Abstract
Purpose of review
Recent advances in T cell biology have shed light on the role of T cell subsets in the pathogenesis of acute kidney injury (AKI). The purpose of this review is to harness our understanding of recent advances in T cell biology in tissue injury and repair and provide a mechanistic insight into the role of T cells in the inflammation of AKI.
Recent findings
New specific reagents and genetic animal models have led to advances in our understanding of the role of T cell subsets involved in renal injury. Whereas some T cells promote innate renal inflammation and injury, other T cells promote protection and repair. Recent studies illuminated the pathogenic mechanisms of invariant natural killer T (NKT) cells and T helper1-type responses, and the beneficial functions of regulatory T cells and NKT cells are just beginning to be explored. Pharmacologic and cell-based therapies that influence T cell responses to experimental AKI suggest that this is a promising approach to preserve renal function.
Summary
The recent insights gained into how T cells modulate renal injury suggest that strategies targeting specific types of T cells, to either inhibit or enhance their activity, may ameliorate renal injury in patients.
Keywords: cisplatin, ischemia-reperfusion, lymphocytes, natural killer T cell, Treg
INTRODUCTION
The incidence of acute kidney injury (AKI) has been steadily increasing in recent decades, and AKI causes significant morbidity and mortality in those who are affected [1]. Common causes of AKI include: sepsis, use of nephrotoxic drugs and conditions resulting in ischemia or hypoperfusion of the kidneys (cardiopulmonary bypass, hemorrhage, severe hypotension, etc.). Ischemia induces a complex series of events that lead to altered hemodynamics, tubular injury and inflammation. Early in the course of AKI, Sutton et al. [2] have proposed an ‘extension phase’ of ischemic AKI in which immune cells play a critical role. This proposal is based upon a long recognized feature that the kidney interstitial microenvironment is a fertile ground for innate immune cells such as dendritic cells and macrophages [3], and following ischemia there is an accumulation and activation of immune cells in the damaged kidney [4]. CD3+ T cells are prominent in the inflammatory infiltrate in human AKI [5] and accumulate in the kidney within 30 min to a few hours in murine models of AKI [6-10]. Studies in experimental AKI have demonstrated a causal role for certain types of T cells in promoting renal injury, whereas other studies have revealed protective roles for other T cell subsets (see below). Immune cells accumulate in the corticomedullary junction leading to vascular congestion, interstitial edema, and diminished nutrient and oxygen delivery.
T CELLS IN THE PATHOGENESIS OF EXPERIMENTAL ACUTE KIDNEY INJURY
The role of T cells in tissue injury is supported by several early studies [11-15]. Zwacka et al. [15] demonstrated an early role of T cells in mouse liver ischemia-reperfusion injury. In this mouse model of liver injury, T cells were detected maximally at 1 h post reperfusion [15]. Using T-cell deficient mice and/or adoptive transfer of T cells, T cells were found to be key mediators of inflammatory responses mediated by neutrophils [15]. In a warm ischemiareperfusion model, using specific markers for inflammatory cells, macrophages, CD4+ T cells and CD8+ T cells have been identified in renal tissue [16]. The appearance of these inflammatory cells began as early as 1 h after ischemia-reperfusion and appeared to peak at around 5 days [16]. Several other studies have demonstrated that CD4+ T cells are involved in kidney ischemia-reperfusion injury (IRI) [17-21]. However, conventional CD4+ T cells are thought to play an obligatory role in antigenspecific, cognate immunity that requires 2–4 days for T cell processing. The kinetics of conventional T cell activation is inconsistent with the rapid, innate immune response following IRI. By contrast, natural killer T (NKT) cells are a T cell sublineage [22] known to participate in innate immunity and may contribute to the early events in IRI (described below).
HOW ARE T CELLS ACTIVATED?
Both kidney parenchymal cells and bone marrow-derived cells make up the renal interstitial microenvironment [3]. Under normal conditions, members of the mononuclear phagocytic system make up the largest population of immune cells in the kidney [23-25]. Many of these mononuclear phagocytes are dendritic cells, based on the expression of phenotypic markers [23-25]. Dendritic cells are professional antigen presenting cells (APCs), specialized for activating T cells. In addition, the uninjured kidney also contains several different types of T cells [CD4+, CD8+, CD4−CD8−, NKT and regulatory T cells (Tregs)] [26]. Following ischemia-reperfusion, vascular endothelial cells and renal tubular epithelial cells are injured and play a critical role in initiating and facilitating inflammation in response to kidney injury [27]. After injury, damage-associated molecular patterns are released by dead or dying cells in the kidney, and these molecules activate dendritic cells through interaction with toll-like receptors and a variety of other proinflammatory receptors [28]. Dendritic cells in turn upregulate positive costimulatory ligands, produce proinflammatory cytokines and activate both innate and adaptive immune cells (including T cells) [29-31]. The injured tubular epithelial cells produce chemokines to attract circulating leukocytes and renal vascular endothelial cells upregulate expression of adhesion molecules to facilitate extravasation of leukocytes [27,32-34]. In summary, the kidney interstitium is inhabited by professional APCs and T cells under normal circumstances and after injury multiple cells contribute to the establishment of a proinflammatory microenvironment.
