Abstract
Current treatment options for ischemia include percutaneous interventions, surgical bypass or pharmacological interventions aimed at slowing the progression of vascular disease. Unfortunately, while each of these treatment modalities provides some benefit for patients in the short-term, many patients have resistant or recurrent disease that is poorly managed by these therapies. A highly appealing strategy for treating ischemic disease is to stimulate the revascularization of the tissue to restore blood flow. While many techniques have been explored in this regard, clinically effective angiogenic therapies remain elusive. Here, we hypothesized that the presence of co-morbid disease states inherently alters the ability of the body to respond to angiogenic therapies. Using a mouse model of diabetes and obesity, we examined alterations in the major components for the signaling pathways for FGF-2, VEGF-A and PDGF under normal and high fat dietary conditions. In skeletal muscle, a high fat diet increased levels of growth factor receptors and co-receptors including syndecan-1, syndecan-4 and PDGFR-□in wild-type mice. These increases did not occur in Ob/Ob mice on a high fat diet and there was a significant decrease in protein levels for neuropilin-1 and heparanase in these mice. With the aim of increasing growth factor effectiveness in the context of disease, we examined whether local treatment with alginate gel-delivered FGF-2 and syndecan-4 proteoliposomes could overcome the growth factor resistance in these mice. This treatment enhanced the formation of new blood vessels in Ob/Ob mice by 6 fold in comparison to FGF-2 delivered alone. Our studies support that disease states cause a profound shift in growth factor signaling pathways and that co-receptor-based therapies have potential to overcome growth factor resistance in the context of disease states.
Keywords: angiogenesis, neovascularization, ischemia, fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), syndecans, neuropilin-1, heparanase, syndecan-4 proteoliposomes, peripheral arterial disease
1. Introduction
Peripheral arterial disease (PAD) affects about 30 million people worldwide and is estimated to affect 16% of the general population over 65 years of age[1]. The increase in prevalence of strong risk factors for PAD, including smoking, diabetes and obesity, indicate that the affected population will continue to grow[2]. A major sequela of PAD is the development of ischemia in the lower limb. Severe PAD has serious clinical consequences for patients including the formation of ulcers, pain from intermittent claudication and, ultimately, increased risk for limb amputation[3]. For many years the dominant clinical treatment for PAD was surgical revascularization through bypass grafting and endarterectomy. More recently, there has been a rapid growth in the number of endovascular treatments such as angioplasty, stenting and catheter-based atherectomy for PAD, although the overall benefit of these treatments versus surgery remains unclear[4]. A major limitation of these therapies is the limited clinical durability due to restenosis and continuation of the atherosclerotic disease process. Thus, an appealing alternative strategy for treating peripheral ischemia is the induction of angiogenesis through the exogenous delivery of growth factors, angiogenic genes or cells[5].
Angiogenesis is an intricate physiological process requiring the intricate coordination of endothelial cells, vascular smooth muscle cells, pericytes and macrophages under the control of environmental cues from the extracellular matrix and a host of growth factors/cytokines[6]. Among these, members of the FGF family bind to cell surface heparan sulfate proteoglycans, interactions that are essential to stabilize the formation of active FGF-FGF receptor complexes[7]. Consequently, cell surface heparan sulfate proteoglycans, such as the syndecans, can serve as essential co-receptors in this pathway. Vascular endothelial growth factor (VEGF) has also been recognized as a potent stimulator of endothelial proliferation/migration and plays an integral role in angiogenesis in-vivo through interactions with its two primary receptors Flt-1 (VEGFR-1) and KDR (VEGFR-2)[8]. Neuropilin-1 is a major co-receptor for VEGF acting to facilitate signaling with both Flt-1 and KDR[9]. In addition, syndecan-2 can bind VEGF and is essential for VEGF-mediated angiogenesis[10]. Platelet-derived growth factor-BB (PDGF-BB) is involved in pericyte recruitment around capillaries during angiogenesis and is consequently involved in blood vessel stabilization during angiogenesis and arteriogenesis[11]. The PDGF-β receptor has the high affinity for PDGF-BB, and this interaction has been linked to the control of cell migration and proliferation[12]. Both neuropilin-1 and the syndecans have been linked to regulation of PDGF activity[13-16]. In addition, PDGF-CC interacts with the PDGF-α and -β receptors, inducing angiogenesis[17] and revascularization of ischemic tissues[18].
Current therapies for peripheral ischemia are composed either of pharmacological interventions aimed at treating the progress of vascular disease/comorbidities or interventional treatments such as angioplasty, stenting, endarterectomy or surgical bypass. However, for a significant portion of the clinical population these methods are insufficient to restore blood flow over the long-term course of their disease[19]. An appealing and potentially revolutionary strategy for treating ischemia is the stimulation of angiogenesis within the ischemic tissue, harnessing the body’s own regenerative capacity to restore blood flow[20]. Previous studies have explored this strategy using exogenous applied growth factors[21-23], viral vectors to express growth factor/angiogenic transcription factor genes[23-32], or the implantation or mobilization of progenitors cells[25]. Unfortunately, while many of these strategies have shown promise in animal studies or small-scale clinical trials, none have found efficacy with significant clinically improvement in large randomized clinical trials[5].
