20–26. [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: Longitudinal changes in immunity and health. Psychosomatic Medicine. 1991;53:345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Sweet RA, Klunk W, Kaufer DI, Saxton J, DeKosky ST. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer's disease. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:346–353. doi: 10.1176/jnp.15.3.346. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the Cardiovascular Health Study. Journal of the American Medical Association. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. doi:10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: Findings from the Cache County Study on Memory in Aging. American Journal of Psychiatry. 2000;157:708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- Marengoni A, Rizzuto D, Wang HX, Winblad B, Fratiglioni L. Patterns of chronic multimorbidity in the elderly population. Journal of the American Geriatrics Society. 2009;57:225–230. doi: 10.1111/j.1532-5415.2008.02109.x. doi:10.1111/j.1532-5415.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Ikeda M, Fukuhara R, Shinagawa S, Ishikawa T, Mori T, Tanabe H. Caregiver burden associated with behavioral and psychological symptoms of dementia in elderly people in the local community. Dementia and Geriatric Cognitive Disorders. 2007;23:219–224. doi: 10.1159/000099472. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Yamada M. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. doi:10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer's disease. Neurology. 2006;67:1592–1599. doi: 10.1212/01.wnl.0000242727.81172.91. doi:10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Ito H, Otsuka T, Kurita H. Caregiver burden in mobile and non-mobile demented patients: A comparative study. International Journal of Geriatric Psychiatry. 2002;17:765–773. doi: 10.1002/gps.694. [DOI] [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. International Psychogeriatrics. 1997;9(Suppl. 1):173–176. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Kukull WA. The uniform data set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer's disease centers. Alzheimer's Disease and Associated Disorders. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Murman DL, Colenda CC. The economic impact of neuropsychiatric symptoms in Alzheimer's disease: Can drugs ease the burden? Pharmacoeconomics. 2005;23:227–242. doi: 10.2165/00019053-200523030-00004. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Benson DF. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Neundorfer MM, McClendon MJ, Smyth KA, Stuckey JC, Strauss ME, Patterson MB. A longitudinal study of the relationship between levels of depression among persons with Alzheimer's disease and levels of depression among their family caregivers. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2001;56:P301–P313. doi: 10.1093/geronb/56.5.p301. [DOI] [PubMed] [Google Scholar]
- Onishi J, Suzuki Y, Umegaki H, Nakamura A, Endo H, Iguchi A. Influence of behavioral and psychological symptoms of dementia (BPSD) and environment of care on caregivers’ burden. Archives of Gerontology and Geriatrics. 2005;41:159–168. doi: 10.1016/j.archger.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Ornstein K, Gaugler JE, Devanand DP, Scarmeas N, Zhu C, Stern Y. The differential impact of unique behavioral and psychological symptoms for the dementia caregiver: How and why do patients’ individual symptom clusters impact caregiver depressive symptoms? American Journal of Geriatric Psychiatry. 2012 doi: 10.1016/j.jagp.2013.01.062. Advance online publication. doi:10.1097/JGP.0b013e31826d6b31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérodeau G, Lauzon S, Lévesque L, Lachance L. Mental health, stress correlates and psychotropic drug use or non-use among aged caregivers to elders with dementia. Aging and Mental Health. 2001;5:225–234. doi: 10.1080/13607860120064998. [DOI] [PubMed] [Google Scholar]
- Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. Journal of the American Medical Association. 2012;307:165–172. doi: 10.1001/jama.2011.1964. doi:10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikkert MG, Tona KD, Janssen L, Burns A, Lobo A, Robert P, Waldemar G. Validity, reliability, and feasibility of clinical staging scales in dementia: A systematic review. American Journal of Alzheimer's Disease and Other Dementias. 2011;26:357–365. doi: 10.1177/1533317511418954. doi:10.1177/1533317511418954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- Schubert CC, Boustani M, Callahan CM, Perkins AJ, Hui S, Hendrie HC. Acute care utilization by dementia caregivers within urban primary care practices. Journal of General Internal Medicine. 2008;23:1736–1740. doi: 10.1007/s11606-008-0711-0. doi:10.1007/s11606-008-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. Journal of the American Medical Association. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Shaji S, Bose S, Kuriakose S. Behavioral and psychological symptoms of dementia: A study of symptomatology. Indian Journal of Psychiatry. 2009;51:38–41. doi: 10.4103/0019-5545.44903. doi:10.4103/0019-5545.44903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, van Reekum R, Cohen T. A review of the cognitive and behavioral symptoms in dementia with Lewy bodies. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:425–450. doi: 10.1176/jnp.12.4.425. [DOI] [PubMed] [Google Scholar]
- Steenland K, Macneil J, Bartell S, Lah J. Analyses of diagnostic patterns at 30 Alzheimer's disease centers in the US. Neuroepidemiology. 2010;35:19–27. doi: 10.1159/000302844. doi:10.1159/000302844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer DL, Levin HS, Mahler ME, High WM, Cummings JL. A comparison of psychiatric symptoms in vascular dementia and Alzheimer's disease. American Journal of Psychiatry. 1993;150:1806–1812. doi: 10.1176/ajp.150.12.1806. [DOI] [PubMed] [Google Scholar]
- Torti FM, Jr., Gwyther LP, Reed SD, Friedman JY, Schulman KA. A multinational review of recent trends and reports in dementia caregiver burden. Alzheimer's Disease and Associated Disorders. 2004;18:99–109. doi: 10.1097/01.wad.0000126902.37908.b2. [DOI] [PubMed] [Google Scholar]