Abstract
We explored the perception of image focus in patients with cataracts, and how this perception changed following cataract removal and implantation of an intraocular lens. Thirty-three patients with immature senile cataract and with normal retinal function were tested before surgery and 2 days after surgery, with 18 of the patients retested again at 2 months following surgery. The subjective focus of natural images was quantified in each session by varying the slope of the image amplitude spectra. At each time, short-term adaptation to the spectral slope was also determined by repeating the measurements after exposure to images with blurred or sharpened spectra. Despite pronounced acuity deficits, before surgery images appeared “best-focused” when they were only slightly blurred, consistent with a strong compensation for the acuity losses. Post-operatively, the image slopes that were judged “in focus” before surgery appeared too sharp. This bias remained strong at 2 months, and was independent of the rapid blur after-effects induced by viewing filtered images. The focus settings tended to renormalize more rapidly in patients with higher post-operative acuity, while acuity differences were unrelated to the magnitude of the short-term blur aftereffects. Our results suggest that subjective judgments of image focus are largely compensated as cataracts develop, but potentially through a very long-term form of adaptation that results in persistent biases after the cataract is removed.
Keywords: Cataract, Blur adaptation, Spatial vision
1. Introduction
Despite substantial optical and neural changes in the aging visual system (Werner, Schefrin, & Bradley, 2010), many aspects of perception remain comparatively stable across the life span (Webster, Werner, & Field, 2005; Werner, 1996), suggesting that visual coding is adjusted to compensate for changes in the observer in order to maintain perceptual constancy. The nature of these compensatory adjustments remains poorly understood. In the present study, we examined perception of blur in patients before and after cataract (an opacity of the crystalline lens that interferes with vision) removal. The perception of blur represents an important and salient attribute of image quality (Watson & Ahumada, 2011). The development and correction for cataracts in elderly patients provides a powerful natural experiment for examining how the senescent visual system adapts to changes in the optical quality of the eye.
Cataracts can severely alter the spatial filtering of the retinal image as a result of increasing light scatter. Sensitivity at higher spatial frequencies is especially affected leading to large losses in visual acuity (Adamsons et al., 1992; Chua, Mitchell, & Cumming, 2004; Elliott, Gilchrist, & Whitaker, 1989; Lasa et al., 1992). The senescent or cataractous lens also selectively absorbs short wavelengths and necessarily alters spectral sensitivity specified at the cornea. In a previous study, Delahunt et al. (2004) examined the relation between spectral transmittance of the ocular media and color appearance in cataract patients. Despite profound attenuation of the short wavelength light reaching the retina, the stimulus that appears achromatic (neutral white) for cataract patients is generally similar to that of normal observers, and much more similar than predicted by the pronounced differences in spectral filtering (Werner & Schefrin, 1993). Cataract patients thus exhibit an extreme form of the color constancy that occurs in normal aging despite the increasing brunescence of their lens. Delahunt et al. (2004) tracked the changes in the achromatic point after cataracts were surgically removed. The settings showed a large initial shift in the yellow direction that gradually returned toward the original white point over several months. These results thus revealed a very long-term renormalization of color vision that was distinct in its time course from the rapid adjustments in color appearance that are routinely revealed by studies of chromatic adaptation.
In the present study we explored whether there are analogous long-term adjustments in spatial vision by examining how cataract influences subjective judgments of image focus, and how these judgments change after cataracts are removed. To assess this, we filtered the amplitude spectra of images to either blur or sharpen them, and then determined the slope of the amplitude spectrum that appeared neither too blurred nor too sharp. Like chromatic adaptation, short-term adaptation to physically blurred or sharpened images can strongly modulate judgments of subjective focus. Specifically, after adapting to images that have been physically blurred or sharpened, a physically focused image appears too sharp or blurred respectively (Elliott, Georgeson, & Webster, 2011; Vera-Diaz, Woods, & Peli, 2010; Webster, Georgeson, & Webster, 2002). Also like chromatic adaptation, blur adaptation can occur very rapidly and thus can lead to a rapid recalibration of perceived focus. The adaptation adjusts to the actual patterns of retinal image blur arising from both low- and high-order aberrations of the eye’s optics (Sabesan & Yoon, 2010; Sawides et al., 2010, 2011a, 2011b) and could function to compensate the perception of focus for the imperfections specific to an individual’s optics. However, it remains unknown whether there are additional long-term adjustments in perceived blur that might be triggered by a long-term change in the optical quality of the eye.
2. Methods
2.1. Subjects
Thirty-three patients with immature senile cataract participated in this experiment (mean age = 67.3, range = 57–81 years, 24 female and 9 male). All patients underwent cataract surgery with phacoemulsification and implantation of a foldable intraocular lens. The observers were tested before (the same day as the cataract extraction) and 2 days after cataract surgery. Eighteen of these observers were also available for testing after 2 months. All patients received retinal examinations before and after cataract removal in the Eye Diseases Clinic “MZERA,” located in Tbilisi, Georgia. These exams revealed no retinal or other ocular diseases that would be expected to cause vision loss. However, participants were not corrected for refractive error before or after cataract surgery, which is the standard of care in the Republic of Georgia. Decimal visual acuity was measured with a Landolt C chart at each visit to the laboratory. The procedure was to ask patients to start reading from the top line and to identify gaps in each letter within the line. This continued until the patient was unable read any C in the line. Acuity before surgery ranged from 0.07 to 0.5, and improved after cataract removal to values ranging from 0.1 to 1.0. The primary aim of the study was to compare how the perception of image focus changed for patients as a result of the surgery, and thus is based on a within-subjects design. However, we also included a control group of six volunteers (ages 25–52 years; 2 males, 4 females) with normal vision and no refractive correction. These participants were not matched in age to the patients, but were included to assess the focus percepts for our experimental conditions by individuals without cataracts.