Given the intense study of the process of angiogenesis and the evidence for the potent induction of angiogenesis by growth factors in experimental models, we hypothesized that the reason for this therapeutic failure may lie in disease mediated alterations in target tissue signaling. In animal models, ischemia is typically induced in a healthy animal by surgically ligating an artery either in the peripheral muscle or coronary arteries. Consequently, ischemia develops acutely in an animal that is often otherwise healthy. In human clinical use, the patient has developed ischemia most often through a long-term disease process. Thus, by the time patients have developed clinically recognizable symptoms they have had the disease for an extended period of time and the compensatory mechanisms of the human body may have been overwhelmed considerably. These mechanisms include, in all likelihood, the induction of the very angiogenic factors that we are attempting to use as therapeutic inducers of blood vessel growth. Accordingly, the presence of long-term ischemic disease in humans likely implies the presence of mechanisms to defeat growth factor therapy without modification. Here, we explored this hypothesis by examining how the expression of signaling components of FGF-2, VEGF-A and PDGF pathways change with diseased state caused by a high fat diet in the Ob/Ob mouse model. Our group has also recently shown that delivery of liposomally embedded co-receptors are effective in enhancing growth factor-induced signaling and trafficking in cell as well as revascularization in healthy rats[33]. To examine if this strategy could overcome disease-induced growth factor resistance, we developed an alginate-based hydrogel system for the local delivery of syndecan-4 (sdc-4) proteoliposomes in combination with FGF-2.
2. Materials and Methods
2.1. Animal studies
All animal studies were performed with the approval of the University of Texas at Austin Institutional Animal Care and Use Committee (IACUC) and in accordance with NIH guidelines “Guide for Care and Use of Laboratory Animals” for animal care. Wild-type mice - C57BL/6J (Jackson Labs) and Ob/Ob mice - B6.Cg-Lepob/J (Jackson Labs) were used in these studies. The animals were fed normal chow diet (Lab Diet - Prolab RMH 1800) and high fat diet (Research Diets - D12331). The animals were fed a particular diet for 10 weeks and then sacrificed so that heart and quadriceps muscle could be harvested for downstream processing, as described below. We fed the animals for 15 weeks prior to the subcutaneous implantation surgery. For the subcutaneous implantation model, the dorsal surface of the mouse was shaved and prepared with three swabs of Betadine and 70% ethanol. A skin incision was made on the back with a scalpel and blunt dissection was used to create a subcutaneous pocket. An alginate bead containing growth factors or a control solution was implanted in the subcutaneous space. The wound was closed using resorbable sutures (Ethicon 5-0 polydioxanone). After seven days, the animals were sacrificed. The alginate gels were imaged and tissue samples were flash frozen in liquid N2 cooled isopentane for subsequent analysis.
2.2. Gene expression analyses
Slices of the tissue were sectioned (10-20μm) with a Leica CM 1850 cryotome equipped with a steel knife. The sections were dissolved in the RLT buffer (Qiagen) using a Qiagen Tissuelyzer with a stainless steel bead. RNA was isolated using the Qiagen RNeasy Midi kit and purity checked on an UV-Vis spectrophotometer (Thermo Scientific Nanodrop 2000c). Pure RNA was reverse transcribed into cDNA using the TaqMan Reverse Transcription reagents (Applied Biosystems). The cDNA was then used with SYBR Green PCR master mix (Life Technologies) for real time qPCR quantification using the Applied Biosystems ViiATM 7 system. GAPDH was used as a reference gene. The cycling conditions used were 95°C for 10 mins for initialization followed by 40 cycles of 95°C for 15 seconds and 60 °C for 60 seconds. Primers for the PCR reactions are listed in Supplemental Table S3.
2.3. Western blotting analyses
The tissues were cryosectioned and the slices lysed in a buffer containing the following: 20mM Tris at pH 8, 150mM NaCl, 1% triton, 0.1% SDS, 2mM sodium orthovanadate, 2mM PMSF, 50mM NaF, and protease inhibitors (Roche). Qiagen Tissuelyzer was used with stainless steel beads to facilitate tissue lysis. The lysates were normalized to the amount of protein loaded into the wells by performing Micro-BCA assay (Thermo-Scientific). The samples were then separated by gel electrophoresis (NuPAGE® Novex 10% Bis-Tris Midi Gel) and transferred to a nitrocellulose membrane using the iBlot system (Life Technologies). The membranes were then blocked for 1h in 5% nonfat milk in PBS with 0.01% Tween-20 and exposed to primary antibodies (see Supplementary Table S4 for details) overnight at 4°C. The membranes were washed and incubated at room temperature for 2h at room temperature with secondary antibody and were detected using a chemiluminescence substrate (Thermo Fisher Scientific). Imaging was performed using the FluorChem HD2 system (Protein Simple). Quantification of the blots was done by densitometric analysis using Metamorph.
2.4. Histological analysis and Immunostaining
Eight-micron thick sections were obtained from frozen tissues using the Leica CM 1850 Cryotome equipped with steel knife. For H&E staining, the sections were fixed in 10% formalin for 10 mins, washed in 1X PBS for 5 mins and then air dried at 60°C for 1h. The standard H&E protocol was then followed and imaged with a upright compound microscope. For immunohistochemical staining the sections were fixed in 4% paraformaldehyde for 5-10 mins, blocked with 25% FBS for 45 mins and then exposed to a 1:50 dilution of primary antibody (see Supplementary Table S4 for details) for overnight at 4°C. The samples were washed three times with PBS and treated with a 1:500 dilution of secondary antibody conjugated to a fluorescent marker (see Supplementary Table S4 for details) for 2h at room temperature. The slides were then rinsed with PBS and cover slipped with using DAPI containing anti-fade mounting medium (Vector Labs). Imaging was performed with the Zeiss Axiovert or Leica SP2 AOBS, and images were analyzed using Photoshop and Metamorph.
2.5. Recombinant Protein Production
A constitutive expression vector containing the full-length syndecan-4 gene (Genecopoeia) was transduced into HEK-293Ta cells (Genecopoeia) using the Lenti-Pac transduction kit (Genecopoeia). Two days post-transduction, cell lysis was performed with a buffer containing the following: 20mM Tris (pH 8.0), 150mM NaCl, 1% Triton X-100, 0.1% SDS, 2mM sodium orthovanadate, 2mM PMSF, 50mM NaF, and protease inhibitors (Roche). The lysates were clarified by centrifugation for 15 mins at 15,000 × g, and the supernatant was collected. The pooled lysates were desalted and separated sequentially using ion exchange and affinity chromatography. The separations were performed with an AKTA FPLC (GE Healthcare) using a Q anion exchange column. The samples were then concentrated using Centriprep (Millipore). SDS-PAGE and silver staining were used to analyze the purity of the final concentrated samples.