Common features of T cells are the expression of the T cell receptor (TCR) and CD3, which is a protein complex that transduces signals from the TCR into the T cell. The TCR allows T cells to recognize specific antigens (peptides, glycolipids, etc.) presented by APCs. At the time of antigen presentation, additional signals provided by APCs influence the type of T cell response that will develop. Costimulation is one such signal provided through cell surface receptors and ligands on APCs and T cells. Positive costimulation through CD28 on T cells and CD80 or CD86 expressed on APCs reinforces the TCR signal and promotes cytokine production and T cell proliferation [35]. On the other hand, negative costimulation through inhibitory receptors on T cells [e.g. cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death 1 (PD-1)] antagonizes the activation of T cells [35]. Some APCs also produce cytokines such as interleukin (IL)-12 and IL-23 that promote and direct the T cell response to antigens.
Nitric oxide is an important signaling molecule and critically related to the pathogenesis of AKI [36]. Depending on its concentration and duration of action nitric oxide can promote survival or death of endothelial and epithelial cells of the kidney [36]. In addition, nitric oxide and inducible nitric oxide synthase (iNOS) can influence the immune response. For example, nitric oxide is required for processing of certain antigens intracellularly prior to presentation to T cells by APCs [37]; however, iNOS-expressing dendritic cells were shown to inhibit T cell activation in an autoimmune myocarditis model [38]. In an ischemic AKI model, iNOS-expressing macrophages contribute to kidney injury, but the role of T cells in this process has not been studied [39]. Given the complexity of the actions of nitric oxide in different contexts and cell types, the influence of nitric oxide on T cell action during AKI requires further study to be understood.
ROLE OF T CELL SUBSETS IN ACUTE KIDNEY INJURY
The family of T cells is large, containing multiple subtypes of lymphocytes with vastly different characteristics. CD3+CD4+ T helper (Th) cells recognize peptide antigens and primarily coordinate and promote the activity of other inflammatory cells. Depending on the context of their activation (costimulation and cytokine milieu), naive CD4 T cells can differentiate into: Th1 cells that produce interferon γ (IFN-γ), Th2 cells that produce IL-4, Th17 cells that produce IL-17, or several other types of Th cells [40]. On the other end of the spectrum of CD3+CD4+ lymphocytes are the Tregs that suppress the activation of most other proinflammatory cells [41]. Tregs are identified by the expression of the transcription factor forkhead box P3 (FoxP3) [42-44] and use a variety of mechanisms to inhibit other immune cells. These include production of anti-inflammatory cytokines (e.g. IL-10) or molecules (e.g. adenosine) or cell contact-dependent mechanisms (CTLA-4, cyclic adenosine monophosphate transfer, etc.) [41]. CD3+CD8+ cytotoxic T cells recognize antigens generated by cancerous or infected cells and kill target cells using perforin, granzymes and/or Fas ligand. NKT cells make up a relatively small proportion of T cells but are robust producers of Th1 and Th2 cytokines. There are two main groups of NKT cells: type I, invariant NKT cells that express very similar TCRs and recognize glycolipid antigens (e.g. α-galactosylceramide) and type II NKT cells that express more diverse TCRs and recognize other lipid antigens (e.g. sulfatide). Depending on the setting, NKT cells can perform proinflammatory or anti-inflammatory functions [45,46]. Thus, T cells are a large family of lymphocytes with diverse functions and phenotypes.
T CELLS THAT PROMOTE INJURY
As mentioned above, CD4+ T cells were initially identified as the primary type of pathogenic T cells in experimental AKI. As there are multiple types of CD4+ T cells, identification of the responsible subset(s), and the ways in which they affect renal function during AKI, is critical to developing therapeutics based on the role of T cells in injury. There are numerous mechanisms by which T lymphocytes can induce injury to other cells and promote renal dysfunction (Fig. 1) [47,48]. The requirement for IFN-γ made by T cells suggests that Th1 cells are involved. However, when signal transducers and activators of transcription 4 knockout mice (which cannot mount Th1 responses) were compared with wild-type mice after IRI, only a mild reduction in serum creatinine was observed, and no difference in tissue damage was noted between groups [49]. Also, the time required for a conventional Th1 response to develop (3 or more days) is not congruent with the inflammation observed in experimental AKI (beginning within hours). In contrast, NKT cells (some but not all of which are CD4+ [46]) once activated can produce large amounts of IFN-γ within hours [45,46]. In support of this, adoptive transfer of α-galactosylceramide (glycolipid antigen specific for type I NKT cells) loaded dendritic cells prior to subthreshold ischemic injury exacerbates renal injury and dysfunction in wild-type mice [33,50■■], but not NKT-cell deficient or IFN-γ-deficient mice [33]. It should be noted that that several studies have observed protective phenotypes in mice either lacking type I NKT cells, or in which glycolipid antigen presentation has been blocked [8,33], whereas another study did not report protection in type I NKT-cell deficient mice [51]. The reasons for this discrepancy are not known, but could be because of the differences in gut flora between different institutions as gut flora has been shown to modulate type I NKT cell phenotype [52].