Written informed consent was provided prior to participation in the experiment. All procedures followed the tenets of the Declaration of Helsinki and were approved by the National Council on Bioethics of Georgia.
2.2. Apparatus and stimuli
Grayscale images of natural scenes (e.g., trees, stones, leaves, etc.) from the study of Webster and Miyahara (1997) were used as stimuli (Fig. 1). The images were presented on a CRT monitor (Samsung SyncMaster 753s) driven by a PC with 8-bit color resolution. Observers adapted to a uniform gray field of 15 cd/m2 for 2 min before proceeding with the experiment. Ten images were used as stimuli, with two chosen at random to be used as test stimuli and the remaining serving as adapting stimuli. The images were filtered by multiplying the amplitude spectra by fα, with α varied between −1 and +1 in steps of 0.01 to allow for finely graded adjustments in the slope. Increasing negative values corresponded to increasing blur while positive values corresponded to sharpened images. After filtering, the root mean square contrast was adjusted to be equivalent to the original image to prevent subjects from using image contrast as a cue, although this does not necessarily equate the overall contrast at the retina.
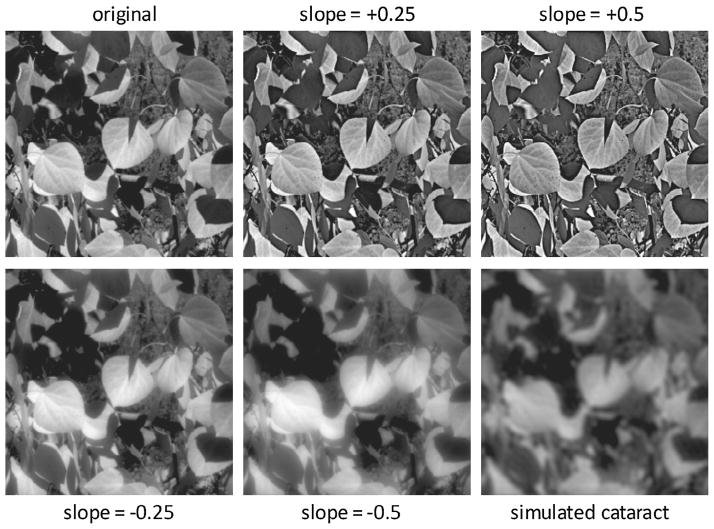
Fig. 1.
An example of the natural images used as adaptation and test stimuli for the experiments. The upper left image shows the original image before filtering (α = 0), while the upper middle and right images illustrate how the image was over-sharpened by biasing the slope of the amplitude spectrum (α = 0.25 or 0.5) to amplify high and attenuate low spatial frequencies. The lower left and middle images show the progressive blurring introduced by filtering to attenuate high spatial frequencies and to amplify low spatial frequencies (α = −0.25 and −0.5). Finally, the bottom-right image shows a simulation of the blur predicted from a typical cortical cataract, based on filtering the original image amplitude spectrum by the average loss in contrast sensitivity estimated for a population of patients (Sakamoto, 2003).
2.3. Procedure
Images were presented in a 4-deg square patch centered on a uniform gray background with the equivalent space-average luminance. The images were 256 by 256 pixels and thus had a maximum sampled horizontal or vertical frequency of 32 c/deg. The observer viewed the display monocularly at a distance of 110 cm in a dimly lit room (~0.5 lx). Pupils were not dilated. Settings were made without the patient’s refractive correction. At each test time, focus judgments were made following adaptation to: (1) a random sequence of blurred images (α = −0.5), (2) a random sequence of sharpened images (α = +0.5), and (3) a uniform field (used as the neutral adaptation condition). The blurred or sharpened images were resampled every 0.25 s during adaptation to avoid local light adaptation. Following the initial adaptation, test images were shown for 0.25 s interleaved with 6-s adaptation top-ups. The subject’s task was to press one of two keys to indicate whether the test image appeared “too blurred” or “too sharp” relative to their individual internal reference for image focus. The slope (α) of successive stimuli was varied with a staircase procedure until the point of subjective neutrality (PSN) was found at which either response was equally probable. The PSN was calculated as the average α of the last 6 of 8 reversals averaged over the two settings per condition. Observers were tested three times: (i) before cataract removal, (ii) 2 days after surgery and (iii) 2 months after surgery. The stimuli and procedure were the same for all test sessions.
Given the low visual acuities of some patients, especially before surgery, it is an issue whether they could in fact judge the subjective focus in the images. However, the task was specifically to judge whether the image itself was physically blurred. This judgment is based on assessing the relative contrast at different frequencies (the spectral slope) and thus is information that is potentially available even at very low frequencies. For the image manipulations used, the shifts in perceived blur with adaptation were on average substantially larger than the discrimination thresholds for judging blur differences between the images, showing both that observers were sensitive to the variations in the stimulus and that the settings could effectively track changes in the perceived neutral point resulting from adaptation.
3. Results
Decimal visual acuity changes measured with a Landolt C chart from before surgery to day 2, and from day 2 to month 2, are shown in Fig. 2 in the left and right panels, respectively. Before surgery acuity was low and ranged from 0.07 to 0.5. Following cataract removal and replacement with the intra-ocular lens, uncorrected visual acuities improved for all but one patient and reached a value of near 1.0 for two individuals. Moreover, they continued to improve for a majority of patients between 2 days and 2 months. However, large differences in acuity persisted after surgery and some patients showed much weaker acuity improvements than would be expected from the lens replacement alone. This raises the possibility that some patients had residual undiagnosed ocular health problems, though we note again that these problems were not apparent in our standard ophthalmologic examinations even after cataract removal, and acuity improvement may have been severely restricted by not correcting refractive error. We consider the impact of the differences in acuity below, but first we summarize the overall trends in the judgments of image focus before and after surgery.
