Abstract
The synthetic cathinones are an emerging class of designer drugs abused for psychostimulant and hallucinogenic effects similar to cocaine, methylenedioxymethamphetamine (MDMA), or other amphetamines. Abuse of synthetic cathinones, frequently included in products sold as ‘bath salts’, became prevalent in early 2009, leading to legislative classification throughout Europe in 2010 and schedule I classification within the United States in 2011. Recent pre-clinical and clinical studies indicate dysregulation of central monoamine systems are a principal mechanism of synthetic cathinone action and presumably underlie the behavioral effects and abuse liability associated with these drugs. This review provides insight into the development of synthetic cathinones as substances of abuse, current patterns of their abuse, known mechanisms of their action and toxicology, and the benefits and drawbacks of their classification.
Keywords: Synthetic Cathinone, Bath Salts, Mephedrone, Methylone, MDPV, Stimulants, Designer Drugs
Introduction
Designer drugs are synthetic compounds developed to provide rewarding effects similar to illicit drugs of abuse (e.g. opioids, amphetamines, and marijuana) while circumventing existing legislative classification and penalty. Recently, designer drug mixtures have been marketed and sold as ‘legal highs’ over the internet and in head shops worldwide. The synthetic cathinones are one of the most prevalent classes of compounds found in these products, frequently sold as 'bath salts' or 'fertilizer’ despite having no such purposes and are insufflated (snorted), ingested, or injected by users seeking psychostimulant effects similar to cocaine, methylenedioxymethamphetamine (MDMA) or other amphetamines. Possession, use, and synthesis of the synthetic cathinones was legal until their emergency schedule I classification in 2011 followed by permanent schedule I classification in the Synthetic Drug Abuse Prevention Act of 2012 (Drug Enforcement Administration, 2011). Schedule I classification will undoubtedly reduce access to and consumption of synthetic cathinones, but will also limit research on these relatively unstudied compounds to a very small number of laboratories and institutions that have been licensed to work with schedule I drugs.
This review aims to provide insight into the development of synthetic cathinones as substances of abuse, current patterns of their abuse, known mechanisms of their action and toxicology, and the benefits and drawbacks of their classification. A brief history of designer drugs will be followed by a description of the manner and prevalence of current synthetic cathinones abuse. This review will then focus on emerging research describing the mechanisms of action, toxicology, and abuse liability of synthetic cathinone compounds, and discuss why categorizing the chemical basis for these substances is important and necessary. Finally, the impact of scheduling upon the ability to research and understand the pharmacology of these drugs will be described.
A Brief History of Designer Drugs
The Controlled Substances Act of 1970 established a framework for regulating substances of abuse within the United States (US) by scheduling them based upon medical use, abuse liability, and risk of developing physical or psychological dependence. Compounds are classified on a scale from schedule I to V, with schedule I drugs considered to have the greatest risk and abuse liability without significant medical application and schedule V drugs having accepted medical use with minimal liability or risk. Following passage of the Controlled Substances Act of 1970, a number of compounds were abused to mimic the effects of popular illicit drugs while avoiding regulation. The term 'designer drug' was coined in the early 1980s to describe such compounds, which were often synthesized in small home laboratories from widely available over-the-counter drugs or chemical precursors and not illegal provided they were structurally different from scheduled drugs (Ziporyn, 1986). Synthetic opioids were the first compounds termed designer drugs, appearing in California as 'China White' in 1979 and produced by fentanyl modification to mimic the effects of heroin and morphine (Henderson, 1988, Kram et al., 1981, Ziporyn, 1986).
Many designer drugs were first synthesized for research or medicinal purposes by chemists in academia or the pharmaceutical industry. The means of synthesis and effects of these compounds were widely available in the research literature, only to be rediscovered at a later date (in some cases decades later) and repurposed as drugs of abuse. For example, MDMA was first synthesized, described and patented by Merck in 1912, but did not appear on the streets until 1970 and was not extensively abused until the mid-1980s (for a fascinating review on the history of MDMA, see (Freudenmann et al., 2006)). Following the widespread abuse of China White and other fentanyl analogues, psychostimulants developed decades earlier emerged and gained popularity as designer drugs, including the amphetamine analogues methylenedioxy- amphetamine (MDA), methamphetamine (METH) and MDMA.