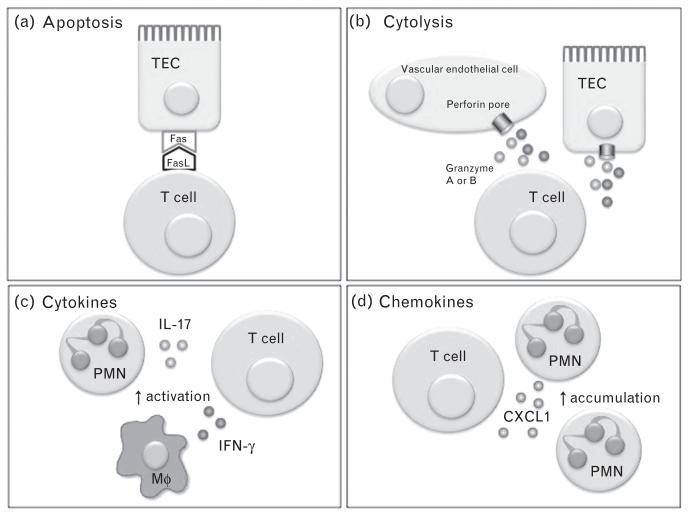
FIGURE 1.
Potential mechanisms of renal injury by T cells. Expression of FasL on bone marrow-derived cells (possibly T cells) promotes renal ischemia-reperfusion injury [47]. FasL interaction with Fas induces apoptosis in Fas bearing cells (e.g. renal TECs (a)). Activated T cells can release granzymes or perforin to injure neighboring cells (b). Release of proinflammatory cytokines promotes the activation of neutrophils (PMN), macrophages (Mφ) and other leukocytes (c). In cisplatin nephrotoxicity, CD4 T cells release the chemokine CXCL1 [48], which can promote PMN recruitment to the injured kidney (d). CXCL1, chemokine (C-X-C motif) ligand 1; FasL, Fas ligand; TECs, tubular epithelial cells.
Some other recent studies have focused more on the mechanisms of activation, and mediators produced by CD4+ T cells in experimental AKI rather than identification of specific subsets. For example, Akcay et al. [48] reported that IL-33 released by renal cells injured by cisplatin activates CD4+ T cells and promotes their recruitment to the kidney. IL-33 promoted chemokine (C-X-C motif) ligand 1 (CXCL1) production by CD4+ T cells in the kidney and in vitro [48], and CXCL1 is known to promote neutrophil recruitment and can directly induce apoptosis [53]. Other recent studies have focused on T-cell immunoglobulin and mucin domain-containing protein-1 [(TIM-1); also known as kidney injury molecule-1 (KIM-1)] expressed on T cells in experimental AKI models [54,55]. TIM-1 is expressed on activated CD4+ T cells as well as injured renal tubular epithelial cells [56■]. Using a blocking antibody to TIM-1 [rat monoclonal TIM-1 antibody (RMT1–10)] was protective against both cisplatin [54] and ischemia-reperfusion [55] induced renal injury in mice. The protective effect was lost in both studies if the antibodies were administered to recombination activating gene-1 (RAG-1) knockout mice (which lack T and B cells) prior to injury [54,55]. Adoptive transfer of splenocytes (containing T and B cells) into RAG-1 knockout recipients restored the protective effect of RMT1–10, demonstrating the role of immune cells in protection from IRI [55]. As pointed out by Ichimura et al. [56■], there are several caveats of these studies to be considered before concluding that TIM-1 on CD4+ T cells is a target to be blocked therapeutically. These include the expression of TIM-1 on immune cells other than just CD4+ T cells, possible stimulatory action of the RMT1–10 antibody on TIM-1 and the very high expression of TIM-1/KIM-1 on tubular epithelial cells vs. intrarenal immune cells after injury (see [56■] and references therein).
In summary, numerous studies support a pathogenic role for T cells in AKI. Progress toward understanding the identity of the T cells involved and the mechanisms by which they promote injury is progressing at a modest pace. Factors hindering progress include the ever growing awareness of the heterogeneity and plasticity of T cell subsets [40] and limitations of the current mouse models and experimental reagents. Persistence and innovative ideas/technologies are needed to advance our knowledge of T cell-based targets for inhibition to ameliorate AKI.
T CELLS THAT PROTECT FROM INJURY OR PROMOTE REPAIR
Research has been progressing more rapidly on the recently discovered protective role of several types of T cells in AKI models. Several subsets of T cells show promise as potential therapeutic agents themselves (as cell-based immunotherapies) or as targets for pharmacological enhancement.
Regulatory T cells
Tregs provide a balance to proinflammatory immune cells by using multiple mechanisms to suppress inflammation (Fig. 2) [41,57]. In 2009, the role of endogenous Tregs to protect against kidney IRI [58] and promote recovery from ischemic injury [59,60] was identified. Since then the critical role for Tregs in the protection afforded by renal ischemic preconditioning has been demonstrated [61,62]. Furthermore, in a nephrotoxic cisplatin-AKI model endogenous Tregs were also shown to protect from injury [63]. Adoptive transfer of isolated Tregs prior to ischemia or cisplatin markedly protects mice from renal dysfunction and tissue injury [58,62,63,64■■]. In addition, adoptive transfer of Tregs after injury accelerates repair and restoration of renal function [59]. Importantly, Treg adoptive transfers have been successfully performed in humans [65-67], suggesting this type of therapy could be used clinically for AKI.