Fig. 2.
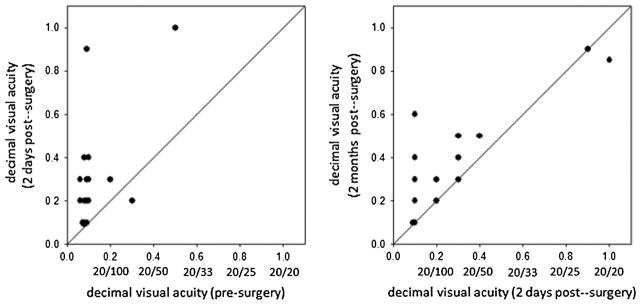
Uncorrected decimal visual acuities (VA) (and corresponding Snellen acuities) of patients before or after surgery. Left: VA at 2 days after surgery vs. VA before surgery. Right: VA 2 months after surgery vs. 2 days after surgery.
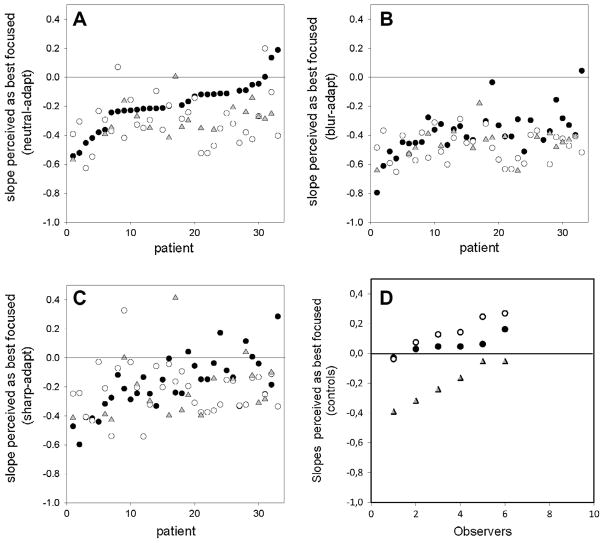
Fig. 3 shows the slopes that were judged as best-focused for individual observers. The first three panels compare the settings at the three different test times when observers were under short-term adaptation to the neutral gray field (Fig. 3A), blurred images (Fig. 3B), or sharpened images (Fig. 3C). The remaining panel (Fig. 3D) shows results for the control group (tested in a single session). In these figures the subjects have been ordered from lowest to highest slopes based on their neutral settings before surgery (Fig. 3A, filled circles). Unfilled circles and filled triangles correspond to the settings at 2 days or 2 months post-surgery. Before surgery, the neutral adaptation judgments were shifted toward negative slopes for almost all observers, with an average value of −0.19. Thus for most observers the images that appeared subjectively focused were physically blurred slightly relative to the original images. In contrast, the slopes chosen by the control group of observers with normal vision were slightly sharpened on average (mean = 0.054; Fig. 3D), which differed significantly from the patients’ settings (t = 6.38, p = 0.001). As discussed further below, this bias of the control group is small compared to the pronounced retinal image blur predicted by the patients’ visual acuities and is similar (though stronger) to the bias in settings observed in a group of older observers with normal vision tested with the same stimuli in a study by Elliott et al. (2007) (see their Fig. 2). For example, the lower right image of Fig. 1 shows a simulation of the level of blur predicted from a “typical” cortical cataract with central opacification, based on measures of the average contrast sensitivity function under daylight viewing conditions for 16 cataract patients by Sakamoto (2003). Again, we did not characterize the contrast sensitivity of our observers, but the curve used for this simulation is likely to underestimate the blurring in many of the present observers because their acuity losses were substantially larger. The simulation nevertheless illustrates that the images should appear profoundly blurred, and much more blurred than expected from the average chosen slope (which is slightly less blurred than the image depicted in the lower left corner of Fig. 1). However, in our results the focus settings before and after surgery represent very small variations compared to the changes predicted by their acuities, perhaps because of compensation for optical blur. If there were no compensation then the images should have appeared extremely blurred to most subjects (since their cataract is predicted to strongly increase the level of retinal image blur), and their pre-surgery focus settings should thus have been strongly shifted toward shallower (sharper) slopes, the opposite of what we observed.
Fig. 3.
Image amplitude spectrum slope that appeared best-focused before or after surgery. (A) Settings under neutral adaptation to the gray field, before surgery (filled circles) or at 2 days (unfilled circles) or 2 months (gray triangles) after surgery. The patients have been ordered based on the lowest to highest focus settings before surgery. (B and C) Settings across the three test times when subjects were instead adapted to blurred images (B) or sharpened images (C). (D) Settings for 6 control observers with normal vision, tested in a single session under the same neutral (filled circles), blurred (gray triangles), or sharpened (unfilled circles) adapting conditions.
After surgery, perceived focus under neutral adaptation was shifted for most observers toward more negative slopes, averaging −0.32 at 2 days and −0.29 at 2 months (Fig. 3A). Thus the image slopes that the observers perceived as in focus through their cataracts now appeared too sharp, consistent with a long-term bias of perceived blur induced by the long-term adaptation to their cataracts. (The leftmost points of Fig. 3A also suggest a trend in the opposite direction for observers who before surgery chose the most negative slopes for their focus settings. This could reflect a second factor such as increased sensitivity to the blur level after surgery, so that there was less inter-observer variance post-operatively. However, this trend was not significant across the three adapting levels or across the two post-surgery times.)