Under the Controlled Substances Act, the government had no authority to prosecute the possession, production, or consumption of illicit drug analogues - the designer drugs - until those specific compounds were scheduled. The growing abuse of designer drugs led to the Controlled Substance Analogue Enforcement Act of 1986, which dictated that “any substance intended for human consumption with a chemical structure similar to a schedule I or II controlled substance that has a similar or greater stimulant, depressive, or hallucinogenic effect shall be treated as a schedule I substance.”(1986) To sidestep prosecution under the Analogue Enforcement Act, two tactics have been employed by designer drug producers. First, designer drugs that achieve the stimulant, depressant or hallucinogenic effects of schedule I substances with little structural analogy have been pursued and developed. Second, designer drugs have been explicitly marketed as products ‘not for human consumption’. Both strategies, in part, have led to the production and abuse of synthetic cathinones in the US without legal ramification until their recent scheduling.
History and Abuse Prevalence
Synthetic cathinones are the most common group of psychoactive compounds, along with the piperazines, found within ‘bath salts’ sold through the internet and in head shops worldwide (Davies et al., 2010). The most widely abused synthetic cathinones - 4-methylmethcathinone (mephedrone), 3,4-methylenedioxymethcathinone (methylone), and 3,4-methylenedioxyprovalerone (MDPV) - are all derivatives of cathinone, a naturally occurring stimulant found in the leaves of khat. Cathinone is an alkaloid, similar in structure and action to amphetamine, whose analogues have been used as stimulants for centuries (Kalix, 1981). Chewing khat dates back to at least the tenth century and continues today, with its origins and popularity associated with East Africa and the Arab Peninsula (Gebissa, 2010). The chemical structures and synthesis of some synthetic cathinones have long been known but only recently abused. Methcathinone, a methylated analogue of cathinone, was synthesized in 1928 (Sanchez, 1929) and was the first synthetic cathinone designer drug, with reports of abuse beginning in the early 1990s (Emerson and Cisek, 1993). Synthesis of mephedrone and MDPV were first described in 1929 (Sanchez, 1929) and 1967 (G.m.b.H., 1967), respectively, but abuse was not reported until the early 2000s. Methylone is more recent analogue, patented 1996 (Jacob Peyton III, 1996).
Following their discovery, the synthetic cathinones were ignored until their abuse as a legal alternative to MDMA was first reported on internet drug websites in 2003 (Morris, 2010) and became prevalent within the United Kingdom in 2009 (BBC, 2009). Mephedrone is the most widely abused synthetic cathinone within Europe, whereas MDPV and methylone are the most frequently abused synthetic cathinones within the US. Synthetic cathinones are most frequently consumed as white powder or crystalline ‘bath salts’ mixtures but are also taken orally in tablet and pill form (Wood et al., 2012). Tablets or pills sold throughout Europe containing mephedrone are marketed as ‘meow meow’, ‘bubbles’, ‘top cat’, ‘4-MMC’, and ‘ecstasy’. Though ecstasy has long been synonymous for MDMA (Freudenmann, Oxler, 2006), mephedrone appears to be replacing MDMA in many tablets marketed as ecstasy (Brunt et al., 2011). Indeed, recent seizures of ecstasy by law enforcement throughout Europe indicate tablets often contain a mixture of mephedrone, MDMA and caffeine, with mephedrone the primary constituent in the majority of tablets (Addiction, 2011, Brunt, Poortman, 2011).
Bath salts are synthetic cathinone powders distributed under trade names such as 'Ivory Wave', 'White Lightning' and 'Vanilla Sky' and labeled as "not for human consumption" to avoid penalty under the Analogue Enforcement Act (Addiction, 2011, Davies, Wood, 2010, Kasick et al., 2012, Winstock et al., 2011). These compounds are most frequently insufflated (snorted), but nasal agitation leads many users to smoke bath salts, take them orally or rectally, or to inject them intravenously or intramuscularly (Addiction, 2011, Kavanagh et al., 2012). Since crystallized synthetic cathinones are water soluble, bath salts are readily dissolved in beverages and orally ingested (Addiction, 2011). As with tablets, mephedrone is more prominent in European bath salts whereas MDPV is more prominent in US bath salts.