2.6. Preparation of Proteoliposomes
We made standard lipid stock solutions for 10mg/ml concentration in chloroform. We used the following lipids: 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), cholesterol, and sphingomyelin (Avanti Polar Lipids) mixed in a ratio of 40:20:20:20 by volume, respectively. The solution mixture was prepared in a round-bottom flask, and the solvent was removed first using a Rotatory Evaporator (Heidolph Collegiate) for 1h and then under a stream of argon gas for 15 mins. The lipids were resuspended in a Hepes-buffered salt solution (10.0mM Hepes and 150mM NaCl in PBS, pH 7.4) by mixing, sonicating, and freeze-thawing to achieve a final solution of 13.2mM total lipid. The final lipid solution was then extruded through a 400nm polycarbonate membrane (Avestin). A detergent, 1% n-octyl-β-D-glucopyranoside (OG), was added to both the 13.2mM lipid and the 25μg/ml syndecan-4 protein solutions. The concentration of the solution was reduced to 40% of the original in 10% increments every 30 mins through dilution with PBS. The detergent and free protein was removed by extensive dialysis in PBS at 4°C. Residual OG was removed by repeated BioBead treatments (SM-2; Bio-Rad).
2.7. Implant preparation
Equal volumes of 4% sodium alginate solution and 0.85% NaCl solution were mixed and the proteoliposomes or growth factors were added to it. The solution was then extruded through a syringe with an 18G needle into a 1.1% CaCl2 solution and cross-linked for 1h at 4°C. One bead was implanted in each of the subcutaneous pocket created. For growth factor delivery, 10μg of the growth factors were encapsulated in 400μl solution that formed 12 beads. For proteoliposomes, 5μg of proteoliposomes and 10 μg of FGF were added to 400μl of the solution that formed 14 beads.
2.8. Statistical Analysis
All results are shown as mean ± SEM. An ANOVA with Duncan post-hoc test was used to make comparisons between groups of continuous variables. Comparisons between only two groups were analyzed using a Student’s t-test. A two tailed probability value p < 0.05 was considered statistically significant.
3. Results
3.1. Increased gene expression of growth factor receptors and co-receptors in diseased skeletal muscle tissue
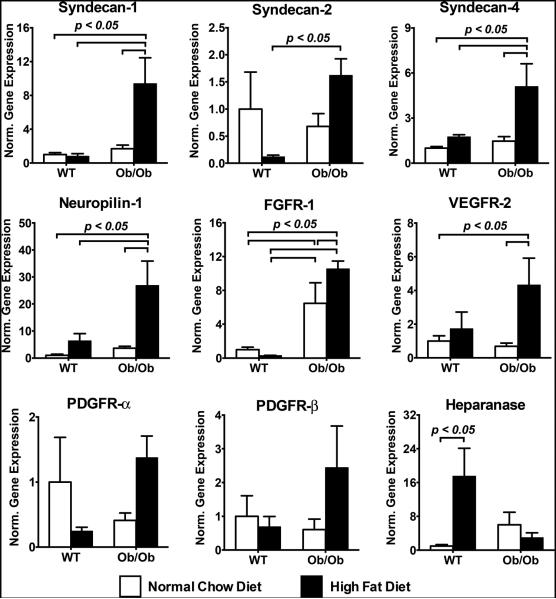
To better understand the potential mechanisms underlying growth factor resistance in diabetic, hyperlipidemic Ob/Ob mice, we exposed these mice to 10 weeks of a high fat or normal chow diet and examined gene expression in the heart and muscle tissue. We first examined quadriceps muscle tissue for gene expression levels of receptors and co-receptors for the FGF-2, VEGF-A and PDGF pathways. At the mRNA expression level, we found a 5- to 28-fold increase in the gene expression for the growth factor receptors/co-receptors including syndecan-1 (sdc-1), syndecan-4 (sdc-4), neuropilin-1 (NRP-1), FGFR-1, and VEGFR-2 (Fig. 1). For WT mice on a high fat diet, we found a significant increase in heparanase, an enzyme that degrades heparan sulfate proteoglycans and mediates the release of angiogenic factor from the extracellular matrix[34]. This trend was not observed in the Ob/Ob mice. An overall summary of the gene expression is shown in Supplemental Table S1.
Figure 1.
Gene expression in skeletal muscle harvested from wild type (WT) and Ob/Ob mice after 10 weeks of normal chow diet or high fat diet. Measurements were made using real time qPCR and are normalized to GAPDH expression and expressed relative to the mRNA levels of WT mice on normal chow diet.