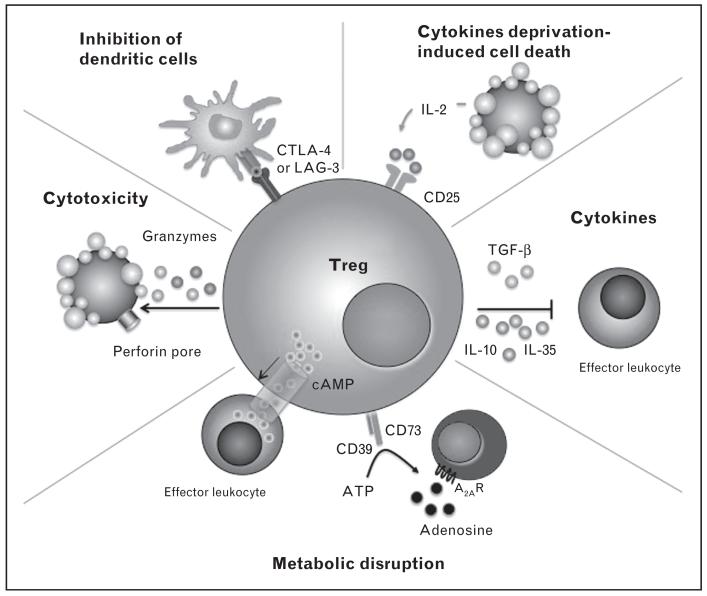
FIGURE 2.
Mechanism by which regulatory T cells suppress immune responses. T regulatory cells (Tregs) target dendritic cells (DCs) to inhibit their maturation through LAG3 (also known as CD223)–MHC-class-II interactions and CTLA-4 interaction with CD80 and CD86 on the surface of DCs. By acting as a sink for IL-2, Tregs can cause cytokine deprivation-induced cell death of effector T cells. Tregs secrete inhibitory cytokines such as IL-10, TGFβ and IL-35. Metabolic disruption can be induced by Tregs through cell transfer of cyclic AMP and by the sequential dephosphorylation of ATP by CD39 and CD73 to generate adenosine, which acts on the adenosine receptor 2A (A2AR) of target cells to mediate suppression. Cytotoxicity to target cells can also be induced through granzyme A and B and perforin-dependent killing. AMP, adenosine monophosphate; ATP, adenosine triphosphate; CTLA-4, cytotoxic T lymphocyte antigen-4; IL-2; interleukin-2; LAG3, lymphocyte-activation gene 3; MHC, major histocompatibility complex; TGFβ, transforming growth factor β. Adapted with permission from [41,57].
An alternative approach is to target endogenous Tregs to promote their proliferation, activity or trafficking to enhance their ability to protect the kidney or promote recovery from injury. Recently, numerous agents that target Tregs in vivo have been utilized in models of AKI with very encouraging results. The sphingosine kinase inhibitor dimethylsphingosine (DMS) protects against IRI in mice by promoting Treg recruitment to the kidney very quickly during reperfusion [68■]. A second sphingosine kinase inhibitor did not replicate this protection [68■], suggesting the enhanced Treg trafficking is mediated by a currently unknown mechanism of action for DMS. CTLA-4 blockade negated the protective effect of DMS in this study suggesting that Tregs utilize CTLA-4 to protect the kidney [68■]. Through CTLA-4, Tregs interact with dendritic cells to downregulate costimulatory molecule expression, and thus inhibit their ability to promote inflammation [69]. In another study, the protective effect of FTY720, a sphingosine-1-phosphate analog, on kidney IRI was shown to require Tregs [70]. Interestingly, bee venom injections increased Treg numbers in the spleen of mice and enhanced the trafficking of Tregs to the kidney shortly after cisplatin administration [71■■]. Bee venom injections reduced renal dysfunction and injury caused by cisplatin in control mice, but not mice in which Tregs were depleted [71■■]. In support of the feasibility of this therapy in cancer patients, bee venom had no effect on cisplatin’s anticancer effects in their mouse model [71■■]. In other disease models, IL-2/anti-IL-2 complexes protect from inflammatory injury by inducing Treg proliferation in vivo [72]. Kim et al. [73■■] used this technique in mice and nicely demonstrated that IL-2/anti-IL-2 complexes can be used to prevent renal IRI and promote recovery from IRI if given after injury. Importantly, IL-2/ anti-IL-2 complexes significantly reduced renal fibrosis at 28 days after injury even when treatment was started 1 day after the ischemic insult [73■■]. Finally, mesenchymal stem cell (MSC) therapy for AKI, which is currently being studied in several clinical trials (NCT00733876, NCT01275612), was shown to depend partially on the interaction between MSCs and Tregs in the spleens of recipient mice, as depletion of Tregs or splenectomy reduced the ability of MSCs to protect the kidney in this experimental IRI model [74■].
