Abstract
Virulence of Trypanosoma cruzi depends on a variety of genetic and biochemical factors. It has been proposed that components of the parasites' antioxidant system may play a key part in this process by pre-adapting the pathogen to the oxidative environment encountered during host cell invasion. Using several isolates (10 strains) belonging to the two major phylogenetic lineages (T. cruzi-I and T. cruzi-II), we investigated whether there was an association between virulence (ranging from highly aggressive to attenuated isolates at the parasitemia and histopathological level) and the antioxidant enzyme content. Antibodies raised against trypanothione synthetase (TcTS), ascorbate peroxidase (TcAPX), mitochondrial and cytosolic tryparedoxin peroxidases (TcMPX and TcCPX) and trypanothione reductase (TcTR) were used to evaluate the antioxidant enzyme levels in epimastigote and metacyclic trypomastigote forms in the T. cruzi strains. Levels of TcCPX, TcMPX and TcTS were shown to increase during differentiation from the non-infective epimastigote to the infective metacyclic trypomastigote stage in all parasite strains examined. Peroxiredoxins were found to be present at higher levels in the metacyclic infective forms of the virulent isolates compared with the attenuated strains. Additionally, an increased resistance of epimastigotes from virulent T. cruzi populations to hydrogen peroxide and peroxynitrite challenge was observed. In mouse infection models, a direct correlation was found between protein levels of TcCPX, TcMPX and TcTS, and the parasitemia elicited by the different isolates studied (Pearson's coefficient: 0.617, 0.771, 0.499; respectively, P < 0.01). No correlation with parasitemia was found for TcAPX and TcTR proteins in any of the strains analyzed. Our data support that enzymes of the parasite antioxidant armamentarium at the onset of infection represent new virulence factors involved in the establishment of disease.
Keywords: Trypanosoma cruzi, Infection, Antioxidant network, Virulence factors
1. Introduction
The unicellular parasite Trypanosoma cruzi is the causative agent of Chagas disease, an infection that afflicts 18–20 million people throughout Mexico, Central and South America. Globally, it is ranked as the third most important parasitic disease in terms of disability adjusted life years (http://www.who.int/tdr/diseases/chagas/swg-chagas.pdf). Part of the complex T. cruzi life cycle involves passage of the parasite through the digestive tract of an invertebrate host (triatomid hematophage arthropod). In the insect's gut, the replicative, non-infective epimastigote form is prevalent. As these pass through the insect towards the rectum, they transform into the infective, non-replicative metacyclic trypomas-tigote form. During this differentiation process (called metacyclo-genesis) the parasite undergoes complex morphological and biochemical changes in order to effectively infect and survive in the hostile environment of the vertebrate host.
As the insect vector takes a blood meal it defecates, depositing metacyclic trypomastigotes in the faecal material. The infective parasites gain access to the vertebrate host via mucosal membranes or through the insect-generated puncture wound. Once inside the body, the trypanosome proceeds to invade different cell types including macrophages, smooth and striated muscle cells and fibroblasts (Andrade and Andrews, 2005). Macrophages are one of the first cellular defences of the vertebrate innate immune response playing a central role in controlling parasite proliferation and dissemination (Kierszenbaum et al., 1974). Upon invasion, metacyclic trypomastigotes must survive and evade the highly oxidative environment found inside the macrophage phagosome in order to establish the infection. The main oxidant species involved in this biochemical assault are hydrogen peroxide (H2O2) and peroxynitrite (ONOO−). During phagocytosis, a macrophage membrane-associated NAD(P)H oxidase is activated resulting in superoxide (O2·−) production. The O2·− can then dis-mutate to H2O2 or react with iNOS-derived nitric oxide (·NO) in a, diffusion control reaction to yield ONOO−, the latter being a strong oxidant and potent cytotoxic effector molecule against T. cruzi (Alvarez et al., 2004). The levels of parasite antioxidant defences at the onset of macrophage invasion may tilt the balance towards pathogen survival, favouring its escape from the vacuole and the establishment of infection (Peluffo et al., 2004; Piacenza et al., 2008). Antioxidant defences in T. cruzi rely on a sophisticated system of linked pathways in which reducing equivalents from NADPH (derived from the pentose phosphate pathway; PPP) are delivered to a variety of enzymatic detoxification systems through the dithiol trypanothione (T(SH)2; N1,N8-bisgluta-thionylspermidine) and the thioredoxin homologue tryparedoxin (TXN). T(SH)2 is synthesized by trypanothione synthetase (TcTS) in a ATP-dependent reaction in which two molecules of glutathione are covalently linked to spermidine and is maintained in its reduced state by the NADPH-dependent flavoenzyme trypano-thine reductase (TcTR) (reviewed in Shames et al. (1986), Carnieri et al. (1993), Irigoin et al. (2008); Krauth-Siegel and Comini (2008)). Five distinct peroxidases have been identified in T. cruzi, differing in their subcellular location and substrate specificity. Glutathione peroxidase-I (TcGPXI, located at the cytosol and gly-cosome) and TcGPXII (located at the endoplasmic reticulum) confer resistance against exogenous hydroperoxides (Wilkinson et al., 2002a, 2002c). The cytosolic and mitochondrial tryparedoxin peroxidases (TcCPX and TcMPX, respectively) have the capacity to detoxify H2O2 and small chain organic hydroperoxides (Wilkinson et al., 2000). Importantly, we have recently shown that the in vivo detoxification of exogenous (macrophage-derived) and endogenously (parasite metabolism-derived) generated per-oxynitrite depends on both TcCPX and TcMPX activities (Piacenza et al., 2008). Finally, a plant-like ascorbate-dependent haemeper-oxidase (TcAPX), is located in the endoplasmic reticulum and confers resistance against H2O2 challenge (Wilkinson et al., 2002b). In addition to the different peroxidases, T. cruzi contains a repertoire of four iron superoxide dismuastes (Fe-SOD) that detoxify O2·− generated in the cytosol, glycosomes and mitochondria (Mateo et al., 2008). Mitochondrial Fe-SODA over-expression has been reported in an in vitro-derived benznidazole-resistant T. cruzi strain (Nogueira et al., 2006) and the existence of a putative extracellular Fe-SOD has been proposed as a diagnostic marker for identifying patients suffering from Chagas disease (Villagran et al., 2005). Due to its unique characteristics compared with the mammalian counterparts, components of the trypanosomatid antioxidant system have been considered good targets for chemotherapy.