3.2. Decreased protein expression for growth factor receptors and co-receptors in diseased skeletal muscle
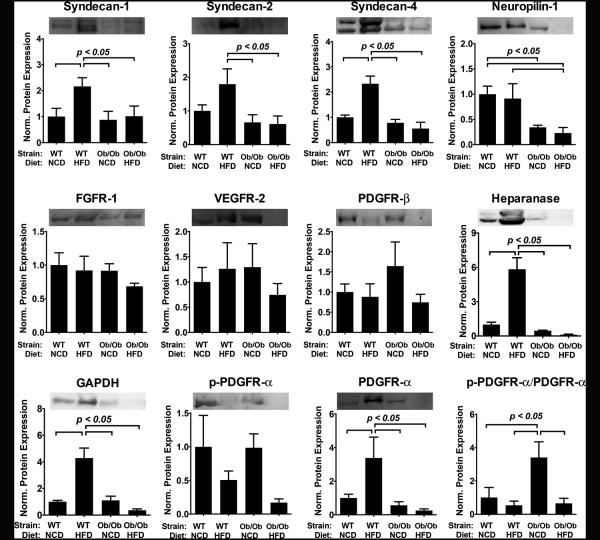
Surprisingly, we observed a markedly different profile at the protein level for the tissue expression of the growth factor receptors and co-receptors. For the co-receptors sdc-1, sdc-2, sdc-4 and PDGFR-βthere was an increase in protein levels in WT with the high fat diet (Fig. 2). Interestingly, this increase did not occur in the Ob/Ob mice. There was a marked reduction in the protein expression for the NRP-1 in comparison to the WT mice on the normal chow diet. All of the protein levels were normalized by the protein mass (mg) in the tissue. We performed a GAPDH blot and found that the portion of GAPDH per unit protein was increased in the high fat diet group of the WT and reduced in the Ob/Ob mice on high fat diet, suggesting a change in the number of cells per mg of protein in the tissue. The levels of heparanase protein corresponded well to the results for mRNA levels in the tissues, with an increase for the WT, high fat diet group and significant decrease in heparanase in the Ob/Ob group with high fat diet. An overall summary of the protein expression is shown in Supplemental Table S2.
Figure 2.
Protein expression of growth factor receptors and co-receptors in skeletal muscle harvested from wild type (WT) and Ob/Ob mice fed with normal chow diet (NCD) or high fat diet (HFD) for 10 weeks. Measurements are performed using densitometric analysis and expressed relative to the protein expression of WT mice on normal chow diet.
3.3. Increased gene expression of growth factor receptors and co-receptors in diseased heart tissue
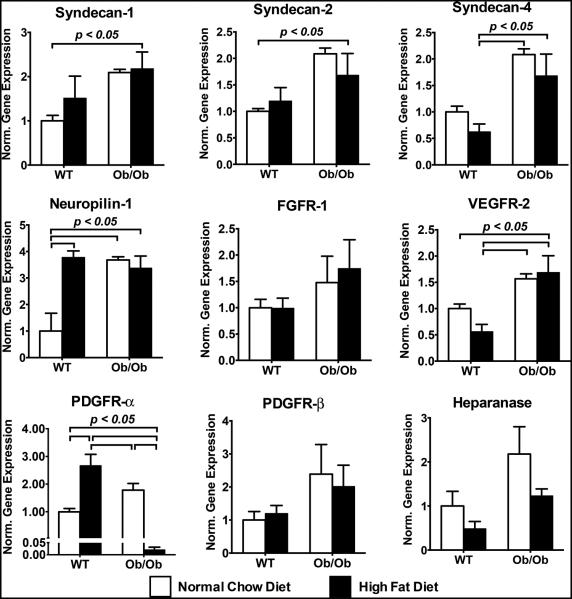
To examine if the tissues were affected in a differential manner, we also analyzed the expression of growth factor receptors and co-receptors in myocardial tissue. We found that there was increased mRNA expression for the syndecans and VEGFR-2 in Ob/Ob mice (Fig. 3). For NRP-1 there was increased gene expression in the Ob/Ob group and the WT group with the high fat diet. The most striking result was a dramatic loss in expression for PDGFR-β (less than 3% of baseline WT levels) in the Ob/Ob group under the high fat diet. A summary of these results can be found in Supplementary Table S1.
Figure 3.
Gene expression in myocardial tissue harvested from wild type (WT) and Ob/Ob mice after 10 weeks of normal chow diet or high fat diet. Measurements were made using real time qPCR and are normalized to GAPDH expression and expressed relative to the mRNA levels of WT mice on normal chow diet.
3.4. Decreased protein expression for growth factor receptors and co-receptors in diseased skeletal muscle
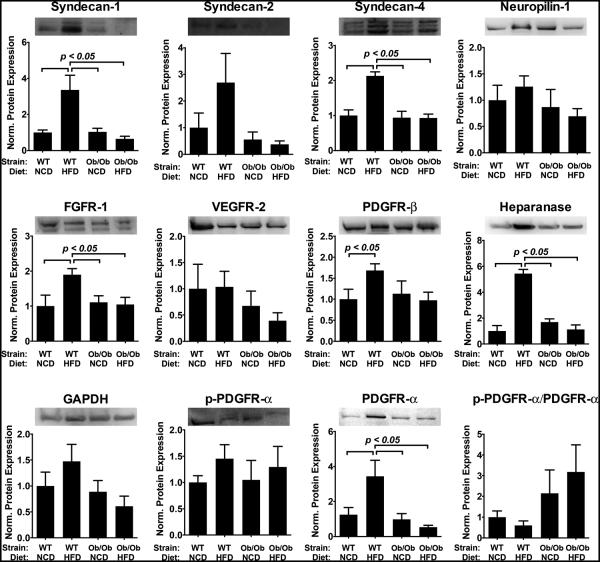
Similar to our results for skeletal muscle, we found a decrease in protein levels of receptors and co-receptors in contrast to gene expression levels in the heart muscle. High fat diet induced an increase in the protein levels of sdc-1, -2 and -4 that was not observed in Ob/Ob mice (Fig. 4). There was a similar trend for PDGFR-α with an over three-fold increase in the receptor for WT mice with high fat diet but a decrease in protein for the Ob/Ob group with high fat diet. Heparanase was also increased in the WT mice with high fat diet but had WT baseline levels for both Ob/Ob mice under normal or high fat diet. The overall summary of these results is shown in Supplementary Table S2.
Figure 4.
Protein expression of growth factor receptors and co-receptors in myocardial tissue harvested from wild type (WT) and Ob/Ob mice fed with normal chow diet (NCD) or high fat diet (HFD) for 10 weeks. Measurements are performed using densitometric analysis and expressed relative to the protein expression of WT mice on normal chow diet.