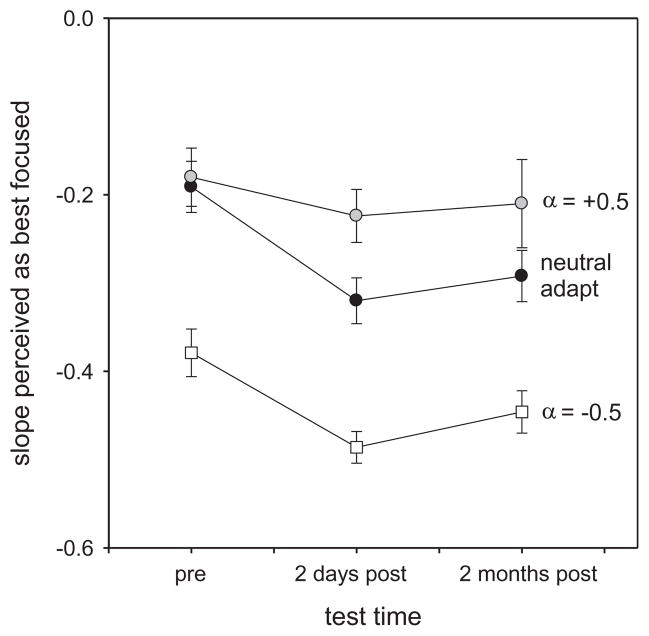
The average bias in the focus settings across the test times was maintained despite short-term changes in blur perception when observers were adapted to different blur levels during each test session. Specifically, adapting to blurred or sharpened images shifted the focus judgments toward more blurred (Fig. 3B) or sharpened (Fig. 3C) neutral points, yet in both cases the settings remained biased toward more blurred images after surgery. This is further illustrated in Fig. 4, which plots the mean settings for the different test times and adaptation conditions. Differences in the mean settings were assessed with a 2-way (test time × adapt condition) repeated measures ANOVA, based on the 18 observers who were evaluated at both 2 days and 2 months. Significant changes occurred both across the different test periods (F(2,34) = 5.22, p = 0.01) and across the different short-term adapting contexts (F(2,34) = 54.96, p < 0.001). Importantly however, there was no significant interaction between the effects of test session (pre- or post-surgery) and the effects of the adapting level during each session (blurred or sharp) (F(4,66) = 1.64, p = 0.175). Posthoc comparisons further showed that different focus settings were made across the three adapting conditions, and that the settings significantly differed before surgery and at either 2 days (t = 2.89, p = 0.017) or 2 months (t = 2.59, p = 0.014) after surgery. In contrast, the mean biases were not different at 2 days and 2 months (t = 0.41, p = 0.686), suggesting that the biases in the percepts induced by the surgery were persistent. (The significant changes in the settings also show that despite their low acuity, as a group the observers must have been sensitive enough to the slope differences in the images so that they were adapted differently to the blurred or sharpened spectra, and so that they could reliably set the perceived point of subjective focus differently for the different adapting conditions. Note also that this cannot be a consequence of differences in effective image contrast (rather than blur level). Both the sharpened and blurred adaptors had more contrast than the gray (zero-contrast) field used for neutral adaptation, but they induced aftereffects in opposite directions.)
Fig. 4.
Mean subjective focus settings across the different adaptation conditions and testing periods. The means are based only on the subset of 18 patients who were tested during all three sessions. Error bars show ±1 SEM.
As indicated above, individuals varied widely in their uncorrected acuity both before and after surgery. To assess the effect of acuity, we examined the correlations between judgments of image focus and the patient’s visual resolution. Notably, acuity did not predict differences in subjective focus at any of the testing times (pre: r(33) = −0.28, p = 0.11; 2 day post: r(33) = −0.28, p = 0.11; 2 month post: r(18) = 0.18, p = 0.47). This again suggests that the observer’s perception or criterion for neutral focus was largely compensated for their spatial sensitivity, potentially analogous to the way that the stimulus that appears neutral in color (i.e., white) is largely compensated for the individual’s spectral sensitivity (Werner & Schefrin, 1993).
There were also large differences across the patients in the strength and pattern of adaptation induced by exposure to the blurred or sharpened images, consistent with a recent report of individual differences in blur adaptation in observers with normal vision (Vera-Diaz, Woods, & Peli, 2010). In the settings before surgery, there was a trend for better acuity to be associated with stronger short-term adaptation, as measured by the difference in the focus settings under adaptation to the sharp vs. blurred adapting images (r(33) = 0.36, p = 0.038). However, differences in acuity were not associated with short-term adaptation strength at either time after surgery (2 day post: r(33) = 0.21, p = 0.24; 2 month post: r(18) = 0.43, p = 0.076). It is thus striking that – despite almost an order of magnitude difference in visual acuity – observers with markedly poorer spatial vision still retained sufficient sensitivity to the differences in the adapting image slopes to generate aftereffects of similar magnitude.