Despite distribution through street-level dealers, head shops, smoke shops, adult book stores, gas stations and internet retailers within Europe, the US and worldwide, the overwhelming majority of synthetic cathinones are produced in China and surrounding South East Asian countries. The synthetic cathinones are commonly transported in powder-form to distributors, where they are then tabletted, pilled or adulterated prior to sale (Addiction, 2011). Producers and sellers claim to provide synthetic cathinones with over 99% purity. However, analyses of seized and purchased products demonstrate purity of around 95% with adulterants including benzocaine, lidocaine, caffeine, piperazines and paracetamol (Addiction, 2011, Davies, Wood, 2010).
The worldwide rise of synthetic cathinone abuse has been rapid and extensive. Mephedrone was the first synthetic cathinone detected by European authorities in late 2007 and by 2010 mephedrone had been detected and seized in 28 European and neighboring countries (Addiction, 2011). Although usage data are limited in scope and self-reported, synthetic cathinone abuse gained prevalence within the UK between 2009 and 2010. Monthly enquiries to the UK National Poisons Information Service regarding cathinone toxicity rose from 0 in 2009 to over 600 in 2010 (James et al., 2011). By 2010, mephedrone was the third most commonly abused drug in the UK (The NHS Information Centre, 2011) and approximately 20% of UK school and college/university aged individuals (between the ages of 14 and 20) reported its use that year (Dargan et al., 2010). By 2012, 128 mephedrone-associated fatalities had been reported within the UK (Schifano et al., 2012).
Within the US, synthetic cathinone drug reports to the National Forensic Laboratory Information System (NFLIS) increased from 34 in 2009 to 628 in 2010 (U.S. Drug Enforcement Administration, 2011). By 2011, the NFLIS reported MDPV as the fifth and methylone as the eleventh most common hallucinogens within the US (U.S. Drug Enforcement Administration, 2012). Meanwhile, poison control center calls regarding bath salts exposure increased from 304 in 2010 to 6136 in 2012 (Centers, 2012) and the Toxicology Investigators Consortium reported bath salts to account for 12% of all toxicology-related cases in 2011(Wiegand et al., 2012).
Motivation for Abuse and Reported Effects
Synthetic cathinones are abused for social and economic reasons in addition to their stimulant and hallucinogenic properties, often serving as a replacement for MDMA, cocaine and the amphetamines. Due to the rapid and recent ascent of abuse, profiles of synthetic cathinone abuse and abusers are limited to case reports of toxicity and surveys of UK mephedrone users. Typical mephedrone users are young adult (mean age of 25.1 years old) men (77%) who are either employed or in school (86%) with a history of stimulant (96% ecstasy use, 92% cocaine use) and polydrug use (Addiction, 2011, Carhart-Harris et al., 2011, Freeman et al., 2012, Schifano, Corkery, 2012). Mephedrone is often taken in binge fashion, with an average of 6 doses over a 9-hour period and 30 minutes to 2 hours between doses, in social settings, such as friends’ homes, house parties or night clubs, and frequently with other drugs (e.g., alcohol, cocaine, ecstasy, cannabis, ketamine). Total mephedrone consumed during any given session ranges enormously, from 25 mg to 9 g (Addiction, 2011, Carhart-Harris, King, 2011, Freeman, Morgan, 2012, Schifano, Corkery, 2012).
Though limited data exist, regular mephedrone users report (in order of decreasing incidence) feelings of intense euphoria, increased concentration, talkativeness, empathy and an "urge to move", as well as heightened sexual desire (Winstock, Mitcheson, 2011). The same users report a number of negative effects associated with mephedrone during its use (in order of decreasing incidence), including jaw clenching, reduced appetite, increased body temperature and sweating, a racing heart, and problems with memory (Winstock, Mitcheson, 2011). Withdrawal effects following mephedrone use most frequently include tiredness, insomnia, nasal congestion and impaired concentration (Winstock, Mitcheson, 2011). A number of social and economic factors concurrent to the hedonic effects of the synthetic cathinones motivate users to abuse these drugs. Most users have a polydrug history of abuse and aren’t necessarily concerned with legality. Instead, perceptions of drug high, drug cost and side effects primarily motivate mephedrone abuse. Mephedrone is perceived as a good value for the money with comparable or better highs and fewer side effects than other stimulants, such as MDMA and cocaine (Addiction, 2011, Freeman, Morgan, 2012, Winstock, Mitcheson, 2011). Importantly, mephedrone is considered a more consistent product than either MDMA or cocaine (Addiction, 2011, Freeman, Morgan, 2012), which coincides with reports of wide variation in MDMA content within ecstasy tablets (Wood et al., 2011). Together, the perceptions of mephedrone being a more consistent and safer product and better value than MDMA or cocaine appear to be driving mephedrone preference over other stimulants.