3.5. Growth factor responsiveness in the diabetic Ob/Ob mouse model
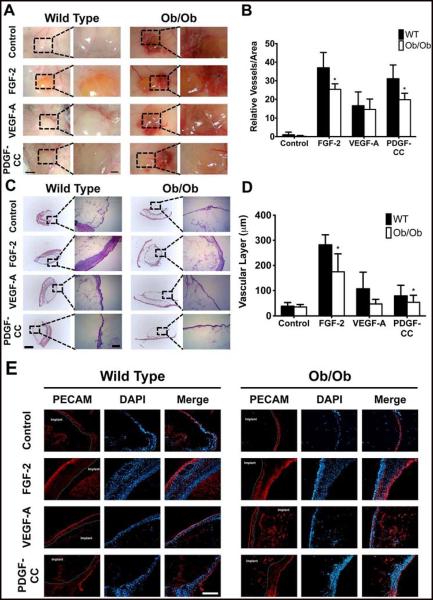
In order to examine whether Ob/Ob mice had growth factor resistance compared to WT mice, we implanted alginate gels subcutaneously in the mice that contained control (PBS), FGF-2, VEGF-A or PDGF-CC. After seven days, we found that there was little response to the control gel, both macroscopically (Fig. 5A) and on histological analysis (Fig. 5C). In WT mice fed a normal diet, gels containing FGF-2 demonstrated increased vascularity (Fig. 5B) as well as a thicker surrounding layer of vascularized tissue (Fig. 5D). In these mice, gels containing VEGF-A and PDGF-CC also had increased vascularity and moderately increased formation of a vascularized cellular layer. In Ob/Ob mice fed a high fat diet, vascularity and vascularized layer thickness was markedly decreased in the FGF-2 and PDGF-CC treated mice compared to the WT mice with the same treatments (Fig. 5B). The decrease in cellular layer thickness (Fig. 5D) was statistically significant in the FGF-2 and PDGF-CC groups. We also performed Movat’s Pentachrome staining to the sections supported that the surrounding layers were primarily cellular (Supplementary Fig. S1). The cellular layer was confirmed to be primarily composed of cells with high vascularity (Fig. 5E). Histochemical staining confirmed the lack of fibrotic tissue (Fig. 5C and Supplementary Fig. S1) and immunostaining for CD45 was negative for the surrounding tissue (data not shown). Together these findings confirmed that WT mice had a more robust growth factor response compared to the Ob/Ob mice and supported the existence of growth factor resistance in this animal model.
Figure 5.
In-vivo animal model to test growth factor resistance by subcutaneously implanting alginate gels with growth factors. Alginate gels containing PBS, FGF-2, VEGF-A and PDGF-CC were subcutaneously implanted on the back of mice. The gels were harvested after seven days and immediately macroscopically imaged (A). Vascularity on the gel was determined for the macroscopic images using Metamorph (B). The gels were then frozen, sectioned and H&E stained (C). The thickness of the vascular layer was quantified by analyzing the H&E stained images using Metamorph (D). The sections were immunostained with anti-PECAM antibody and imaged with an epifluorescence microscope (E). Panel A - size bar = 3mm. Mag. size bar = 1mm. Panel B - size bar = 1mm. Mag. size bar = 250μm. Panel E - size bar = 250μm. *Statistically different from Ob/Ob group (p < 0.05). n=6
3.6. Overcoming growth factor resistance in the diabetic Ob/Ob mouse model
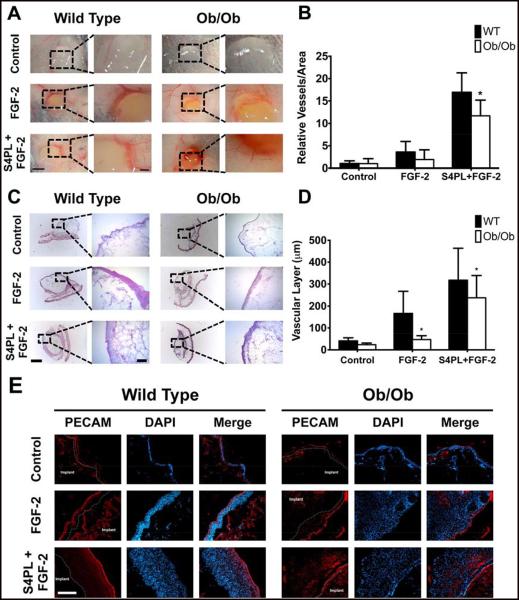
The failure of many human trials for growth factor therapies has highlighted the need for new treatments that can overcome growth factor resistance[5]. Our finding of decreased cell surface co-receptors in a diseased state suggested that this could be an amenable target for increasing growth factor activity in disease states. We have recently shown that growth factor activity can be enhanced in healthy animals by co-delivering them with sdc-4 embedded in a liposomal carrier[33]. Here, we formulated sdc-4 proteoliposomes into an injectable alginate carrier and examined whether this treatment could overcome disease-induced resistance to growth factor stimulation. We subcutaneously implanted gels containing PBS, FGF-2 and FGF-2 with sdc-4 proteoliposomes in Ob/Ob mice after 15 weeks of high fat diet. The addition of sdc-4 proteoliposomes markedly enhanced vascularity (Fig. 6A,B) as well as the vascularized layer thickness (Fig. 6C,E). We observed similar alterations for the vascularized layer thickness for both WT and Ob/Ob mice. We also performed Movat’s Pentachrome Staining on the tissue sections that confirmed the cellular nature of the tissue surrounding the implant (Supplementary Fig. S2). Together these studies support that the delivery of the co-receptor proteins, which are reduced in the diseased tissue, can enhance the growth factor response and significantly overcome the growth factor resistance in this animal model of diabetes and obesity.
Figure 6.