Natural killer T cells
NKT cells have the unique ability to produce Th1 (IFN-γ) and/or Th2 (IL-10 and IL-4) cytokines rapidly and may promote or inhibit immune-mediated renal injury. Yang et al. [51] found that adoptive transfer of activated type II NKT cells (those which respond to the lipid antigen sulfatide) inhibited renal injury induced by ischemia-reperfusion. To offer protection, the NKT cells needed to traffic into the injured kidney [51]. In-vitro coculture experiments revealed that suflatide-activated NKT cells suppress hypoxia-induced tubular epithelial cell death in an IL-10-dependent manner [51]. Using a separate methodology to target the protective potential of type I NKT cells, our laboratory has loaded wild-type dendritic cells with α-galactosylceramide in the presence of an immunosuppressive adenosine 2A receptor (A2A R) agonist [75■■], or loaded sphingosine-1-phosphate receptor 3 (S1P3R)-deficient dendritic cells with α-galactosylceramide [50■■] prior to adoptive transfer into mice. A2AR agonist-treated α-galactosylceramide-loaded wild-type dendritic cells and α-galactosylceramideloaded S1P3R knockout dendritic cells induced long lasting (up to 7 days after injection) protection from IRI [50■■,75■■]. In addition, significant functional protection was observed when the ex-vivo manipulated dendritic cells were injected 3–6 h after ischemia [50■■,75■■]. The protective effects of A2AR agonist-treated α-galactosylceramide-loaded dendritic cells were dependent on IL-10 expression in the recipient [75■■] and the α-galactosylceramide loaded S1P3R knockout dendritic cells required IL-4 [50■■]. These studies show that activation of NKT cells in the appropriate context can endow the NKT cells with renal protective properties, likely based on their ability to produce the Th2 cytokines IL-10 and IL-4. These findings are in line with the previous observation that Th2 responses are protective in renal IRI [49]. Similar to Tregs and MSCs, dendritic cells have been adoptively transferred to humans in numerous clinical trials [76] and may represent an effective way to target intrinsic T cells in AKI.
Splenic T cells
The spleen is a rich source of immune cells including T cells. Recent studies suggest that the spleen plays an important role in modulating organ ischemia-reperfusion. Evidence for the importance of the spleen in protecting organs comes from studies of preconditioning [77] and ischemia reperfusion to the kidney [78]. In these studies, splenectomy increases the injury or inflammation in organs subjected to ischemia-reperfusion or in distant organs [77,78]. The mechanism for this effect is unknown, however recent studies by Gigliotti et al. [79■■] have shed some light on this issue. In this study, exposure of mice to ultrasound led to marked tissue protection and this protective effect required the presence of CD4+ cells. Protection was lost following splenectomy or in Rag1−/− mice that lacked T and B cells. The tissue protective effect of ultrasound was reconstituted following adaptive transfer of CD4+ cells into Rag1−/− mice [79■■]. Thus, a novel concept and paradigm has evolved that extrarenal splenic T cells may have an important modulatory role in kidney IRI (Fig. 3) [79■■].
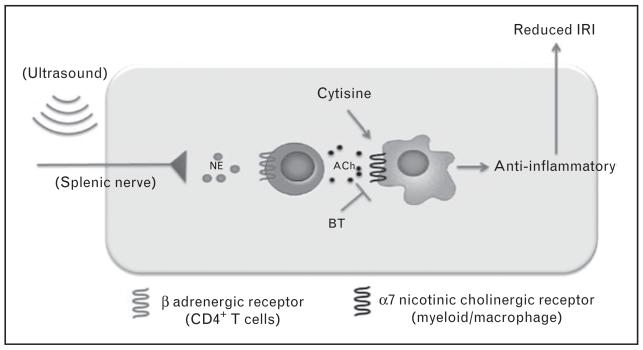
FIGURE 3.
Splenic T cells are critical for an anti-inflammatory pathway that reduces kidney ischemia-reperfusion injury. CD4+ T cells in the spleen are activated by ultrasound to produce acetylcholine (ACh), which activates the α7 nicotinic acetylcholine receptor, expressed on myeloid cells, initiating an anti-inflammatory response promoting protection from kidney ischemia-reperfusion injury (IRI). NE, norepinephrine. Adapted with permission from Gigliotti et al. [79■■].
CONCLUSION
The role of T cells in AKI has become much more complicated than originally envisioned. Some progress has been made in understanding the detrimental mechanisms of T cells in promoting injury and a previously unrecognized protective role of some T cell subsets has emerged. The complexity of the T cell family and the characteristics of T cells that are yet unknown make this a challenging area of investigation. However, as our understanding of how T cells modulate renal injury grows this should facilitate development of specifically targeted therapies to protect the kidney without causing unwanted global immunosuppression.
KEY POINTS.
Some T cells use proinflammatory cytokines (e.g. IFN-γ) and chemokines (e.g. CXCL1) to promote acute renal injury.
Tregs promote protection from injury by production of IL-10 and expression of anti-inflammatory proteins on their cell surface.
Activation of endogenous Tregs or cell-based therapy using Tregs may be used in the treatment of AKI.
NKT cells can induce renal inflammation and injury or protect from it depending on the context of their activation.
Acknowledgements
This work was supported in part from grants from the National Institutes of Health R01 to M.D.O.: DK062324, DK085259, and G.R.K.: K01 DK088967. G.R.K. was previously supported by T32 DK072922.