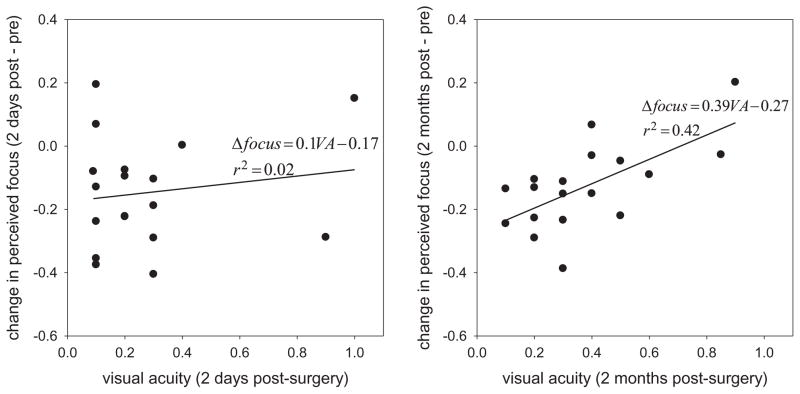
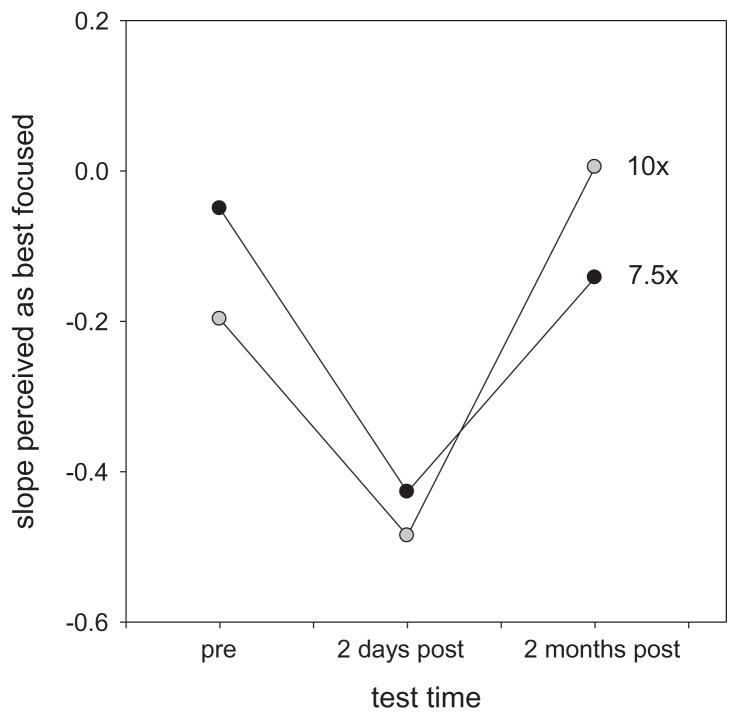
Finally, we examined the effect of acuity on the long-term adjustments to blur, as represented by the change in the neutral focus settings after surgery. Scatter plots of these effects are shown in Fig. 5. Neither post-operative acuity nor the post vs. pre-acuity change predicted the change in perceived focus after 2 days (r(33) = 0.025, p = 0.89). In contrast, a significant positive correlation was found between the absolute acuity and the focus changes at 2 months (r(18) = 0.65, p = 0.004) (and also when the subjective focus changes were compared to the relative improvements in acuity; r(18) = 0.55, p = 0.018, though not when the two observers with the greatest acuity improvements were excluded; r(16) = 0.38, NS). This relationship held for each short-term adaptation state at 2 months, so that the relationship pooled across the neutral and short-term adapting conditions was highly significant (r(54) = 0.54, p < 0.0001). Because acuity was unrelated to the absolute focus settings, and unrelated to the change in focus at 2 days, this effect cannot be accounted for simply by the improvements in vision alone. Instead, the pattern implies that observers with better visual acuity after surgery tended to show stronger or more rapid renormalization of their focus settings toward their pre-surgery levels. For example, Fig. 6 shows the settings for the two individuals who showed the largest improvements in their uncorrected acuity (10× or 7.5× their pre-surgery values). Both show a strong perceptual oversharpening 2 days after the operation but returned to their pre-surgery settings by 2 months.
Fig. 5.
Shift in perceived focus under neutral adaptation (best focused slope after–before surgery) as a function of visual acuity after surgery. Left: Change in perceived focus at 2 days. Right; Focus changes at 2 months. Lines and equations show the linear regression of the subjective focus on post-operative acuity.
Fig. 6.
Subjective focus settings of the two observers who showed the largest improvements (10× or 7.5× ) in uncorrected acuity following surgery. The slopes perceived as focused under neutral adaptation are plotted for pre-surgery or at 2 days or 2 months post-surgery.
4. Discussion
Before considering the implications of these results, it is important to address some potential limitations in how we measured the perception of focus. First, the image filtering we used was not designed to mimic optical changes with cataracts or optical blur in general. However, the slope changes do provide a sensitive measure of subjective focus and one that is largely independent of contrast, and induce adaptation effects that behave in very similar ways to simulations of actual aberrations (Sawides et al., 2010). The cataract patients were similar to normally-sighted observers in reliably judging the focus of these patterns, and it is unlikely that this could be based on a cue other than the perceived blur or sharpness in the image. Second, we did not characterize the observers’ spatial sensitivity beyond the measurement of acuity, and thus have only restricted information on how their spatial vision changed before and after surgery. However, the focus settings before and after – though different – in fact represented very small variations compared to the changes predicted by their acuities. If there were no compensation then the images should have appeared extremely blurred to most subjects (since their cataract is predicted to strongly increase the level of retinal image blur), and their pre-surgery focus settings should thus have been strongly shifted toward shallower (sharper) slopes, the opposite of what we observed. The results suggest that to a large extent the cataract patients’ judgments were compensated for their degraded spatial vision. The basis for the slight “over-compensation” is unclear, but could be related to the overshoot of sharpness constancy found in the normal visual periphery (Galvin et al., 1997). In the periphery, physically blurred edges appear sharp, and thus are matched by physically sharper edges in the fovea, perhaps reflecting the absence of evidence for blur under conditions of reduced acuity. Third, observers were tested with their natural pupils, and we did not measure or record the patients’ individual pupil sizes. While there is evidence that phacoemulsification surgery produces a transient, small reduction (~0.5 mm diameter) in pupil diameter, it is not significant (clinically or statistically) at 1 month (Hayashi & Hayashi, 2004), and this small effect is not likely to have a significant effect on image contrast. Finally, we tested observers who did not use a refractive correction in order to assess focus perception through their native optics, since as noted it is rare for this population to use a correction before or after surgery. As a result, our findings may not generalize to other populations where corrections are normally used. However, even if we instead examined observers who were fully adapted to a correction (so that physically focused images seen through it appeared in focus), then testing without their lenses should have caused the stimuli to appear too blurred rather than too sharp, again the opposite of the aftereffect we observed. Together this suggests that while the images and the blurring were unlikely to appear “natural” to the observers, the procedures we used did reveal perceptual judgments closely related to subjective focus and how those judgments shifted, both over long time scales before and after surgery, and over short time scales with adaptation to blurred or sharpened images.