Trypanosoma cruzi consists of a mixed population of strains classified into two major phylogenetic lineages T cruzi I and T cruzi II (subgroups IIa to IIe) that circulate in the domestic and sylvatic cycles (Souto et al., 1996). The existing heterogeneity between strains is in part responsible for the diverse clinical manifestations of the disease ranging from asymptomatic to severe cardiac and digestive presentations (Luquetti et al., 1986). It has been postulated that parasite and host genetic variability controls virulence, tissue tropism and the ability to maintain long-term infections in the vertebrate host. To date, different proteomic analyses have suggested the up-regulation of members of the T cruzi antioxidant network (TcTS; TcMPX; TXN; Fe-SODA and TcAPX) in the infective metacyclic trypomastigote compared with the non-infective epi-mastigote stage (Atwood et al., 2005; Parodi-Talice et al., 2007). At the cellular level, differentiation from the epimastigote to the metacyclic trypomastigote stage correlates with increased levels of TcCPX, making infective parasites more resistant to peroxyni-trite challenge (Piacenza et al., 2008). The association of the oxidative defence system with the pre-adaption mechanisms required for intracellular survival is complicated by the heterogeneous nature of T. cruzi populations.
In this study we performed a systematic analysis of TcTS, TcCPX, TcMPX, TcAPX and TcTR levels during metacyclogenesis in several T. cruzi strains. In addition, resistance to oxidative challenge and the virulence of the T. cruzi isolates in experimental animal infections were determined. The data were used to investigate whether there was a correlation between the levels of enzymes analyzed and parasite virulence. Overall, the results support the hypothesis that enzymes of the parasite antioxidant armamentarium represent novel virulence factors involved in the establishment of disease.
2. Materials and methods
2.1. Parasites
Epimastigotes from 10 different T. cruzi strains (Table 1) were cultured at 28 °C in brain heart infusion medium (BHI) as previously described (Piacenza et al., 2001). Trypanosoma cruzi strains were classified as belonging to lineages I or II by lineage-specific multilocus enzyme electrophoresis (MLEE) (Diosque et al., 2003). Metacyclogenesis was performed by adding 1% triatomine gut homogenate to epimastigote cultures (Isola et al., 1986). Parasites were harvested after 10 days and complement resistant (CR) forms were purified by incubating them with normal human serum (non-heat inactivated). Male Swiss mice (1.–52 months old) were inoculated i.p. with 1 × 103 CR metacyclic forms. Blood (10 ul) drawn from the tail tips of anesthetized mice was used to determine the parasite load and the number of trypomastigotes per 100 fields of view (Parasitemia, Table 1) recorded. After 90 days, tissue samples (heart and quadriceps muscle) of infected mice were recovered, fixed in 10% (v/v) fresh formaldehyde and histological H & E-stained sections analyzed (Zago et al., 2008). Lesions found were scored blindly as severe, moderate, slight or no detectable by two observers. Three sections of 53 mm2 (heart) and 38 mm2 (muscle) were screened for amastigote nests. Animal ethics approval was provided by the School of Health Science, National University of Salta, Argentina and the work strictly followed animal care guidelines adopted by the institution (Grossblatt, 1996).
Table 1.
Characteristics of Trypanosoma cruzi strains.
| Trypanosoma cruzi strain | Lineage | Isolated from | Parasitemiaa | Histopathologyb | Strain classification |
|---|---|---|---|---|---|
| TUL-0 | I | Laboratory strain, spontaneously attenuated clone from TUL-2 strain | 1 | No detectable | Attenuated |
| Y NULL | ND | Gp72 null-mutant, laboratory clone | 1 | No detectable | Attenuated |
| Y | ND | Human acute infection | 2 | Slightc (heart and vesicle) | Attenuated |
| TCC | I | Laboratory strain, Tul-R clone maintained in axenic culture | 2 | No detectable | Attenuated |
| TUL-2 | I | Triatoma infestans | 5 | No detectable | Attenuated |
| TULAHUEN | IIe | Laboratory strain, spontaneously attenuated clone from Tul-R strain | 40 | Moderate (heart and muscle) | Virulent |
| CL-wilde type (CL-WT) | IIe | Triatoma infestans | 170 | Severe (heart and muscle) | Virulent |
| CHIÑATA | I | Triatoma infestans | 200 | Severe (heart) | Virulent |
| TUL ROSARIO (Tul-R) | IIe | Triatoma infestans | 300 | Severe (heart and muscle) | Virulent |
| COLOMBIANA | I | Human chronic infection | 600 | Severe (heart) | Virulent |
Blood trypomastigotes/100 fields at the peak of parasitemia.
Histopathological lesions found in experimental mouse infections.
Lesions observed in immunocompromised mice, no detectable lesions in immunocompetent mice.
2.2. Specific antibodies
Specific antibodies were raised against purified recombinant TcAPX and TcTS. Briefly, the histidine-tagged recombinant proteins were produced in Escherichia coli BL-21 cells and purified by affinity chromatography using HiTrap™ chelating HP (GE healthcare). Purified fractions (50 μg) were used for the intradermal immunization of New Zealand white rabbits in FCA (priming, day 0) and Freund's incomplete adjuvant 2 weeks later (booster). Four weeks after priming, blood samples were collected and polyclonal IgG antibodies purified by standard methods (ammonium sulphate precipitation and affinity chromatography in Affi-Gel protein-A agarose, Bio-Rad). Specific antibodies against TcAPX and TcTS were further purified by antigen-affinity chromatography by coupling purified TcAPX or TcTS to cyanogen-activated Se-pharose beads. Anti-TcMPX and anti-TcCPX antibodies were kindly provided by Dr. Carlos Robello (Pineyro et al., 2008). Anti-TcTR antibody was kindly provided by Dr. Marcelo Comini (Comini et al., 2007).