Footnotes
Conflicts of interest
M.O. has the following disclosures: Daiichi-Sankyo, PGX Health/Adenosine Therapeutics, LLC; UVA Patent Office.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 2.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaissling B, Le Hir M. The renal cortical interstitium: morphological and functional aspects. Histochem Cell Biol. 2008;130:247–262. doi: 10.1007/s00418-008-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 6.Fiorina P, Ansari MJ, Jurewicz M, et al. Role of CXC chemokine receptor 3 pathway in renal ischemic injury. J Am Soc Nephrol. 2006;17:716–723. doi: 10.1681/ASN.2005090954. [DOI] [PubMed] [Google Scholar]
- 7.Lai LW, Yong KC, Igarashi S, Lien YH. A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int. 2007;71:1223–1231. doi: 10.1038/sj.ki.5002203. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Huang L, Sung SS, et al. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol. 2007;178:5899–5911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 9.Liu M, Chien CC, Burne-Taney M, et al. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J Am Soc Nephrol. 2006;17:765–774. doi: 10.1681/ASN.2005010102. [DOI] [PubMed] [Google Scholar]
- 10.Rabb H. Immune modulation of acute kidney injury. J Am Soc Nephrol. 2006;17:604–606. doi: 10.1681/ASN.2006010060. [DOI] [PubMed] [Google Scholar]
- 11.Horie Y, Chervenak RP, Wolf R, et al. Lymphocytes mediate TNF-alpha-induced endothelial cell adhesion molecule expression: studies on SCID and RAG-1 mutant mice. J Immunol. 1997;159:5053–5062. [PubMed] [Google Scholar]
- 12.Kokura S, Wolf RE, Yoshikawa T, et al. T-lymphocyte-derived tumor necrosis factor exacerbates anoxia-reoxygenation-induced neutrophil-endothelial cell adhesion. Circ Res. 2000;86:205–213. doi: 10.1161/01.res.86.2.205. [DOI] [PubMed] [Google Scholar]
- 13.Le Moine O, Louis H, Demols A, et al. Cold liver ischemia-reperfusion injury critically depends on liver T cells and is improved by donor pretreatment with interleukin 10 in mice. Hepatology. 2000;31:1266–1274. doi: 10.1053/jhep.2000.7881. [DOI] [PubMed] [Google Scholar]
- 14.Nikbakht-Sangari M, Qayumi AK, Keown PA. The role of inflammatory mediators in the mechanism of the host immune response induced by ischemia-reperfusion injury. Immunol Invest. 2000;29:13–26. doi: 10.3109/08820130009105141. [DOI] [PubMed] [Google Scholar]
- 15.Zwacka RM, Zhang Y, Halldorson J, et al. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takada M, Nadeau KC, Shaw GD, et al. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P-selectin ligand. J Clin Invest. 1997;99:2682–2690. doi: 10.1172/JCI119457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burne MJ, Daniels F, El Ghandour A, et al. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001;108:1283–1290. doi: 10.1172/JCI12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burne-Taney MJ, Liu M, Ascon D, et al. Transfer of lymphocytes from mice with renal ischemia can induce albuminuria in naive mice: a possible mechanism linking early injury and progressive renal disease? Am J Physiol Renal Physiol. 2006;291:F981–F986. doi: 10.1152/ajprenal.00229.2005. [DOI] [PubMed] [Google Scholar]
- 19.Burne-Taney MJ, Yokota-Ikeda N, Rabb H. Effects of combined T- and B-cell deficiency on murine ischemia reperfusion injury. Am J Transplant. 2005;5:1186–1193. doi: 10.1111/j.1600-6143.2005.00815.x. [DOI] [PubMed] [Google Scholar]
- 20.Day YJ, Huang L, Ye H, et al. Renal ischemia-reperfusion injury and adenosine 2a receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol. 2006;176:3108–3114. doi: 10.4049/jimmunol.176.5.3108. [DOI] [PubMed] [Google Scholar]
- 21.Ysebaert DK, De Greef KE, De Beuf A, et al. T cells as mediators in renal ischemia/reperfusion injury. Kidney Int. 2004;66:491–496. doi: 10.1111/j.1523-1755.2004.761_4.x. [DOI] [PubMed] [Google Scholar]
- 22.Seino K, Motohashi S, Fujisawa T, et al. Natural killer T cell-mediated antitumor immune responses and their clinical applications. Cancer Sci. 2006;97:807–812. doi: 10.1111/j.1349-7006.2006.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Huang L, Sung SS, et al. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008;74:1526–1537. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson PJ, Rees AJ, Griffin MD, et al. The renal mononuclear phagocytic system. J Am Soc Nephrol. 2012;23:194–203. doi: 10.1681/ASN.2011070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soos TJ, Sims TN, Barisoni L, et al. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 2006;70:591–596. doi: 10.1038/sj.ki.5001567. [DOI] [PubMed] [Google Scholar]
- 26.Ascon DB, Ascon M, Satpute S, et al. Normal mouse kidneys contain activated and CD3+CD4-CD8-double-negative T lymphocytes with a distinct TCR repertoire. J Leukoc Biol. 2008;84:1400–1409. doi: 10.1189/jlb.0907651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109:e102–e107. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011;22:416–425. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong X, Swaminathan S, Bachman LA, et al. Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int. 2005;68:1096–1108. doi: 10.1111/j.1523-1755.2005.00502.x. [DOI] [PubMed] [Google Scholar]
- 30.Dong X, Swaminathan S, Bachman LA, et al. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 2007;71:619–628. doi: 10.1038/sj.ki.5002132. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol. 2010;30:268–277. doi: 10.1016/j.semnephrol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly KJ, Williams WW, Jr, Colvin RB, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Huang L, Vergis AL, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okusa MD, Linden J, Huang L, et al. A(2a) adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol Renal Physiol. 2000;279:F809–F818. doi: 10.1152/ajprenal.2000.279.5.F809. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goligorsky MS, Brodsky SV, Noiri E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002;61:855–861. doi: 10.1046/j.1523-1755.2002.00233.x. [DOI] [PubMed] [Google Scholar]