Chemical Structure
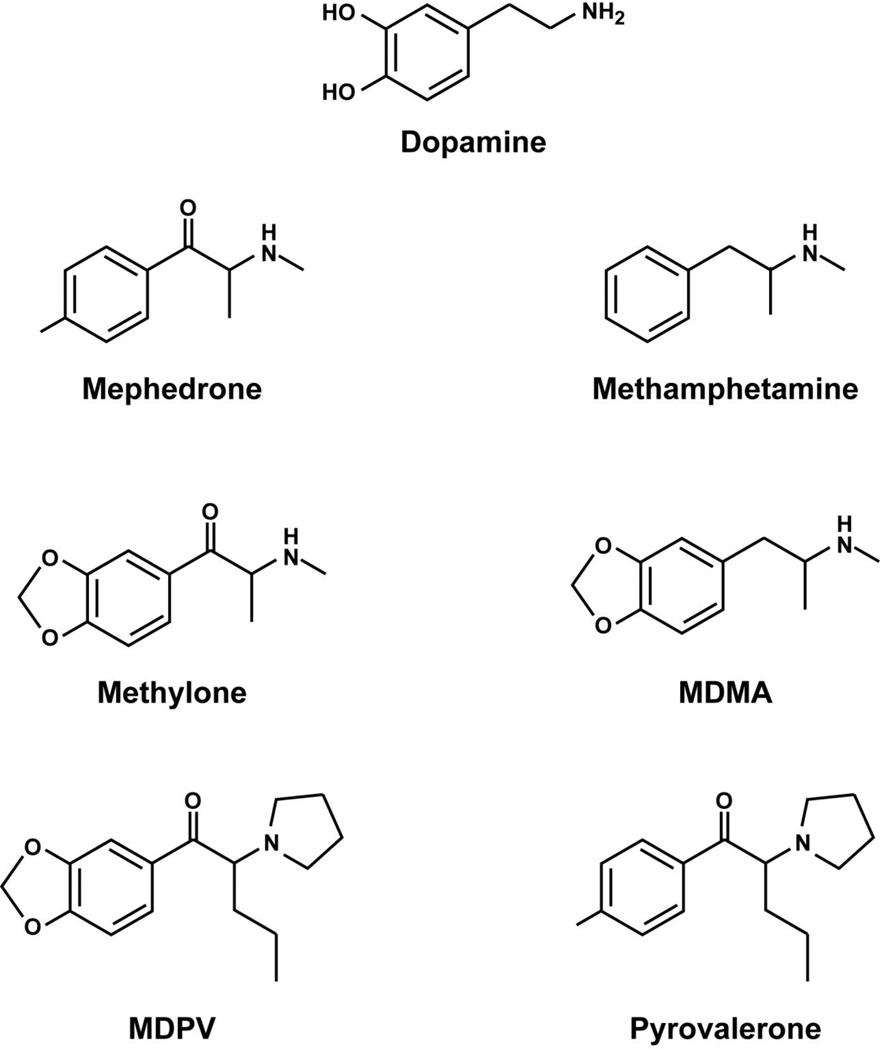
The synthetic cathinones are β-ketoamphetamines with structural similarity to dopamine, methamphetamine, MDMA, and pyrovalerone (Figure 1). The backbone of mephedrone, methylone and MDPV is a phenethylamine with a ketone group at the β carbon. Mephedrone is methylated on the amine group, α carbon, and aromatic ring of this backbone, forming a structure similar to methamphetamine and methcathinone. Methylone is also methylated on the amine group and α carbon of this β-ketophenethylamine backbone, but has a methylenedioxy ring attached to the aromatic ring, forming a structure similar to MDMA. MDPV has the greatest structural divergence from other synthetic cathinones, with a methylenedioxy ring attached to the aromatic ring of the β-ketophenethylamine backbone and a pyrrolidinyl ring and propane attached to the α carbon, forming a structure similar to pyrovalerone. Structural semblance between the synthetic cathinones and other stimulants in many ways accounts for shared function, such as stimulating monoamine neurotransmitter release and inhibiting its reuptake from the synaptic cleft (as discussed below). Despite structural and functional analogy with other stimulants, the synthetic cathinones have just as many differences and should be considered a unique family of compounds.