2.3. Parasite extracts and Western blotting
Epimastigotes (3 × 108 cells) in the logarithmic phase of growth (3-days or 6-days) were collected at 800g for 10 min at 25 °C, washed three times in Dulbecco's PBS (dPBS) pH 7.3, resuspended in 250 ul lysis buffer (Tris-HCl 10 mM, 1 mM EDTA and 0.5% (v/v), Triton X-100) and incubated on ice for 15 min. Cell extracts were clarified (13,000g for 30 min at 4 °C) and supernatants supplemented with loading buffer (30 mM Tris-HCl, pH 6.6, 1% (w/v) SDS and 5% (v/v) glycerol) stored at −80°C until used. Metacyclic trypomastigotes from different T. cruzi strains were obtained in vitro by incubation in triatomine artificial urine media as previously described (Bonaldo et al., 1988; Piacenza et al., 2008). Protein concentration in parasite extracts was determined by the bicinch-oninic acid protein assay kit (BCA, Pierce, Rockford, Ill.). Protein extracts (50 μg), were resolved by SDS-PAGE and then blotted onto nitrocellulose membranes (Hybond-C extra, Amersham). After transfer, proteins were stained with Ponceau-S solution (Aplichem, Daermstadt, Germany) and blocked using 5% (w/v) BSA and 0.1% (v/v) Tween 20 (Sigma) in Tris-buffered saline (TBS, 25 mM Tris-HCl pH 7.6, 140 mM NaCl and 3 mM KCl) for 1 h at 25 °C. Membranes were then probed with anti-TcTS (1:8000), anti-TcAPX (1:4000), anti-TcTR (1:2000), anti-TcMPX (1:2000) and anti-TcCPX (1:8000) diluted in TBS 0.1% (v/v) Tween 20 for 1 h at 25°C following 1 h incubation with anti-rabbit IgG peroxidase conjugate (Cal-biochem) diluted 1:10,000 in TBS containing 1% (w/v) BSA and 0.1% (v/v) Tween 20. Immunoreactive proteins were detected using the Immun-Star™ Chemiluminescence Kit (Bio-Rad). Protein content relative to total protein loaded (Ponceau-S staining (Klein et al., 1995; Moore and Viselli, 2000)) in the different extracts analyzed was determined by densitometric techniques using ImageJ (National Institute of Health, USA). Results are expressed as relative enzyme content respect to total protein content.
2.4. Oxidant sensitivity experiments
Epimastigotes (3 × 108 cells) in the logarithmic phase of growth (5-days) from the different T. cruzi strains were incubated in sterile dPBS, pH 7.3 and exposed to H2O2 (350 uM) or ONOO− (synthesized, quantitated and handled as previously described (Denicola et al., 1993)). ONOOT− (200 μM) was added under vigorous vor-texing to parasite suspensions as a single dose. Parasites were incubated in the presence of the different oxidants for 1 h at 28 °C in dPBS pH 7.3. After treatment, parasite viability was evaluated by the [3H]-Thymidine (American Radiolabeled Chemicals) incorporation assay as previously described (Piacenza et al., 2001). Briefly, following parasite exposure to the different oxidants an aliquot containing 5 × 106 cells was incubated overnight at 28 °C in BHI medium containing 1 μCi [3H]-Thymidine.
After incubation cells were harvested over glass fibre filters and washed several times with distilled water. Radioactivity on the filters was measured in a liquid scintillation counter Trilux 1450, (Wallac Instruments). Results are expressed as the percentage of [3H]-Thymidine incorporation with respect to the control condition (no oxidant addition) for each cell line. After parasite treatment, peroxynitrite-dependent oxidative modifications to proteins were evaluated by Western blot using rabbit anti-nitro-tyrosine antiserum produced in our laboratory and diluted 1:2000 as above (Brito et al., 1999).
2.5. Data analysis
All experiments were performed at least three times on independent days. Results are expressed as means ± SEM. The Student's t-test was performed for comparison between two groups and AN-OVA (Pagano and Gauvreau, 2000) was used for comparison between more than two groups. For post hoc analysis, the least significant difference (LSD) method was used. P < 0.05 was considered significant. For correlation analysis SPSS software was used. Briefly, data from at least 10 independent experiments corresponding to the relative content of TcAPX, TcTS, TcTR, TcCPX and TcMPX from each strain were plotted against the parasitemia elicited in experimental infections by the corresponding strain. A bivariate correlation was performed and the Pearson's coefficient was calculated as the estimator of the correlation. A value of P < 0.01 was considered significant.
3. Results
3.1. Virulence of T. cruzi strains
The T. cruzi strains used in this study were classified based on their behaviour in experimental mouse infections using parasite-mia and the histopathological lesions found in heart and quadriceps muscles as the grouping variables. In this way, the degree of parasitemia and the magnitude of lesions were taken as virulence indicators, allowing us to classify the strains as attenuated or virulent. Strains that elicited parasitemia <5 blood trypomastig-otes/100 fields and with no detectable to slight lesions in heart and muscle were considered attenuated (Table 1). Strains were classified as virulent when the parasitemia was >30 blood try-pomastigotes/100 fields and with moderate to severe heart and muscle histopathological lesions. Table 1 summarises the characteristics of all the strains and includes the T. cruzi lineage (I or II) and the origin of each isolate. The parasitemia and inflammatory infiltrates in heart tissue elicited in mice by the infection with a typical attenuated (TCC) or virulent (CL-WT) T. cruzi strain is shown in Fig. 1.
Fig. 1.

Behaviour of an attenuated (TCC) and virulent (CL-WT) Trypanosoma cruzi strain in experimental infections. Two month-old Swiss mice were infected by i.p. inoculation with 1 × 103 complement resistant (CR) metacyclic trypomastigote forms. Parasitemia and histopathology were analyzed as described in Section 2. (A) Parasitemia (trypomastigotes/100 fields) levels elicited by the attenuated strain TCC and the virulent CL-WT strain. Values are given as means ± SEM. (B) Microphotographs of heart tissue sections from mice inoculated with the attenuated strain TCC (a) and the virulent strain CL-WT (b,c). Note the amastigote nest inside the inflammatory infiltrate (c) elicited by the inoculation of the virulent strain CL-WT (magnification 25×).
3.2. Expression of antioxidant enzymes during differentiation to the infective metacyclic trypomastigote
Our previous work has shown that metacyclic trypomastigotes from the strain CL-Brener were more resistant to oxidative challenge than their non-infective epimastigote counterparts, with increased expression of both TcCPX and TcMPX contributing to this observation (Piacenza et al., 2008). We have built on this finding to explore the levels of TcTS, TcAPX, TcCPX, TcMPX and TcTR by Western blot analysis in extracts (3-days epimastigotes and metacyclic trypomastigotes) derived from several T. cruzi strains. For detection of TcTS and TcAPX, purified recombinant proteins were used as antigens to generate rabbit anti-TcTS and anti-TcAPX polyclonal antibodies. The anti-TcTS antibody recognized a band of ∼60 kDa whereas the anti-TcAPX antibody detected a band of ∼36 kDa in total protein extracts from epimastigotes in agreement with the expected molecular weight for both protein monomers (Fig. 2A). Due to the divergence that exists among the T. cruzi strains, none of the typical constitutive proteins, actin and/or tubulin (using commercially available antibodies), could be used to normalize the antioxidant enzyme content in each parasite extract. Therefore, protein loadings were judged by Ponceau-S staining of nitrocellulose membranes as before (Klein et al., 1995; Moore and Viselli, 2000; Piacenza et al., 2008). Fig. 2B shows an illustrative typical experiment carried out in epimastigotes extracts probed with the set of antibodies used throughout this study and the corresponding Ponceau S-stained nitrocellulose membrane used to normalize. Following this approach, with at least 10 independent determinations, enzyme levels were measured in non-infective epimastigotes and infective metacyclic trypomastigotes for all of the T. cruzi strains.