- 37.Mazmanian SK, Kasper DL. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Immunol. 2006;6:849–858. doi: 10.1038/nri1956. [DOI] [PubMed] [Google Scholar]
- 38.Kania G, Siegert S, Behnke S, et al. Innate signaling promotes formation of regulatory nitric oxide-producing dendritic cells limiting T-cell expansion in experimental autoimmune myocarditis. Circulation. 2013;127:2285–2294. doi: 10.1161/CIRCULATIONAHA.112.000434. [DOI] [PubMed] [Google Scholar]
- 39.Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fontenot JD, Gavin MA, Rudensky AY. FoxP3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 43.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor FoxP3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 44.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 45.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 47.Ko GJ, Jang HR, Huang Y, et al. Blocking fas ligand on leukocytes attenuates kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2011;22:732–742. doi: 10.1681/ASN.2010010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akcay A, Nguyen Q, He Z, et al. IL-33 exacerbates acute kidney injury. J Am Soc Nephrol. 2011;22:2057–2067. doi: 10.1681/ASN.2010091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yokota N, Burne-Taney M, Racusen L, Rabb H. Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2003;285:F319–F325. doi: 10.1152/ajprenal.00432.2002. [DOI] [PubMed] [Google Scholar]
- 50■■.Bajwa A, Huang L, Ye H, et al. Dendritic cell sphingosine 1-phosphate receptor-3 regulates Th1-Th2 polarity in kidney ischemia-reperfusion injury. J Immunol. 2012;189:2584–2596. doi: 10.4049/jimmunol.1200999. This article shows that the context in which type I NKT cells are activated in vivo with their ligand α-galactosylceramide can determine whether AKI is exacerbated or inhibited. S1PR3-deficient dendritic cells activate NKT cells in such a way that a Th2 response is predominant and this is protective against kidney IRI.
- 51.Yang SH, Lee JP, Jang HR, et al. Sulfatide-reactive natural killer T cells abrogate ischemia-reperfusion injury. J Am Soc Nephrol. 2011;22:1305–1314. doi: 10.1681/ASN.2010080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokote H, Miyake S, Croxford JL, et al. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol. 2008;173:1714–1723. doi: 10.2353/ajpath.2008.080622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gangadharan C, Thoh M, Manna SK. Late phase activation of nuclear transcription factor kappaB by doxorubicin is mediated by interleukin-8 and induction of apoptosis via FasL. Breast Cancer Res Treat. 2010;120:671–683. doi: 10.1007/s10549-009-0493-z. [DOI] [PubMed] [Google Scholar]
- 54.Nozaki Y, Nikolic-Paterson DJ, Yagita H, et al. Tim-1 promotes cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 2011;301:F1098–F1104. doi: 10.1152/ajprenal.00193.2011. [DOI] [PubMed] [Google Scholar]
- 55.Rong S, Park JK, Kirsch T, et al. The TIM-1: YIM-4 pathway enhances renal ischemia-reperfusion injury. J Am Soc Nephrol. 2011;22:484–495. doi: 10.1681/ASN.2010030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56■.Ichimura T, Brooks CR, Bonventre JV. Kim-1/Tim-1 and immune cells: shifting sands. Kidney Int. 2012;81:809–811. doi: 10.1038/ki.2012.11. This editorial gives a nice summary of what is known about KIM-1/TIM-1 on renal cells, T cells and other immune cells and their potential roles in AKI. It also explains the caveats of several reagents used to investigate the KIM-1/TIM-1 pathway.
- 57.Bodor J, Bopp T, Vaeth M, et al. Cyclic AMP underpins suppression by regulatory T cells. Eur J Immunol. 2012;42:1375–1384. doi: 10.1002/eji.201141578. [DOI] [PubMed] [Google Scholar]
- 58.Kinsey GR, Sharma R, Huang L, et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20:1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gandolfo MT, Jang HR, Bagnasco SM, et al. FoxP3(+) regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76:717–729. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 60.Monteiro RMM, Camara NOS, Rodrigues MM, et al. A role for regulatory T cells in renal acute kidney injury. Transpl Immunol. 2009;21:50–55. doi: 10.1016/j.trim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Cho WY, Choi HM, Lee SY, et al. The role of Tregs and CD11c(+) macrophages/dendritic cells in ischemic preconditioning of the kidney. Kidney Int. 2010;78:981–992. doi: 10.1038/ki.2010.266. [DOI] [PubMed] [Google Scholar]
- 62.Kinsey GR, Huang L, Vergis AL, et al. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 2010;77:771–780. doi: 10.1038/ki.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee H, Nho D, Chung HS, et al. CD4+CD25+ cisplatin-induced regulatory T cells attenuate nephrotoxicity in mice. Kidney Int. 2010;78:1100–1109. doi: 10.1038/ki.2010.139. [DOI] [PubMed] [Google Scholar]
- 64■■.Kinsey GR, Huang L, Jaworska K, et al. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J Am Soc Nephrol. 2012;23:1528–1537. doi: 10.1681/ASN.2012010070. This work shows that in order to protect the kidney from IRI, Tregs must generate their own adenosine, via CD73, which acts on Treg A2ARs to promote PD-1 expression on the cell surface of Tregs. Ex-vivo activation of the A2AR enhances the ability of Tregs to suppress kidney IRI in a PD-1-dependent manner.