Figure 1.
Chemical structures of dopamine, the synthetic cathinones, and closely related stimulants.
Mechanisms of Action, Toxicology and Abuse Liability
Few, if any, preclinical or clinical studies evaluating the pharmacology, toxicology or physiologic effects of synthetic cathinones had been performed prior to their widespread abuse. Given their close structural similarity to methcathinone, MDMA and other amphetamines, the synthetic cathinones were predicted to act as stimulants through disruption of central monoamine systems. Indeed, recent preclinical evidence indicates dysregulation of central and peripheral monoamine systems is a primary mechanism of synthetic cathinone action. Due to the nascent nature of synthetic cathinone research, little consensus on dosages and treatment paradigms has formed, leading to a wide range of treatment parameters and variability within the reported results.
Two mechanisms are known to underlie stimulant-induced increases in extracellular monoamines: blocked transporter reuptake and elevated presynaptic release. The dopamine (DA) transporter (DAT) and serotonin (5-HT) transporter (SERT) tightly regulate the amount of neurotransmitter within the synaptic cleft, influencing the extent and duration of signaling between neurons. Loss of transporter function has been linked to both acute and long-term deficiencies in the DA and 5-HT systems following exposure to toxic levels of stimulants, primarily the amphetamines (Fleckenstein et al., 2007). In addition, monoamine release may occur from increased synaptic vesicle release, driven by presynaptic input from other neurotransmitter systems such as the cholinergic or glutamatergic systems, and from the drug itself acting as a substrate for DAT and/or SERT and reversing the direction of neurotransmitter transport. For example, amphetamine is a DAT substrate that is actively transported into the presynaptic terminal, driving DA efflux through the DAT by rapidly increasing the concentration of substrates within the presynaptic terminal (Connor and Kuczenski, 1986, Kahlig et al., 2005). Repeated large doses of mephedrone (10 or 25 mg/kg/injection, subcutaneous, 4 injections, 2-hour intervals), which mimic the binge patterns observed in human consumption, reduce DAT and SERT function by approximately 20% within rat striatal and hippocampal synaptosomes, respectively, one hour following the last injection (Hadlock et al., 2011). Multiple smaller doses (1 or 3 mg/kg/injection, subcutaneous, 4 injections, 2-hour intervals) of mephedrone have no effect upon DAT or SERT function within this same time period. In vitro transporter assays further confirm that mephedrone and methylone, when applied to synaptosomes, not only block DAT and SERT uptake (Baumann et al., 2012a, Martinez-Clemente et al., 2012) but act as DAT and SERT substrates, releasing neurotransmitter from synaptosomes (Baumann, Ayestas, 2012a, Baumann et al., 2012b, Cameron et al., 2012, Hadlock, Webb, 2011). In contrast, MDPV does not act as a DAT and SERT substrate but functions solely as a blocker of monoamine uptake through DAT and SERT (Baumann, Partilla, 2012b, Cameron, Kolanos, 2012). Importantly, microdialysis studies have confirmed synthetic cathinone-induced loss of DAT and SERT function to coincide with rapid increases in extracellular DA and 5-HT within DAT and SERT-rich brain regions following administration of even small doses of mephedrone (3 mg/kg, subcutaneous) (Baumann, Ayestas, 2012a, Kehr et al., 2011).