The biases in perceived focus we observed exhibit a number of parallels to the effects of cataracts on color appearance. First, before surgery, subjective focus corresponded to stimuli that were close to the physical focus in the images. Thus the perception, or at least labeling, of the stimuli was largely compensated for the pronounced blur in the retinal images. The similarity among observers is striking given the enormous variation in optical quality and acuity in our sample. Tadmor and Tolhurst (1994) estimated that blurring by steepening the slope of the amplitude spectra by −0.5 was visually equivalent to approximately 1.5 diopters of blur. Thus the mean slope chosen of −0.19 was many times smaller than the range of equivalent dioptric blur among the patients, and differences in perceived focus were uncorrelated with the differences in acuity. The results before surgery are thus consistent with a substantial compensation of perceived focus for the observer’s contrast sensitivity. Nevertheless, this compensation was again not complete, because the settings for the patients did significantly differ from the control group.
A second parallel is that after surgery there was an aftereffect, so that the previously focused images appeared too sharp (analogous to the shift in color appearance toward blue after removing the yellow lens). Notably this aftereffect was not pronounced (an average shift in the focus to a slope of ~−0.3), was many times smaller than the shift predicted by the optical changes, and again was uncorrelated with the magnitude of the acuity change. This suggests that even within the 2 days before testing there was some recalibration of perceived focus, again because the subjective focus settings changed less than would be expected from the optical changes. A rapid initial adjustment has also been reported for color appearance changes after cataract surgery (Delahunt et al., 2004; Kitakawa et al., 2009).
Third, we found that the post-surgery bias in the focus settings was maintained for many patients even at 2 months. While observers with better acuity did tend to show more renormalization (e.g. Figs. 5 and 6), the settings for many remained biased, so that there was no difference in the overall settings at 2 days or 2 months (Fig. 4). This suggests that the surgery produced a persistent shift in the perception of image focus. These long-lasting biases were a characteristic feature of the changes in achromatic settings after cataract removal, which Delahunt et al. (2004) found persisted for months.
In the present study, we further found that the longer-term biases appeared superimposed on the more fleeting adaptation when observers viewed blurred or sharpened images. These short-term aftereffects were robust both before and after surgery. That these short-term blur aftereffects were robust for the present population confirms that the neural response changes underlying the adaptation remain largely intact in the normal elderly observer (Elliott et al., 2007), and is also consistent with previous findings that the strength of adaptation is unrelated to more moderate variations in refractive errors (Vera-Diaz, Woods, & Peli, 2010). The present results extend these studies to further show that short-term adaptation to blur also remains surprisingly unaffected even in observers – like our patients – with highly compromised optics and thus highly reduced spatial sensitivity.
Finally, the pattern of aftereffects further suggests that the biases at the short and long timescales are not only distinct but are largely independent, and thus may reflect separate neural responses. This finding also parallels previous results for color. For example, exposing observers for prolonged periods to reddish or greenish environments (by altering the room lighting or wearing colored contact lenses) leads to prolonged color aftereffects, e.g., in the wavelength that appears pure yellow (Neitz et al., 2002). Belmore and Shevell (2010) showed that these long-term color shifts were additive with the brief color aftereffects produced by chromatic adaptation to red or green fields. Similarly, the observers’ focus settings could be rapidly altered by adaptation to blur, but this brief aftereffect did not interact with the long-term shift in perceptual focus after surgery.
Compared to the extensive literature on short-term visual after-effects, the nature of long-term adaptation remains largely unexplored (Webster, 2011). However, there are a number of spatial aftereffects that point to slow timescales of adaptation in spatial vision. For example, adaptation to blur not only changes perceived focus, but can lead to improvements in visual acuity when observers are exposed for an hour or more to blurred vision induced by wearing defocusing lenses (Mon-Williams et al., 1998; Pesudovs & Brennan, 1993; Rajeev & Metha, 2010; Rosenfield, Hong, & George, 2004). Recent studies have also demonstrated changes in contrast sensitivity (Zhang et al., 2009) and contrast perception (Kwon et al., 2009) following exposure to reduced contrast over a period of hours, and have found long lasting dynamics in adaptation to the perceptual distortions induced by astigmatic lenses (Yehezkel et al., 2010). In a cataract patient, MacLeod and Anstis (submitted for publication) found that suprathreshold contrasts were largely compensated before surgery so that perceived contrasts in the cataractous eye were closer to the fellow eye than predicted by the differences in threshold sensitivity. This is analogous to the contrast constancy across different spatial frequencies demonstrated by Georgeson and Sullivan (1975). In the patient described by MacLeod and Anstis, this constancy led to an overshoot of perceived contrast when the cataract was removed. Fine et al. (2002) examined spatial perception in an individual with congenital cataracts that were not corrected until age 43. They similarly reported an overshoot in perceived focus following surgery, such that square-wave edges appeared too sharp. This oversharpening persisted for the 2 years over which the patient was tracked, suggesting that the spatial compensation developed under the cataract was relatively permanent. In our patients the cataracts developed late in life, and the percepts instead showed some plasticity, but still at a very slow timescale. Notably, this plasticity was greater in patients with better post-surgery acuity.