- 65.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di Ianni M, Falzetti F, Carotti A, et al. Immunoselection and clinical use of T regulatory cells in HLA-haploidentical stem cell transplantation. Best Pract Res Clin Haematol. 2011;24:459–466. doi: 10.1016/j.beha.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, et al. Administration of CD4+CD25highCD127-regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–1820. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68■.Lai L-W, Yong K-C, Lien Y-HH. Pharmacologic recruitment of regulatory T cells as a therapy for ischemic acute kidney injury. Kidney Int. 2012;81:983–992. doi: 10.1038/ki.2011.412. In this article, the authors show that administration of DMS rapidly promotes Treg trafficking to the postischemic kidney and reduces injury. The protective effect is inhibited if Tregs are partially depleted or if CTLA-4 blocking antibodies are administered.
- 69.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over FoxP3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 70.Kim M-G, Lee SY, Ko YS, et al. CD4+ CD25+ regulatory T cells partially mediate the beneficial effects of FTY720, a sphingosine-1-phosphate analogue, during ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2011;26:111–124. doi: 10.1093/ndt/gfq480. [DOI] [PubMed] [Google Scholar]
- 71■■.Kim H, Lee G, Park S, et al. Bee venom mitigates cisplatin-induced nephrotoxicity by regulating CD4+CD25+FoxP3+ regulatory T cells in mice. Evidence-Based Complementary and Alternative Medicine. 2013;2013:10. doi: 10.1155/2013/879845. Bee venom is shown to induce expansion of Tregs in vivo and promote early trafficking of Tregs into the kidney after cisplatin administration. Bee venom mitigated cisplatin-induced renal injury in a Treg-dependent manner but did not affect cisplatin’s ability to retard tumor growth in vivo.
- 72.Webster KE, Walters S, Kohler RE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to eae and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73■■.Kim MG, Koo TY, Yan JJ, et al. IL-2/anti-IL-2 complex attenuates renal ischemia-reperfusion injury through expansion of regulatory T cells. J Am Soc Nephrol. 2013;24:1529–1536. doi: 10.1681/ASN.2012080784. IL-2/anti-IL-2 complexes cause Treg expansion in the spleen and kidney and when given prior to IRI protect against kidney injury. Importantly, when IL-2/anti-IL-2 complexes are given 24 h after IRI they promote functional recovery and inhibit renal fibrosis.
- 74■.Hu J, Zhang L, Wang N, et al. Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions. Kidney Int. 2013;84:521–531. doi: 10.1038/ki.2013.114. The protective effect of MSC administration in a mouse model of AKI is shown to partially depend on their interaction with Tregs inside the spleen of the recipient. Splenectomy or Treg depletion inhibits the protective ability of MSCs.
- 75■■.Li L, Huang L, Ye H, et al. Dendritic cells tolerized with adenosine A(2)AR agonist attenuate acute kidney injury. J Clin Invest. 2012;122:3931–3942. doi: 10.1172/JCI63170. The presence of an A2AR agonist at the time that dendritic cells are loaded with the type I NKT cell antigen α-galactosylceramide endows these dendritic cells with the ability to protect against kidney IRI even when given up to 6 h after ischemia. Adoptive transfer of these anti-inflammatory dendritic cells promotes IL-10 production in the recipient, which is required for the observed protection.
- 76.Mantia-Smaldone GM, Chu CS. A review of dendritic cell therapy for cancer: progress and challenges. BioDrugs. 2013;27:453–468. doi: 10.1007/s40259-013-0030-9. [DOI] [PubMed] [Google Scholar]
- 77.Patschan D, Krupincza K, Patschan S, et al. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol Renal Physiol. 2006;291:F176–F185. doi: 10.1152/ajprenal.00454.2005. [DOI] [PubMed] [Google Scholar]
- 78.Andres-Hernando A, Altmann C, Ahuja N, et al. Splenectomy exacerbates lung injury after ischemic acute kidney injury in mice. Am J Physiol Renal Physiol. 2011;301:F907–F916. doi: 10.1152/ajprenal.00107.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79■■.Gigliotti JC, Huang L, Ye H, et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol. 2013;24:1451–1460. doi: 10.1681/ASN.2013010084. Ultrasound treatment of mice markedly protects them from subsequent kidney IRI. Ultrasound-mediated protection requires splenic CD4 T cells that initiate a protective anti-inflammatory response.