Short-term loss of DAT and SERT function is associated with the long-term DA and 5-HT neurotoxicity following exposure to other stimulants, namely the amphetamines (Fleckenstein, Volz, 2007). Despite synthetic cathinone-induced short-term loss of both DAT and SERT function, persistent deficits have only been reported in the 5-HT system and under conditions that promote hyperthermia (Hadlock, Webb, 2011). Seven days following high-dose binge mephedrone treatments, hippocampal SERT activity and 5-HT content are reduced as much as 60% and 45%, respectively (Hadlock, Webb, 2011). However, a number of other studies have reported no loss in 5-HT content several days and weeks following mephedrone administrations, though fewer administrations, lower dosage and/or greater time periods between injections were employed under conditions that do not promote hyperthermia (Baumann, Ayestas, 2012a, den Hollander et al., 2013, Motbey et al., 2012). Although persistent 5-HT deficits were absent in these studies, significant behavioral consequences followed mephedrone use (discussed below) (den Hollander, Rozov, 2013). In contrast to the 5-HT system, mephedrone causes no observable persistent deficits in the DA system. Striatal DAT activity was completely restored seven days following a binge mephedrone treatment and no loss of DA content, DAT content or tyrosine hydroxylase (TH; synthesizes DA) activity was detected within this time period (Angoa-Perez et al., 2012b, Hadlock, Webb, 2011). Mephedrone did not appear to activate striatal microglia or astrocytes, events indicative of methamphetamine-induced dopaminergic deficits (Bowyer et al., 2008). Surprisingly, mephedrone has a greater binding affinity for DAT than SERT.
The underlying causes of persistent synthetic cathinone-induced deficits in the 5-HT system, and lack thereof in the DA system, are unknown. Prolonged loss (greater than 24 h) of function of the vesicular monoamine transporter-2 (VMAT2), which packages cytoplasmic dopamine into synaptic vesicles, is associated with long-term stimulant-induced DA and 5-HT toxicity (Fleckenstein, Volz, 2007). Reduced VMAT2 uptake presumably increases unsequestered DA or 5-HT within the presynaptic cytoplasmic space, which may be converted into reactive oxygen species or quinones and damage terminals (Fleckenstein et al., 1997a, Fleckenstein et al., 1997b, LaVoie and Hastings, 1999). Some evidence indicates mephedrone administration in vivo disrupts ex vivo VMAT2 DA uptake, but the duration and specificity of reduced VMAT2 uptake (dopaminergic vs. serotonergic terminals) has not been thoroughly assessed (Lopez-Arnau et al., 2012). If synthetic cathinones selectively and persistently reduce VMAT2 function within the 5-HT system but not the DA system, large amounts of unsequestered 5-HT may form reactive oxygen species or induce excitotoxicity that lead, in part, to selective 5-HT neurotoxicity.
Hyperthermia likely influences the extent of synthetic cathinone toxicity. The degree of hyperthermia experienced during MDMA, amphetamine and METH exposure directly impacts the magnitude of acute and long-term deficits in the DA and 5-HT systems. Lower doses of mephedrone (up to 10mg/kg) do not cause substantial or life-threatening hyperthermia (Aarde et al., 2013, Miller et al., 2013, Wright et al., 2012a), with core body temperatures rarely exceeding 38.0 °C. In contrast, repeated high-dose mephedrone treatment (4 × 50 mg/kg/injection, s.c., 2-h intervals) in a warm environment causes significant and prolonged hyperthermia of up to 40.0 ± 0.1 °C average core body temperature over the 8 hour treatment period (Hadlock, Webb, 2011). Importantly, persistent serotonergic deficits have only been reported under these hyperthermic conditions (Hadlock, Webb, 2011). Emergency room and poison control case reports indicate hyperthermia is a key symptom of human MDPV and mephedrone overdose (Borek and Holstege, 2012, Forrester, 2013, Levine et al., 2013, Ross et al., 2012), leading to core body temperatures of up to 42.1 °C (Levine, Levitan, 2013). Consequently, investigation into the role hyperthermia plays in synthetic cathinone toxicity is warranted.
Concurrent use of other stimulants (cocaine, MDMA, other amphetamines) with synthetic cathinones is prevalent amongst abusers (Addiction, 2011, Carhart-Harris, King, 2011), and emerging evidence suggests synthetic cathinones enhance the toxicity of some stimulants. Mephedrone given prior to and during binge METH, amphetamine or MDMA treatments synergistically increased the loss of DA, DAT and TH content several days following drug exposure (Angoa-Perez et al., 2012a). Other stimulants are frequently detected in toxicology reports (discussed in further detail below) of mephedrone-associated human fatalities (Schifano, Corkery, 2012), suggesting simultaneous use of synthetic cathinones with other stimulants may amplify toxicity and enhance the rate of fatality.