Fig. 2.

Detection of trypanothione synthetase (TcTS), ascorbate peroxidase (TcAPX), mitochondrial and cytosolic tryparedoxin peroxidases (TcMPX and TcCPX) and trypanothione reductase (TcTR) in parasite extracts by specific antibodies. (A) Anti-TcTS and anti-TcAPX antibodies. Western blot analysis in protein extracts of Trypanosoma cruzi epimastigotes (6-days). Proteins (50 μg) from TCC, CL-WT, TUL-0 and Chiñata strains were probed using the anti-TcTS (a) and anti-TcAPX (b) antibodies generated in this study. (c) Ponceau-S protein staining of purified recombinant rTcTS and rTcAPX proteins (2 μg, lane one) and parasite extracts loaded. (B) Enzyme expression in epimastigotes. An illustrative Western blot for the detection of TcAPX, TcTS, TcMPX, TcCPX and TcTR in parasite extracts (50 μg) of epimastigotes (6-days) of the different T. cruzi strains analyzed.
The expression levels of TcCPX, TcMPX, TcTS, TcTR and TcAPX in epimastigotes (3-days) and metacyclic trypomastigotes were then compared. When the average pooled data from all strains (Tul-0, Y null, Y, TCC, Tul-2, Tulahuen, CL-WT, Chiñata and Tul-R), independent of their virulence, was investigated, a significant increase in expression of TcCPX, TcMPX and TcTS was observed (Fig. 3A) whereas no difference was detected in TcTR and TcAPX (not shown). Enzyme levels were then evaluated individually for each T. cruzi strain (Fig. 3B). All strains (except for Tul-0) showed a similar alteration in their expression profiles after metacyclogenesis; indeed, in the infective metacyclic stage, an up-regulation of TcTS and at least one of the two peroxiredoxins was observed. The absolute values reached for both TcCPX and TcMPX in the virulent strains (Chiñata, CL-WT and Tul-R) was higher than that achieved in the attenuated ones (TCC, Tul-0 and Y) (Fig. 3B). Peroxiredoxin contents in the 3-days epimastigote stage of attenuated and virulent strains did not show statistical differences although a trend, that indicated higher levels in the virulent ones could be observed; for TcTS, a significantly higher content in the virulent strains was found (Fig. 3B). No significant differences were observed in the TcTR and TcAPX expression levels for any of the strains, independent of the parasites stage analyzed (not shown).
Fig. 3.
Antioxidant enzyme levels in the non-infective epimastigotes and infective metacyclic trypomastigotes of Trypanosoma cruzi. (A) Enzyme levels from epimastigotes in the logarithmic phase of growth (three-days) and metacyclic trypomastigotes from all strains (Tul-0, Y null, Y, TCC, Tul-2, Tulahuen, CL-WT, Chiñata and Tul-R) evaluated were analyzed by Western blot using specific antibodies as for Fig. 2. Results are means ± SEM of relative densitometric quantitation of at least 10 independent determinations (Western blot signal/total protein). *P < 0.05. Mitochondrial and cytosolic tryparedoxin peroxidases (TcMPX and TcCPX). (B) Individual strain analysis of the relative enzyme content in epimastigotes in the logarithmic phase of growth (3-days) and metacyclic trypomastigotes by Western blot as performed for A. Results are means ± SEM of relative densitometric quantitation of at least 10 independent determinations (specific Western blot signal/total protein loaded). *P < 0.05 with respect to epimastigote enzyme content. #P < 0.05 with respect to trypanothione synthetase (TcTS) epimastigote content of TCC, Tul-0 and Y.
3.3. Virulent T. cruzi strains are resistant to oxidative challenge
Western blot analysis of peroxiredoxins from epimastigotes of attenuated and virulent strains showed a trend indicating higher content in at least one of the components in the virulent strains. We therefore evaluated, by [3H]-Thymidine incorporation, the susceptibility of the epimastigotes forms from the attenuated and virulent strains against H2O2 and peroxynitrite challenge. The sensitivity, of the proliferation assay provides insights into the antioxidant network acting as a whole including enzymes that we did not evaluate in the present study such as FeSODs, GPX-I, GPX-II and the PPP.
Epimastigotes from the virulent T. cruzi strains, Chiñata, CL-WT and Tulahuen, were significantly resistant towards H2O2 and/or peroxynitrite challenge compared with the epimastigotes from the attenuated strains TCC, Tul-0 and Y (Fig. 4A). Moreover, oxida-tive modifications to proteins due to peroxynitrite-treatment were assayed by detection of protein 3-nitro-tyrosine in parasite extracts following oxidant addition. Previously we have shown that peroxynitrite is preferentially catabolised by T. cruzi peroxiredox-ins before its homolysis to secondary radical products responsible for protein oxidation and nitration (Radi et al., 2001; Trujillo et al., 2004; Piacenza et al., 2008). After peroxynitrite addition (100 μM), protein tyrosine nitration was observed in the attenuated strains TCC and Tul-0 while in the virulent strain CL-WT protein nitration at the same oxidant concentration was largely inhibited (Fig. 4B). At higher peroxynitrite concentrations (250 μM), the detoxification system is overwhelmed and no differences in protein nitration, were observed between strains. The ability to inhibit peroxyni-trite-dependent oxidative modifications to proteins is an indicator at the cellular level of increased peroxiredoxin activity (Piacenza et al., 2008).
Fig. 4.