A final important parallel with color is that, like “white,” the perception of image focus is an important example of a perceptual norm in spatial vision, in that the stimulus that appears focused is a perceptually unique neutral point in the continuum from blurred to sharp (Elliott, Georgeson, & Webster, 2011). We typically experience the world as “in focus” despite the fact that spatial sensitivity is affected by “blur” imposed by both optical and neural limits. Thus, as with the perception of white, the subjective experience of blur may be corrected for the intrinsic spatial resolution limits in visual coding. For example, observers experience images blurred by their own aberrations as correctly focused (Artal et al., 2004; Sawides et al., 2011b), and experience blur aftereffects when exposed to weaker or stronger aberrations or to the wave aberrations characterizing a different observer’s eyes (Sawides et al., 2010, 2011a). These results suggest that individuals are not simply learning different criteria for judging focus (e.g., so that what they report as in focus is simply the blur they are used to seeing). Instead, the perceptual norm for focus is likely to reflect a norm in the responses of the neural mechanisms encoding blur (Webster & Leonard, 2008). Such renormalizations may be critical for maintaining visual experience as the visual system ages (Enoch et al., 1999). Our results build on other studies in revealing that these processes may operate over multiple timescales.
Acknowledgments
This work was supported by CRDF grant (GEB1-2930-TB-08), the National Eye Institute (EY10834), the National Institute on Aging (AG04058) and Research to Prevent Blindness.
References
- Adamsons I, Rubin GS, Vitale S, Taylor HR, Stark WJ. The effect of early cataracts on glare and contrast sensitivity. Archives of Ophthalmology. 1992;110:1081–1086. doi: 10.1001/archopht.1992.01080200061025. [DOI] [PubMed] [Google Scholar]
- Artal P, Chen L, Fernandez EJ, Singer B, Manzanera S, Williams DR. Neural compensation for the eye’s optical aberrations. Journal of Vision. 2004;4(4) doi: 10.1167/4.4.4. http://dx.doi.org/10.1167/4.4.4 (article no. 4) [DOI] [PubMed] [Google Scholar]
- Belmore SC, Shevell SK. Very-long-term and short-term chromatic adaptation: Are their influences cumulative? Vision Research. 2010;51:362–366. doi: 10.1016/j.visres.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua BE, Mitchell P, Cumming RG. Effects of cataract type and location on visual function: The Blue Mountains Eye Study. Eye. 2004;18:765–772. doi: 10.1038/sj.eye.6701366. [DOI] [PubMed] [Google Scholar]
- Delahunt PB, Webster MA, Ma L, Werner JS. Long-term renormalization of chromatic mechanisms following cataract surgery. Visual Neuroscience. 2004;21:301–307. doi: 10.1017/S0952523804213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott SL, Georgeson MA, Webster MA. Response normalization and blur adaptation: Data and multi-scale model. Journal of Vision. 2011;11(2) doi: 10.1167/11.2.7. http://dx.doi.org/10.1167/11.2.7 (article no. 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DB, Gilchrist J, Whitaker D. Contrast sensitivity and glare sensitivity changes with three types of cataract morphology: Are these techniques necessary in a clinical evaluation of cataract? Ophthalmic Physiological Optics. 1989;9:25–30. doi: 10.1111/j.1475-1313.1989.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Elliott SL, Hardy JL, Webster MA, Werner JS. Aging and blur adaptation. Journal of Vision. 2007;7(6) doi: 10.1167/7.6.8. http://dx.doi.org/10.1167/7.6.8 (article no. 8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch JM, Werner JS, Haegerstrom-Portnoy G, Lakshminarayanan V, Rynders M. Forever young: Visual functions not affected or minimally affected by aging: A review. Journal of Gerontology: Biological Sciences. 1999;54A:336–351. doi: 10.1093/gerona/54.8.b336. [DOI] [PubMed] [Google Scholar]
- Fine I, Smallman HS, Doyle P, MacLeod DIA. Visual function before and after the removal of bilateral congenital cataracts in adulthood. Vision Research. 2002;42:191–210. doi: 10.1016/s0042-6989(01)00266-8. [DOI] [PubMed] [Google Scholar]
- Galvin SJ, O’Shea RP, Squire AM, Govan DG. Sharpness overconstancy in peripheral vision. Vision Research. 1997;37:2035–2039. doi: 10.1016/s0042-6989(97)00016-3. [DOI] [PubMed] [Google Scholar]
- Georgeson MA, Sullivan GD. Contrast constancy: Deblurring in human vision by spatial frequency channels. Journal of Physiology. 1975;252:627–656. doi: 10.1113/jphysiol.1975.sp011162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Hayashi H. Pupil size before and after phacoemulsification in nondiabetic and diabetic patients. Journal of Cataract and Refractive Surgery. 2004;30:2543–2550. doi: 10.1016/j.jcrs.2004.04.045. [DOI] [PubMed] [Google Scholar]
- Kitakawa T, Nakadomari S, Kuriki I, Kitahara K. Evaluation of early state of cyanopsia with subjective color settings immediately after cataract removal surgery. Journal of the Optical Society of America. A Optics, Image Science, and Vision. 2009;26:1375–1381. doi: 10.1364/josaa.26.001375. [DOI] [PubMed] [Google Scholar]
- Kwon M, Legge GE, Fang F, Cheong AM, He S. Adaptive changes in visual cortex following prolonged contrast reduction. Journal of Vision. 2009;9(2) doi: 10.1167/9.2.20. http://dx.doi.org/10.1167/9.2.20 (article no. 20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa MS, Datiles MB, Podgor MJ, Magno BV. Contrast and glare sensitivity. Association with the type and severity of the cataract. Ophthalmology. 1992;99:1045–1049. [PubMed] [Google Scholar]