Macroscopic images with vessels per unit area and histochemically stained images with thickness of the vascular layer on the subcutaneously implanted alginate gels with FGF-2 with syndecan-4 (sdc-4) proteoliposomes. Alginate gels containing PBS, FGF-2 and sdc-4 proteoliposomes with FGF-2 were subcutaneously implanted on the back of mice. The gels were harvested after seven days and macroscopically imaged (A). Vascularity around the gel was determined for the macroscopic images using Metamorph (B). The gels were frozen, sectioned and H&E stained (C). The thickness of the vascular layer was quantified by analyzing the H&E stained images on Metamorph (D). The sections were immunostained with anti-PECAM antibody and imaged with an epifluorescence microscope (E). Panel A - size bar = 3mm. Mag. size bar = 1mm. Panel C - size bar = 1mm. Mag. size bar = 250μm. Panel E - size bar = 250μm. *Statistically different from Ob/Ob group (p < 0.05). n=5
4. Discussion
Diabetes and metabolic syndrome are major contributors to cardiovascular risk and mortality. Both diabetes and obesity have been associated with loss of microvascular density and reduced formation of collateralization in ischemic tissues[35, 36]. For many years, there has been acute awareness of insulin resistance in diabetic and pre-diabetic patients. Insulin resistance can be defined as a sub-physiological response to insulin at a given concentration and has been linked to multiple defects in the signaling and receptor dynamics[37]. Owing to the failure of a number of clinical trials in using exogenously delivered growth factors or growth factor gene expressing vectors, we hypothesized that resistance exists not only for insulin but for many growth factor including FGF-2, VEGF and PDGF isoforms. Previous studies have indirectly supported this hypothesis by demonstrating reduced wound healing in diabetic animals[38], a reduced capacity for ischemic pre-conditioning in reperfusion injury in Ob/Ob mice[39] and a defective response to angiogenic gene therapy in Db/Db mice[40]. A previous study examined the broad set of gene expression following ischemia in WT and Db/Db mice using microarray analysis[41], which supported the occurrence of reduced vascularization in these mice and alterations in angiogenic gene regulatory networks.
Here, we show that the Ob/Ob mouse model has growth factor resistance in the context of therapeutic angiogenesis to multiple growth factors including FGF-2 and PDGF-CC. The results of this study also reveal a previously unrecognized loss in co-receptor levels in Ob/Ob mice and have broad implications in both understanding the mechanisms of growth factor resistance and designing new effective angiogenic therapies. One of the key findings of this study is the potential pitfall of drawing conclusions of growth factor pathway status through the use of gene expression analysis, particularly for receptors and co-receptors. In both the heart and muscle samples we found a paradoxical increase in mRNA levels in contrast to the protein levels of the receptors/co-receptors. An implication of these findings is that there are either post-transcriptional mechanisms of regulation for these proteins or that increased degradation/shedding of these molecules is removing the protein from the cell surface. Syndecans, in particular, are known to be shed from the surface through the action of proteases[18]. In either case, is clear from our studies that a proteomic rather than genomic approach to studying these pathways is needed to capture the full complexity of the regulation of these processes by diabetes/high fat diet.
The regulation of the growth factor receptors and co-receptors had a remarkably similar regulation for many of the pathways studied. The archetypal pattern was an increase in the WT mice for the protein with exposure to high fat diet with no change for in the Ob/Ob mice with high fat diet. One possible explanation for these findings is that the WT mice, being analogous to healthy patients, up-regulate their angiogenic pathways when challenged with a high fat diet that likely causes the initial stages of endothelial dysfunction and vascular disease. The Ob/Ob mice, which are compromised by their additional metabolic defects, do not compensate by increasing their receptor levels. This finding would be consistent with the finding of previous studies that show an increase in NRP-1 in WT mice following ischemia and no change in NRP-1 in Db/Db mice after induction of ischemia[41]. Thus, Ob/Ob mice have altered compensation to disease, which may in part recapitulate the long-term disease state in human patients. In our studies, heparanase was found to increase with high fat diet in the WT mice. This finding is consistent with a previous study that demonstrated up-regulation of heparanase by fatty acids[42]. Our group has recently linked heparanase to restenosis and thrombosis following stenting[43, 44] and in the pathogenesis of atherosclerosis[45]. Heparanase is also linked to angiogenesis and is thought to release angiogenic growth factor from sequestration in heparan sulfate proteoglycans[34]. Interestingly, elevated heparanase levels were not observed in Ob/Ob mice fed with high fat diet in our studies. This would suggest that the elevation of heparanase levels is important for compensatory revascularization under the high fat diet in WT mice. However this mechanism does not occur with the additional defects in the Ob/Ob mice.
In our studies aimed at overcoming growth factor resistance, we focused our therapeutic strategy on restoring sdc-4 as a means for enhancing angiogenesis in the disease state. Syndecan-4 is known to regulate FGFR-1 signaling and macropinocytosis[33, 46]. It also has pro-angiogenic activities through interactions with thrombospondin-1[47] and regulates FGF-2 signaling[48]. Due to the continued challenges in applying exogenous gene delivery in humans[7], we developed a strategy to deliver co-receptors that are absent due to disease by using recombinant proteins embedded in a liposomal carrier[33]. Our major aim here was to test whether exogenously delivered co-receptors could overcome growth factor resistance that is, in part, caused by loss of co-receptors. In our studies, the sdc-4 proteoliposomes were highly effective in enhancing the effectiveness of FGF-2 stimulation in mice with disease. Our findings suggest an approach for combating growth factor resistance at the level of co-receptor/receptor loss and illustrate an example of the efficacy of this strategy in an animal disease model. Moreover, these findings suggest that liposomal co-receptor therapies may have the potential to treat many diseases in which the enhancement or reduction of signaling is caused by the loss of a co-receptor. From our findings on the reduction in other receptors such as NRP-1, these would be appealing targets to explore for the development of additional therapeutics.