Susceptibility of attenuated and virulent strains to hydrogen peroxoide (H2O2) and peroxynitrite (ONOO_) challenge. (A) Epimastigotes (3×108 cells ml_1) were exposed to H2O2 (350 μM) or peroxynitrite (200 μM) in Dulbecco's PBS (dPBS) pH 7.2 for 1 h. After treatment, cells (5×106) were cultured in brain heart infusion (BHI) medium and pulsed with 1 μCi [3H]-Thymidine for 18 h at 28 °C. Results are expressed as the percentage of [3H]-Thymidine incorporation compared with the control condition (no oxidant addition). Results are means ± SEM of three independent determinations. *P < 0.05. (B) Protein 3-nitro tyrosine detection. Epimastigotes (3×108 cells ml_1) of the virulent (CL-WT) and attenuated strains (TCC and Y) were treated with ONOO_ (0–0.25 mM). After treatment equal amounts of cell extracts (50 μg) were run on, SDS–PAGE (13% gels). Equal protein loading was checked by Ponceau-S staining of the transferred nitrocellulose membrane before Western blot analysis using antinitrotyrosine, antibody. The Western blots were performed in parallel and membranes were developed in the same autoradiography cassette with equal exposure time, allowing accurate comparison between Western blots.
3.4. Correlation between antioxidant enzyme levels and virulence of T. cruzi strains
In order to assess the magnitude of the association between the enzymes of the antioxidant network with virulence, we used the parasitemia elicited in mice as a surrogate indicator of virulence, which could be input as a continuous variable into a correlation model. To minimize bias, parasitemia were determined independently in a blind fashion. Therefore, each of the strains explored in this work corresponds to a parasitemia value in the mouse model (Table 1). A positive correlation was found between the levels of, TcCPX, TcMPX, TcTS in the infective trypomastigote stage with the parasitemia elicited in mice by each strain with Pearson's correlation coefficients of 0.617, 0.771 and 0.499, respectively (P < 0.01) (Table 2 and Fig. 5). No correlation was observed for TcTR and TcAPX levels (Pearson's correlation coefficients of 0.081 and −0.006, respectively, Table 2). Relative protein contents of TcCPX, TcMPX, TcTS, TcAPX and TcTR in the metacyclic trypomastigote stage versus parasitemia are shown in Fig. 5.
Table 2.
Correlation between the enzyme contents of Trypanosoma cruzi metacyclic trypom-astigotes and the parasitemia elicited in mice.
| TcMPX | TcCPX | TcAPX | TcTS | TcTR | |
|---|---|---|---|---|---|
| Pearson's coefficient | 0.771a | 0.617a | −0.006 | 0.499a | 0.081 |
| Determinations (n) | 59 | 105 | 79 | 75 | 49 |
Correlation is significant at the 0.01 level (two tailed). Mitochondrial and cytosolic tryparedoxin peroxidases (TcMPX and TcCPX), trypanothione synthetase (TcTS), ascorbate peroxidase (TcAPX) and trypanothione reductase (TcTR).
Fig. 5.
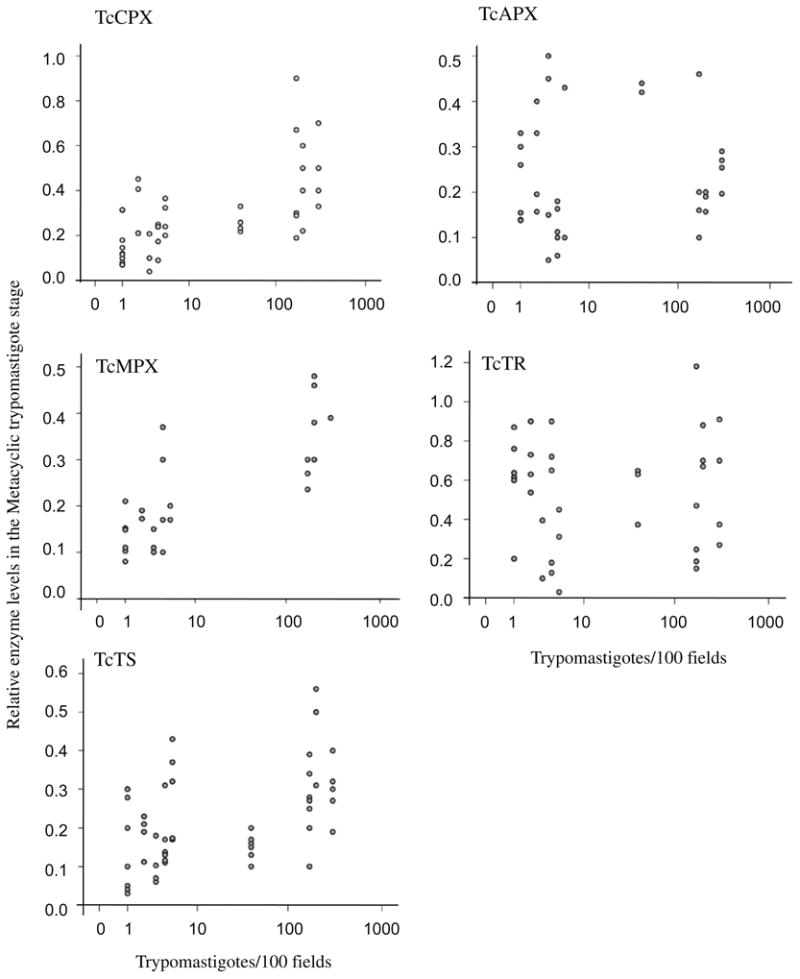
Two-way scatter plot assessing the relationship between metacyclic trypomastigote enzyme levels and parasitemia. The relative enzyme content of the mitochondrial and cytosolic tryparedoxin peroxidases (TcMPX and TcCPX), trypanothione synthetase (TcTS), ascorbate peroxidase (TcAPX) and trypanothione reductase (TcTR) in metacyclic, trypomastigote stage (expressed as specific Western blot signal/total protein loaded) were plotted against the parasitemia (blood trypomastigotes/100 fields) developed by, each Trypanosoma cruzi strain described in Table 1. The corresponding statistical analysis is shown in Table 2.
Together, the data presented in Figs. 3B and 5 clearly indicate that virulent T. cruzi strains contain higher levels of TcTS, TcCPX and TcMPX compared with the attenuated ones, especially in their infective metacyclic stage.