Peripheral monoamines, particularly norepinephrine (NE) and 5-HT, are important regulators of autonomic nervous system function (for review, see (Berger et al., 2009)) and disruption of these systems by stimulants frequently coincides with profound changes in peripheral organ systems, such as the cardiovascular and digestive systems. Studies in rat brain synaptosomes indicate mephedrone and methylone are NE transporter (NET) substrates while MDPV blocks NE reuptake through the NET, likely increasing extracellular NE (Baumann, Ayestas, 2012a, Baumann, Partilla, 2012b). Dysregulation of the NE and 5-HT systems by synthetic cathinones suggests these drugs affect peripheral autonomic organ systems. Though few studies have evaluated the peripheral effects of synthetic cathinones, mephedrone is known to significantly and acutely affect cardiovascular functions. Mephedrone has little effect upon cardiac ion channels or myocyte action potentials in whole cell in vitro patch clamp experiments at drug concentrations consistent with those found in the blood stream of abusers (Meng et al., 2012). However, mephedrone dose-dependently increases heart rate, stroke volume, cardiac output and cardiac contraction; effects consistent with sympathomimetic stimulation (Meng, Cao, 2012). Surprisingly, reserpine pretreatment, which prevents monoamine release by inhibiting their uptake into VMAT2 synaptic vesicles, has no effect upon the cardiovascular effects of mephedrone, indicating peripheral 5-HT or NE release is unlikely to be directly involved (Meng, Cao, 2012).
The synthetic cathinones, presumably through actions on the central monoamine systems, induce profound behavioral changes in animals. Mephedrone, methylone and MDPV all rapidly increase locomotor activity and stereotypy in a manner described as recurrent bouts of explosive activity separated by brief periods of rest (Angoa-Perez, Kane, 2012b, Baumann, Ayestas, 2012a, Baumann, Partilla, 2012b, den Hollander, Rozov, 2013, Fantegrossi et al., 2012, Kehr, Ichinose, 2011, Marusich et al., 2012, Wright, Angrish, 2012a). Stereotypic behaviors most frequently associated with the synthetic cathinones are head weaving, head circling, and stimulation (sudden muscle clenching and darting) (Marusich, Grant, 2012). Unfocused and frenetic movement is significantly increased by all synthetic cathinones, but voluntary locomotor activity is disparately affected by mephedrone and MDPV. Mephedrone dose-dependently decreases the intensity and duration of wheel running, whereas low doses of MDPV increase the intensity and duration of wheel running (Huang et al., 2012). Voluntary locomotor activity, specifically wheel running, is gated by DA D2 receptor activation within the striatum (Klinker et al., 2013). Consequently, activation or inhibition of these receptors by MDPV and mephedrone, respectively, is likely responsible for observed differences in voluntary locomotor activity.
Performance in complex motor and memory tasks are also affected by the synthetic cathinones, though few studies have been performed. Mephedrone dose-dependently degrades non-human primate performance on the rotating turntable and bimanual motor skill tasks, two measures of complex motor skill and procedural learning, shortly following drug exposure (Wright et al., 2012b). Despite the loss of fine motor skill, modest improvements in visuospatial learning and memory were observed in mephedrone treated animals (Wright, Vandewater, 2012b). Short-term gains in visuospatial memory may not persist over time or during chronic drug exposure, however. Multi-day mephedrone treatment impaired recall performance in mice tasked with the Morris Water Maze, a measure of long-term working memory(den Hollander, Rozov, 2013). Adolescent rats given multi-day mephedrone treatment failed to discriminate novel from familiar objects thirty-five days later, further indicating that mephedrone degrades long-term working memory (Motbey, Karanges, 2012). Many of these findings were recapitulated in humans. When intoxicated, mephedrone diminished performance in working memory tests but increased psychomotor skills, enhancing verbal and category fluency (Freeman, Morgan, 2012). Unfortunately, the long-term consequences of mephedrone use upon human memory and behavior are unknown.