- MacLeod DIA, Anstis S. Contrast gain control before and after cataract surgery: A case study. Perception. 2013 (submitted for publication) [Google Scholar]
- Mon-Williams M, Tresilian JR, Strang NC, Kochhar P, Wann JP. Improving vision: Neural compensation for optical defocus. Proceedings of the Royal Science, B. 1998;265:71–77. doi: 10.1098/rspb.1998.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitz J, Carroll J, Yamauchi Y, Neitz M, Williams DR. Color perception is mediated by a plastic neural mechanism that is adjustable in adults. Neuron. 2002;35:783–792. doi: 10.1016/s0896-6273(02)00818-8. [DOI] [PubMed] [Google Scholar]
- Pesudovs K, Brennan NA. Decreased uncorrected vision after a period of distance fixation with spectacle wear. Optometry & Vision Science. 1993;70:528–531. doi: 10.1097/00006324-199307000-00002. [DOI] [PubMed] [Google Scholar]
- Rajeev N, Metha A. Enhanced contrast sensitivity confirms active compensation in blur adaptation. Investigative Ophthalmology & Vision Science. 2010;51:1242–1246. doi: 10.1167/iovs.09-3965. [DOI] [PubMed] [Google Scholar]
- Rosenfield M, Hong SE, George S. Blur adaptation in myopes. Optometry & Vision Science. 2004;81:657–662. doi: 10.1097/01.opx.0000144743.34976.da. [DOI] [PubMed] [Google Scholar]
- Sabesan R, Yoon G. Neural compensation for long-term asymmetric optical blur to improve visual performance in keratoconic eyes. Investigative Ophthalmology & Vision Science. 2010;51:3835–3839. doi: 10.1167/iovs.09-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A. Optical properties and visual functions of patients with cortical cataract: Relationship to guideline for medical treatment of cataracts. Journal of Kanazawa Medical University. 2003;28:151–159. [Google Scholar]
- Sawides L, de Gracia P, Dorronsoro C, Webster M, Marcos S. Adapting to blur produced by ocular high-order aberrations. Journal of Vision. 2011a;11(7) doi: 10.1167/11.7.21. http://dx.doi.org/10.1167/11.7.21 (article no. 21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawides L, de Gracia P, Dorronsoro C, Webster MA, Marcos S. Vision is adapted to the natural level of blur present in the retinal image. PLoS One. 2011b;6(11):e27031. doi: 10.1371/journal.pone.0027031. http://dx.doi.org/10.1371/journal.pone.0027031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawides L, Marcos S, Ravikumar S, Thibos L, Bradley A, Webster M. Adaptation to astigmatic blur. Journal of Vision. 2010;10(12) doi: 10.1167/10.12.22. http://dx.doi.org/10.1167/10.12.22 (article no. 22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadmor Y, Tolhurst DJ. Discrimination of changes in the second-order statistics of natural and synthetic images. Vision Research. 1994;34:541–554. doi: 10.1016/0042-6989(94)90167-8. [DOI] [PubMed] [Google Scholar]
- Vera-Diaz FA, Woods RL, Peli E. Shape and individual variability of the blur adaptation curve. Vision Research. 2010;50:1452–1461. doi: 10.1016/j.visres.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AB, Ahumada AJ. Blur clarified: A review and synthesis of blur discrimination. Journal of Vision. 2011;11(5) doi: 10.1167/11.5.10. http://dx.doi.org/10.1167/11.5.10 (article no. 10) [DOI] [PubMed] [Google Scholar]
- Webster MA. Adaptation and visual coding. Journal of Vision. 2011;11(5) doi: 10.1167/11.5.3. http://dx.doi.org/10.1167/11.5.3 (article no. 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MA, Georgeson MA, Webster SM. Neural adjustments to image blur. Nature Neuroscience. 2002;5:839–840. doi: 10.1038/nn906. [DOI] [PubMed] [Google Scholar]
- Webster MA, Leonard D. Adaptation and perceptual norms in color vision. Journal of the Optical Society of America A Optics, Image Science, and Vision. 2008;25:2817–2825. doi: 10.1364/josaa.25.002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MA, Miyahara E. Contrast adaptation and the spatial structure of natural images. Journal of the Optical Society of America. A Optics, Image Science and Vision. 1997;14:2355–2366. doi: 10.1364/josaa.14.002355. [DOI] [PubMed] [Google Scholar]
- Webster MA, Werner JS, Field DJ. Adaptation and the phenomenology of perception. In: Clifford C, Rhodes G, editors. Fitting the mind to the world: Adaptation and aftereffects in high level vision. Advances in visual cognition series. Vol. 2. Oxford: Oxford University Press; 2005. pp. 241–277. [Google Scholar]
- Werner JS. Visual problems of the retina during ageing: Compensation mechanisms and colour constancy across the life span. In: Osborne NN, Chader J, editors. Progress in retinal and eye research. 15/2. Oxford: Pergamon Press; 1996. pp. 621–645. [Google Scholar]
- Werner JS, Schefrin BE. Loci of achromatic points throughout the life span. Journal of the Optical Society of America. A Optics and Image Science. 1993;10:1509–1516. doi: 10.1364/josaa.10.001509. [DOI] [PubMed] [Google Scholar]
- Werner JS, Schefrin BE, Bradley A. Optics and vision of the aging eye. In: Bass M, editor. OSA handbook of optics. Vision and vision optics. III. New York: McGraw Hill, Inc; 2010. pp. 14-1–14-38. [Google Scholar]
- Yehezkel O, Sagi D, Sterkin A, Belkin M, Polat U. Learning to adapt: Dynamics of readaptation to geometrical distortions. Vision Research. 2010;50:1550–1558. doi: 10.1016/j.visres.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bao M, Kwon M, He S, Engel SA. Effects of orientation-specific visual deprivation induced with altered reality. Current Biology. 2009;19:1956–1960. doi: 10.1016/j.cub.2009.10.018. [DOI] [PubMed] [Google Scholar]