4. Discussion
In most eukaryotic cells, catalase and selenium glutathione peroxidases represent the major enzymatic components involved in, hydroperoxide metabolism. However trypanosomes lack these activities, leading to the proposal that these parasites are deficient in their ability to deal with such oxidative insults (Boveris et al., 1980). During the last decade, the antioxidant network of T. cruzi has emerged as a rather complex, efficient and well-compartmentalized series of biochemical pathways that occupy a pivotal role in the detoxification of reactive oxygen and nitrogen species produced during parasite-host cell interactions (reviewed in Irigoin et al., 2008; Krauth-Siegel and Comini, 2008). Moreover, some of these proteins that are central to this system (TS, TR, TXN) are absent or are structurally distinct from the host counterparts, making them excellent pharmacological targets (Flohe et al., 1999; Comini et al., 2004). Therefore functional analysis of the parasites' antioxidant network within T. cruzi itself may unravel the role played by this system in critical biological processes such as virulence. The global analysis of the 10 strains studied in this work (Table 1) revealed that during differentiation from the non-infective epimasti-gote stage to the infective metacyclic trypomastigote forms, there is a significant elevation in the TcCPX, TcMPX and TcTS content (Fig. 3A). This increase is in agreement with the pre-adaptation of infective parasites to cope with the host immune cell-derived oxidants upon macrophage invasion (Alvarez et al., 2004; Peluffo et al., 2004; Atwood et al., 2005; Piacenza et al., 2008). Changes in parasite antioxidant enzyme expression are also suggestive of an increase of insect-derived oxidant production during metacy-clogenesis, a process in which the molecular and biochemical mediators are still not well-defined. The evaluation of antioxidant enzymes in each T. cruzi clone revealed that the protein contents observed for TcCPX, TcMPX and TcTS in the infective metacyclic trypomastigotes was higher in the virulent strains compared with the attenuated ones (Figs. 3B and 5). The levels of TcTR and TcAPX remained constant between the different parasites stages evaluated. TcTR, a key enzyme in the parasite antioxidant network, is essential for all trypanosomatids studied to date (Schmidt and Krauth-Siegel, 2002). TR is normally present in excess and its levels must be lowered >90% to render Trypanosoma brucei more susceptible to H2O2-killing and render them avirulent in mouse infections compared with wild type parasites (Krieger et al., 2000). Indeed, protein expression levels measured in this study show that this enzyme is highly expressed in all of the clones evaluated (Fig. 5), in line with its fundamental role in trypanosomatid viability. Other important members of the parasite antioxidant defences are FeS-ODs, located at different subcellular compartments including mitochondria (Piacenza et al., 2007). It has been shown that over-expression of the mitochondrial isoform FeSOD-A protects cells against mitochondrial O2·− -dependent programmed cell death signalling, revealing its key role in parasite survival (Piacenza et al., 2007). Evaluation of FeSOD-A levels and its relationship with parasite virulence proved to be difficult due to the lack of specificity shown by TcFeSOD-A antibodies raised against the recombinant enzyme (not shown). The present work was designed to evaluate the enzymes located at the first line of the parasite antioxidant defences, detoxifying oxidants themselves rather than oxidation products. Further studies are needed to address the role of GPX-I and GPX-II in virulence, which represents a secondary line of defence, since they mainly react with organic hydroperoxides.
Virulence is a complex function involving the highly evolved ability of T. cruzi to transform into the infective stage, penetrate epithelia and invade cells evading the complex immunological reaction of the vertebrate host (Andrade and Andrews, 2005). It has been postulated that structural and biochemical heterogeneity between the different strains may modulate T. cruzi pathogenecity (Brisse et al., 1998). The study of virulence factors can identify new targets that have potential in the development of novel trypanocidal treatments. Several T. cruzi virulence factors have been characterised using targeted gene deletion but only a few of those have, been proved to be related to the virulence phenotype (product of the gene LYT, LYT-1(Zago et al., 2008), surface glycoprotein, gp72 (Basombrio et al., 2002), Oligopeptidase-B (Caler et al., 1998) and T. cruzi released protein, Tc52 (Garzon et al., 2003)). Variations within T. cruzi strains with regard to oxidative metabolism were previously observed (Mancilla and Naquira, 1964). The glucose flux through the PPP responsible for providing NADPH for the antioxi-dant network was found to be higher in the virulent Tulahuen strain than in the attenuated Peruana strain (Mancilla and Naquira, 1964). Moreover, parasites with higher resistance to H2O2 challenge (Tulahuen-2) showed elevated levels of glucose-6-phosphate dehydrogenase and TcCPX than susceptible ones (Y strain) (Miel-niczki-Pereira et al., 2007). At the cellular level the results are supported by the fact that epimastigotes of the virulent T. cruzi strains present higher resistance to the oxidative damage generated by two biologically-relevant oxidants, peroxynitrite and H2O2, as assessed by thymidine incorporation studies and oxidative modifications to cellular proteins (Fig. 4A and B). In this regard, the expression of antioxidant enzymes seems to be accompanied by an increased expression of the PPP pathway as was previously shown (Mielniczki-Pereira et al., 2007). Finally, in this study we linked the heterogeneity observed in virulence between the T. cruzi strains with the expression levels of parasite antioxidant enzymes in the metacyclic infective form. The positive correlation found between the parasitemia and the contents of TcCPX, TcMPX, and TcTS strongly suggest the intertwined relationship of these two variables (Fig. 5 and Table 2). In summary, the present work shows that TcCPX, TcMPX and TcTS are up-regulated in the infective metacy-clic trypomastigote stage in all of the strains evaluated in what appears to be general pre-adaptation strategy, although additional studies with other strains need to be performed to confirm this observation. Furthermore, a significant correlation between virulence and the aforementioned enzymes was found, which underscores the importance of the antioxidant network for a successful infection and adds to the concept that these enzymes can be pharmacological targets for new drug development.
Acknowledgments
This work was supported by grants from the Howard Hughes Medical Institute (HHMI) to R.R., Fondo Clemente Estable (PDT) and Comisión Sectorial de Investigación Científica (CSIC), Uruguay to L.P. and M.N.A, Consejo Nacional de Investigaciones Cientificas y Técnias (CONICET), Argentina, to P.Z. and PICT 2005-32739 to M.A.B. R.R. is an International Research Scholar of the H.H.M.I. We thank Dr. Shane Wilkinson for critical reading and editing of this manuscript.