5. Conclusions
Angiogenic therapies have generated great interest both in animal studies and in clinical trials. However, despite these many promising animal studies, no therapies have shown clinically meaningful benefits in controlled clinical trials. Our findings here suggest that this may be due to the development of growth factor resistance in patients with long-term disease, which is absent when using healthy animal models. In this work, we have identified multiple defects in growth factor signaling pathways that involve loss of receptors and co-receptors at the protein level. In addition, we have demonstrated that these blocked pathways can be recovered by the delivery of the missing components in a liposomal carrier. Thus, therapies of this type may be amenable for the treatment of ischemia in human patients that have developed growth factor resistance of over the long-term course of their disease.
Supplementary Material
Acknowledgments
The authors would like to thank the services provided by the ICMB (Institute of Cellular and Molecular Biology) core facility at University of Texas at Austin. The authors would like to acknowledge support through the American Heart Association (10SDG2630139) and the NIH Director’s New Innovator Grant (1DP2 OD008716-01).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures. The authors have filed a patent application on the compounds described in this work.
REFERENCES
- 1.Shammas NW. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vasc Health Risk Manag. 2007;3:229–34. doi: 10.2147/vhrm.2007.3.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooi JD, Kester AD, Stoffers HE, Overdijk MM, van Ree JW, Knottnerus JA. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. Am J Epidemiol. 2001;153:666–72. doi: 10.1093/aje/153.7.666. [DOI] [PubMed] [Google Scholar]
- 3.Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23. doi: 10.1161/CIRCULATIONAHA.110.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudagi VS, White CJ. Endovascular stents: a review of their use in peripheral arterial disease. Am J Cardiovasc Drugs. 2013;13:199–212. doi: 10.1007/s40256-013-0023-6. [DOI] [PubMed] [Google Scholar]
- 5.Annex BH. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol. 2013;10:387–96. doi: 10.1038/nrcardio.2013.70. [DOI] [PubMed] [Google Scholar]
- 6.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–78. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 7.Ornitz DM. FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays. 2000;22:108–12. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 9.Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem J. 2008;411:211–26. doi: 10.1042/BJ20071639. [DOI] [PubMed] [Google Scholar]
- 10.Chen E, Hermanson S, Ekker SC. Syndecan-2 is essential for angiogenic sprouting during zebrafish development. Blood. 2004;103:1710–9. doi: 10.1182/blood-2003-06-1783. [DOI] [PubMed] [Google Scholar]
- 11.Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994;125:917–28. doi: 10.1083/jcb.125.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 13.Ball SG, Bayley C, Shuttleworth CA, Kielty CM. Neuropilin-1 regulates platelet-derived growth factor receptor signalling in mesenchymal stem cells. Biochem J. 2010;427:29–40. doi: 10.1042/BJ20091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pellet-Many C, Frankel P, Evans IM, Herzog B, Junemann-Ramirez M, Zachary IC. Neuropilin-1 mediates PDGF stimulation of vascular smooth muscle cell migration and signalling via p130Cas. Biochem J. 2011;435:609–18. doi: 10.1042/BJ20100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Lee JH, Park HS, Hwang J, Han IO, Bae YS, et al. Syndecan-4 regulates platelet-derived growth factor-mediated MAP kinase activation by altering intracellular reactive oxygen species. FEBS Lett. 2008;582:2725–30. doi: 10.1016/j.febslet.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 16.Fukai N, Kenagy RD, Chen L, Gao L, Daum G, Clowes AW. Syndecan-1: an inhibitor of arterial smooth muscle cell growth and intimal hyperplasia. Arterioscler Thromb Vasc Biol. 2009;29:1356–62. doi: 10.1161/ATVBAHA.109.190132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao R, Brakenhielm E, Li X, Pietras K, Widenfalk J, Ostman A, et al. Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-alphaalpha and -alphabeta receptors. FASEB J. 2002;16:1575–83. doi: 10.1096/fj.02-0319com. [DOI] [PubMed] [Google Scholar]
- 18.Moriya J, Wu X, Zavala-Solorio J, Ross J, Liang XH, Ferrara N. PDGF-C promotes revascularization in ischemic limbs of diabetic mice. J Vasc Surg. 2013 doi: 10.1016/j.jvs.2013.04.053. [DOI] [PubMed] [Google Scholar]
- 19.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 20.Tirziu D, Simons M. Angiogenesis in the human heart: gene and cell therapy. Angiogenesis. 2005;8:241–51. doi: 10.1007/s10456-005-9011-z. [DOI] [PubMed] [Google Scholar]
- 21.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–93. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 22.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–65. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 23.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–8. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 24.Lederman RJ, Tenaglia AN, Anderson RD, Hermiller JB, Rocha-Singh K, Mendelsohn FO, et al. Design of the therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (TRAFFIC) trial. Am J Cardiol. 2001;88:192–5. doi: 10.1016/s0002-9149(01)01622-8. A6-7. [DOI] [PubMed] [Google Scholar]
- 25.Udelson JE, Dilsizian V, Laham RJ, Chronos N, Vansant J, Blais M, et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 improves stress and rest myocardial perfusion abnormalities in patients with severe symptomatic chronic coronary artery disease. Circulation. 2000;102:1605–10. doi: 10.1161/01.cir.102.14.1605. [DOI] [PubMed] [Google Scholar]