References
- Alvarez MN, Piacenza L, Irigoin F, Peluffo G, Radi R. Macrophage-derived peroxynitrite diffusion and toxicity to Trypanosoma cruzi. Arch Biochem Biophys. 2004;432:222–232. doi: 10.1016/j.abb.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Andrade LO, Andrews NW. The Trypanosoma cruzi–host cell interplay: location, invasion, retention. Nat Rev Microbiol. 2005;3:819–823. doi: 10.1038/nrmicro1249. [DOI] [PubMed] [Google Scholar]
- Atwood JA, 3rd, Weatherly DB, Minning TA, Bundy B, Cavola C, Opperdoes FR, Orlando R, Tarleton RL. The Trypanosoma cruzi proteome. Science. 2005;309:473–476. doi: 10.1126/science.1110289. [DOI] [PubMed] [Google Scholar]
- Basombrio MA, Gomez L, Padilla AM, Ciaccio M, Nozaki T, Cross GA. Targeted deletion of the gp72 gene decreases the infectivity of Trypanosoma cruzi for mice and insect vectors. J Parasitol. 2002;88:489–493. doi: 10.1645/0022-3395(2002)088[0489:TDOTGG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bonaldo MC, Souto-Padron T, de Souza W, Goldenberg S. Cell-substrate adhesion during Trypanosoma cruzi differentiation. J Cell Biol. 1988;106:1349–1358. doi: 10.1083/jcb.106.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Sies H, Martino EE, Docampo R, Turrens JF, Stoppani AO. Deficient metabolic utilization of hydrogen peroxide in Trypanosoma cruzi. Biochem J. 1980;188:643–648. doi: 10.1042/bj1880643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisse S, Barnabe C, Banuls AL, Sidibe I, Noel S, Tibayrenc M. A phylogenetic analysis of the Trypanosoma cruzi genome project CL Brener reference strain by multilocus enzyme electrophoresis and multiprimer random amplified polymorphic DNA fingerprinting. Mol Biochem Parasitol. 1998;92:253–263. doi: 10.1016/s0166-6851(98)00005-x. [DOI] [PubMed] [Google Scholar]
- Brito C, Naviliat M, Tiscornia AC, Vuillier F, Gualco G, Dighiero G, Radi R, Cayota AM. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol. 1999;162:3356–3366. [PubMed] [Google Scholar]
- Caler EV, Vaena de Avalos S, Haynes PA, Andrews NW, Burleigh BA. Oligopeptidase B-dependent signaling mediates host cell invasion by Trypanosoma cruzi. EMBO J. 1998;17:4975–4986. doi: 10.1093/emboj/17.17.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnieri EG, Moreno SN, Docampo R. Trypanothione-dependent peroxide metabolism in Trypanosoma cruzi different stages. Mol Biochem Parasitol. 1993;61:79–86. doi: 10.1016/0166-6851(93)90160-y. [DOI] [PubMed] [Google Scholar]
- Comini MA, Guerrero SA, Haile S, Menge U, Lunsdorf H, Flohe L. Validation of Trypanosoma brucei trypanothione synthetase as drug target. Free Radic Biol Med. 2004;36:1289–1302. doi: 10.1016/j.freeradbiomed.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Comini MA, Krauth-Siegel RL, Flohe L. Depletion of the thioredoxin homologue tryparedoxin impairs antioxidative defence in African trypanosomes. Biochem J. 2007;402:43–49. doi: 10.1042/BJ20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denicola A, Rubbo H, Rodriguez D, Radi R. Peroxynitrite-mediated cytotoxicity to Trypanosoma cruzi. Arch Biochem Biophys. 1993;304:279–286. doi: 10.1006/abbi.1993.1350. [DOI] [PubMed] [Google Scholar]
- Diosque P, Barnabe C, Padilla AM, Marco JD, Cardozo RM, Cimino RO, Nasser JR, Tibayrenc M, Basombrio MA. Multilocus enzyme electrophoresis analysis of Trypanosoma cruzi isolates from a geographically restricted endemic area for Chagas' disease in Argentina. Int J Parasitol. 2003;33:997–1003. doi: 10.1016/s0020-7519(03)00139-5. [DOI] [PubMed] [Google Scholar]
- Flohe L, Hecht HJ, Steinert P. Glutathione and trypanothione in parasitic hydroperoxide metabolism. Free Radic Biol Med. 1999;27:966–984. doi: 10.1016/s0891-5849(99)00172-0. [DOI] [PubMed] [Google Scholar]
- Garzon E, Borges MC, Cordeiro-da-Silva A, Nacife V, Meirelles Mde N, Guilvard E, Bosseno MF, Guevara AG, Breniere SF, Ouaissi A. Trypanosoma cruzi carrying a targeted deletion of a Tc52 protein-encoding allele elicits attenuated Chagas' disease in mice. Immunol Lett. 2003;89:67–80. doi: 10.1016/s0165-2478(03)00112-3. [DOI] [PubMed] [Google Scholar]
- Grossblatt N. Guide for the Care and Use of Laboratory Animals. National Academic Press; Washington, DC: 1996. [Google Scholar]
- Irigoin F, Cibils L, Comini MA, Wilkinson SR, Flohe L, Radi R. Insights into the redox biology of Trypanosoma cruzi: Trypanothione metabolism and oxidant detoxification. Free Radic Biol Med. 2008;45:733–742. doi: 10.1016/j.freeradbiomed.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Isola EL, Lammel EM, Gonzalez Cappa SM. Trypanosoma cruzi: differentiation after interaction of epimastigotes and Triatoma infestans intestinal homogenate. Exp Parasitol. 1986;62:329–335. doi: 10.1016/0014-4894(86)90039-1. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F, Knecht E, Budzko DB, Pizzimenti MC. Phagocytosis: a defense mechanism against infection with Trypanosoma cruzi. J Immunol. 1974;112:1839–1844. [PubMed] [Google Scholar]
- Klein D, Kern RM, Sokol RZ. A method for quantification and correction of proteins after transfer to immobilization membranes. Biochem Mol Biol Int. 1995;36:59–66. [PubMed] [Google Scholar]
- Krauth-Siegel RL, Comini MA. Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim Biophys Acta. 2008;1780:1236–1248. doi: 10.1016/j.bbagen.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Krieger S, Schwarz W, Ariyanayagam MR, Fairlamb AH, Krauth-Siegel RL, Clayton C. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Mol Microbiol. 2000;35:542–552. doi: 10.1046/j.1365-2958.2000.01721.x. [DOI] [PubMed] [Google Scholar]
- Luquetti AO, Miles MA, Rassi A, de Rezende JM, de Souza AA, Povoa MM, Rodrigues I. Trypanosoma cruzi: zymodemes associated with acute and chronic Chagas' disease in central Brazil. Trans R Soc Trop Med Hyg. 1986;80:462–470. doi: 10.1016/0035-9203(86)90347-0. [DOI] [PubMed] [Google Scholar]
- Mancilla R, Naquira C. Comparative metabolism of C14-glucose in two strains of Trypanosoma cruzi. J Protozool. 1964;11:509–513. doi: 10.1111/j.1550-7408.1964.tb01790.x. [DOI] [PubMed] [Google Scholar]
- Mateo H, Marin C, Perez-Cordon G, Sanchez-Moreno M. Purification and biochemical characterization of four iron superoxide dismutases in Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 2008;103:271–276. doi: 10.1590/s0074-02762008000300008. [DOI] [PubMed] [Google Scholar]
- Mielniczki-Pereira AA, Chiavegatto CM, Lopez JA, Colli W, Alves MJ, Gadelha FR. Trypanosoma cruzi strains, Tulahuen 2 and Y, besides the difference in resistance to oxidative stress, display differential glucose-6-phosphate and 6-phosphogluconate dehydrogenases activities. Acta Trop. 2007;101:54–60. doi: 10.1016/j.actatropica.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Moore MK, Viselli SM. Staining and quantification of proteins transferred to polyvinylidene fluoride membranes. Anal Biochem. 2000;279:241–242. doi: 10.1006/abio.2000.4482. [DOI] [PubMed] [Google Scholar]
- Nogueira FB, Krieger MA, Nirde P, Goldenberg S, Romanha AJ, Murta SM. Increased expression of iron-containing superoxide dismutase-A (TcFeSOD-A) enzyme in Trypanosoma cruzi population with in vitro-induced resistance to benznidazole. Acta Trop. 2006;100:119–132. doi: 10.1016/j.actatropica.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Pagano M, Gauvreau K. Analysis of variance. In: Pagano M, Gauvreau K, editors. Principles of Biostatistics. Duxbury; Pacific Grove: 2000. pp. 285–301. [Google Scholar]
- Parodi-Talice A, Monteiro-Goes V, Arrambide N, Avila AR, Duran R, Correa A, Dallagiovanna B, Cayota A, Krieger M, Goldenberg S, Robello C. Proteomic analysis of metacyclic trypomastigotes undergoing Trypanosoma cruzi metacyclogenesis. J Mass Spectrom. 2007;42:1422–1432. doi: 10.1002/jms.1267. [DOI] [PubMed] [Google Scholar]
- Peluffo G, Piacenza L, Irigoin F, Alvarez MN, Radi R. l-arginine metabolism during interaction of Trypanosoma cruzi with host cells. Trends Parasitol. 2004;20:363–369. doi: 10.1016/j.pt.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Piacenza L, Peluffo G, Radi R. l-arginine-dependent suppression of apoptosis in Trypanosoma cruzi: contribution of the nitric oxide and polyamine pathways. Proc Natl Acad Sci USA. 2001;98:7301–7306. doi: 10.1073/pnas.121520398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacenza L, Irigoin F, Alvarez MN, Peluffo G, Taylor MC, Kelly JM, Wilkinson SR, Radi R. Mitochondrial superoxide radicals mediate programmed cell death in Trypanosoma cruzi: cytoprotective action of mitochondrial iron superoxide dismutase overexpression. Biochem J. 2007;403:323–334. doi: 10.1042/BJ20061281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacenza L, Peluffo G, Alvarez MN, Kelly JM, Wilkinson SR, Radi R. Peroxiredoxins play a major role in protecting Trypanosoma cruzi against macrophage- and endogenously-derived peroxynitrite. Biochem J. 2008;410:359–368. doi: 10.1042/BJ20071138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineyro MD, Parodi-Talice A, Arcari T, Robello C. Peroxiredoxins from Trypanosoma cruzi: virulence factors and drug targets for treatment of Chagas disease? Gene. 2008;408:45–50. doi: 10.1016/j.gene.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med. 2001;30:463–488. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Krauth-Siegel RL. Enzymes of the trypanothione metabolism as targets for antitrypanosomal drug development. Curr Top Med Chem. 2002;2:1239–1259. doi: 10.2174/1568026023393048. [DOI] [PubMed] [Google Scholar]
- Shames SL, Fairlamb AH, Cerami A, Walsh CT. Purification and characterization of trypanothione reductase from Crithidia fasciculata, a newly discovered member of the family of disulfide-containing flavoprotein reductases. Biochemistry. 1986;25:3519–3526. doi: 10.1021/bi00360a007. [DOI] [PubMed] [Google Scholar]
- Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol Biochem Parasitol. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- Trujillo M, Budde H, Pineyro MD, Stehr M, Robello C, Flohe L, Radi R. Trypanosoma brucei and Trypanosoma cruzi tryparedoxin peroxidases catalytically detoxify peroxynitrite via oxidation of fast reacting thiols. J Biol Chem. 2004;279:34175–34182. doi: 10.1074/jbc.M404317200. [DOI] [PubMed] [Google Scholar]
- Villagran ME, Marin C, Rodriguez-Gonzalez I, De Diego JA, Sanchez-Moreno M. Use of an iron superoxide dismutase excreted by Trypanosoma cruzi in the diagnosis of Chagas disease: seroprevalence in rural zones of the state of Queretaro, Mexico. Am J Trop Med Hyg. 2005;73:510–516. [PubMed] [Google Scholar]
- Wilkinson SR, Temperton NJ, Mondragon A, Kelly JM. Distinct mitochondrial and cytosolic enzymes mediate trypanothione-dependent peroxide metabolism in Trypanosoma cruzi. J Biol Chem. 2000;275:8220–8225. doi: 10.1074/jbc.275.11.8220. [DOI] [PubMed] [Google Scholar]
- Wilkinson SR, Meyer DJ, Taylor MC, Bromley EV, Miles MA, Kelly JM. The Trypanosoma cruzi enzyme TcGPXI is a glycosomal peroxidase and can be linked to trypanothione reduction by glutathione or tryparedoxin. J Biol Chem. 2002a;277:17062–17071. doi: 10.1074/jbc.M111126200. [DOI] [PubMed] [Google Scholar]
- Wilkinson SR, Obado SO, Mauricio IL, Kelly JM. Trypanosoma cruzi expresses a plant-like ascorbate-dependent hemoperoxidase localized to the endoplasmic reticulum. Proc Natl Acad Sci USA. 2002b;99:13453–13458. doi: 10.1073/pnas.202422899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SR, Taylor MC, Touitha S, Mauricio IL, Meyer DJ, Kelly JM. TcGPXII, a glutathione-dependent Trypanosoma cruzi peroxidase with substrate specificity restricted to fatty acid and phospholipid hydroperoxides, is localized to the endoplasmic reticulum. Biochem J. 2002c;364:787–794. doi: 10.1042/BJ20020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago MP, Barrio AB, Cardozo RM, Duffy T, Schijman AG, Basombrio MA. Impairment of infectivity and immunoprotective effect of a LYT1 null mutant of Trypanosoma cruzi. Infect Immun. 2008;76:443–451. doi: 10.1128/IAI.00400-07. [DOI] [PMC free article] [PubMed] [Google Scholar]