- 26.Rajagopalan S, Mohler ER, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. (3rd) 2003;108:1933–8. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 27.Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–7. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 28.Kusumanto YH, van Weel V, Mulder NH, Smit AJ, van den Dungen JJ, Hooymans JM, et al. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double-blind randomized trial. Hum Gene Ther. 2006;17:683–91. doi: 10.1089/hum.2006.17.683. [DOI] [PubMed] [Google Scholar]
- 29.Comerota AJ, Throm RC, Miller KA, Henry T, Chronos N, Laird J, et al. Naked plasmid DNA encoding fibroblast growth factor type 1 for the treatment of end-stage unreconstructible lower extremity ischemia: preliminary results of a phase I trial. J Vasc Surg. 2002;35:930–6. doi: 10.1067/mva.2002.123677. [DOI] [PubMed] [Google Scholar]
- 30.Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, et al. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377:1929–37. doi: 10.1016/S0140-6736(11)60394-2. [DOI] [PubMed] [Google Scholar]
- 31.Shigematsu H, Yasuda K, Iwai T, Sasajima T, Ishimaru S, Ohashi Y, et al. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17:1152–61. doi: 10.1038/gt.2010.51. [DOI] [PubMed] [Google Scholar]
- 32.Creager MA, Olin JW, Belch JJ, Moneta GL, Henry TD, Rajagopalan S, et al. Effect of hypoxia-inducible factor-1alpha gene therapy on walking performance in patients with intermittent claudication. Circulation. 2011;124:1765–73. doi: 10.1161/CIRCULATIONAHA.110.009407. [DOI] [PubMed] [Google Scholar]
- 33.Jang E, Albadawi H, Watkins MT, Edelman ER, Baker AB. Syndecan-4 proteoliposomes enhance fibroblast growth factor-2 (FGF-2)-induced proliferation, migration, and neovascularization of ischemic muscle. Proc Natl Acad Sci U S A. 2012;109:1679–84. doi: 10.1073/pnas.1117885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol. 2006;38:2018–39. doi: 10.1016/j.biocel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Adeghate E. Molecular and cellular basis of the aetiology and management of diabetic cardiomyopathy: a short review. Mol Cell Biochem. 2004;261:187–91. doi: 10.1023/b:mcbi.0000028755.86521.11. [DOI] [PubMed] [Google Scholar]
- 36.Skilton MR, Chin-Dusting JP, Dart AM, Brazionis L, Lantieri O, O'Dea K, et al. Metabolic health, obesity and 9-year incidence of peripheral arterial disease: the D.E.S.I.R. study. Atherosclerosis. 2011;216:471–6. doi: 10.1016/j.atherosclerosis.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 37.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 38.Seitz O, Schurmann C, Hermes N, Muller E, Pfeilschifter J, Frank S, et al. Wound healing in mice with high-fat diet- or ob gene-induced diabetes-obesity syndromes: a comparative study. Exp Diabetes Res. 2010;2010:476969. doi: 10.1155/2010/476969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouhidel O, Pons S, Souktani R, Zini R, Berdeaux A, Ghaleh B. Myocardial ischemic postconditioning against ischemia-reperfusion is impaired in ob/ob mice. Am J Physiol Heart Circ Physiol. 2008;295:H1580–6. doi: 10.1152/ajpheart.00379.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emanueli C, Caporali A, Krankel N, Cristofaro B, Van Linthout S, Madeddu P. Type-2 diabetic Lepr(db/db) mice show a defective microvascular phenotype under basal conditions and an impaired response to angiogenesis gene therapy in the setting of limb ischemia. Front Biosci. 2007;12:2003–12. doi: 10.2741/2205. [DOI] [PubMed] [Google Scholar]
- 41.Schiekofer S, Galasso G, Sato K, Kraus BJ, Walsh K. Impaired revascularization in a mouse model of type 2 diabetes is associated with dysregulation of a complex angiogenic-regulatory network. Arterioscler Thromb Vasc Biol. 2005;25:1603–9. doi: 10.1161/01.ATV.0000171994.89106.ca. [DOI] [PubMed] [Google Scholar]
- 42.Chen G, Wang D, Vikramadithyan R, Yagyu H, Saxena U, Pillarisetti S, et al. Inflammatory cytokines and fatty acids regulate endothelial cell heparanase expression. Biochemistry. 2004;43:4971–7. doi: 10.1021/bi0356552. [DOI] [PubMed] [Google Scholar]
- 43.Baker AB, Groothuis A, Jonas M, Ettenson DS, Shazly T, Zcharia E, et al. Heparanase alters arterial structure, mechanics, and repair following endovascular stenting in mice. Circ Res. 2009;104:380–7. doi: 10.1161/CIRCRESAHA.108.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker AB, Gibson WJ, Kolachalama VB, Golomb M, Indolfi L, Spruell C, et al. Heparanase regulates thrombosis in vascular injury and stent-induced flow disturbance. J Am Coll Cardiol. 2012;59:1551–60. doi: 10.1016/j.jacc.2011.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker AB, Chatzizisis YS, Beigel R, Jonas M, Stone BV, Coskun AU, et al. Regulation of heparanase expression in coronary artery disease in diabetic, hyperlipidemic swine. Atherosclerosis. 2010;213:436–42. doi: 10.1016/j.atherosclerosis.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elfenbein A, Lanahan A, Zhou TX, Yamasaki A, Tkachenko E, Matsuda M, et al. Syndecan 4 regulates FGFR1 signaling in endothelial cells by directing macropinocytosis. Sci Signal. 2012;5:ra36. doi: 10.1126/scisignal.2002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunes SS, Outeiro-Bernstein MA, Juliano L, Vardiero F, Nader HB, Woods A, et al. Syndecan-4 contributes to endothelial tubulogenesis through interactions with two motifs inside the pro-angiogenic N-terminal domain of thrombospondin-1. J Cell Physiol. 2008;214:828–37. doi: 10.1002/jcp.21281. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Li J, Partovian C, Sellke FW, Simons M. Syndecan-4 modulates basic fibroblast growth factor 2 signaling in vivo. Am J Physiol Heart Circ Physiol. 2003;284:H2078–82. doi: 10.1152/ajpheart.00942.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.