Human and animal studies indicate significant abuse liability with the synthetic cathinones. Rodents self-administer mephedrone over saline (Aarde, Angrish, 2013, Hadlock, Webb, 2011, Shortall et al., 2012), discriminate mephedrone, and develop conditioned place preferences for the cage regions associated with drug infusions (Lisek et al., 2012). Further, mephedrone potentiates responding for intracranial self-stimulation in a dose-dependent fashion to a degree comparable with cocaine (Robinson et al., 2012). MDPV demonstrates similar degree of drug discrimination, self-administration and reduction in intracranial self-stimulation (Fantegrossi, Gannon, 2012, Watterson et al., 2012). These models of self-administration and place preference are thought to reflect the positive reinforcing, or rewarding, effects of drugs while discrimination is presumed to mimic the subjective effects of drugs in humans (Schuster and Johanson, 1988).
To the extent that the behavioral effects in animals reflect the subjective effects of synthetic cathinones in humans, mephedrone users report significantly increased desire and craving for the drug following use paired with the development of withdrawal effects and tolerance, affirming the significant abuse liability of these drugs (Freeman, Morgan, 2012, Winstock, Mitcheson, 2011). However, how synthetic cathinone abuse liability compares to other stimulants has only received limited investigation.
Human Toxicology
The clinical presentations of synthetic cathinone intoxicated humans coincide with the monoamine dysfunction observed in animal studies. Neurologic and cardiovascular sympathomimetic symptoms are most frequently associated with synthetic cathinone toxicity, which is not surprising given the effect these drugs have upon the monoamine systems. Agitation, paranoia, hallucinations, psychosis, myoclonus and headaches are the most frequent neurologic symptoms reported in patients experiencing synthetic cathinone toxicity (James, Adams, 2011, Kasick, McKnight, 2012, Spiller et al., 2011, Stoica and Felthous, 2013, Thornton et al., 2012). Synthetic cathinone hallucinations are frequently auditory and tactile in nature and paired with psychoses that can be severe and long lasting; many patients are admitted days after cessation of drug use and psychotic symptoms persist for several days thereafter while receiving treatment (Kasick, McKnight, 2012, Stoica and Felthous, 2013). The anxiolytic and antipsychotic drugs lorazepam, haloperidol, diazepam, and risperidone have been used alone or in combination to successfully treat synthetic cathinone-induced neurologic disorders (Kasick, McKnight, 2012, Mas-Morey et al., 2012, Stoica and Felthous, 2013). However, haloperidol should be used with caution since it may exacerbate hyperthermia and trigger the development of neuroleptic malignant syndrome (Mas-Morey, Visser, 2012, Shalev and Munitz, 1986).
Peripherally, the most common symptoms of synthetic cathinone toxicity include hyperthermia, hypertension, tachycardia, hyponatremia, nausea, vomiting and chest pains. The more serious symptoms of synthetic cathinone toxicity that require substantial and prolonged medical treatment, and in some cases lead to death, include liver failure, kidney failure, rhabdomyolysis, and the development of compartment syndrome (swelling in muscular fascia compartments) (Adebamiro and Perazella, 2012, Borek and Holstege, 2012, Levine, Levitan, 2013, Stoica and Felthous, 2013).
Acute drug toxicity is the leading cause of synthetic cathinone-induced fatality. Concomitant consumption of synthetic cathinones and other drugs have been reported in numerous fatalities (Aromatario et al., 2012, Marinetti and Antonides, 2013, Maskell et al., 2011, Schifano, Corkery, 2012). As discussed above, concurrent use of synthetic cathinones with other stimulants leads to significantly greater monoamine toxicity (Angoa-Perez, Kane, 2012a), which may underlie the high frequency of polydrug use fatality associated with the synthetic cathinones.
Of concern, self-harm and bizarre/at risk behavior without evidence of psychosis or depression comorbidity is the second leading cause of death associated with the synthetic cathinones. Hangings are the most common form of fatal self harm, though gunshots, self-stabbings, repeated self-lacerations including slitting of one's own throat, and jumping from bridges have all been reported (Marinetti and Antonides, 2013, Schifano, Corkery, 2012). Limited observations of self-harm, including self-biting stereotypy and excessive grooming, have occurred in rodent models (Fantegrossi, Gannon, 2012, Marusich, Grant, 2012), though the synthetic cathinones are not unique amongst the stimulants in causing self-mutilation (Shishido et al., 2000, Wagner et al., 2004). The prevalence of self-harm in synthetic cathinone fatalities and presence of self-mutilation in animal studies certainly warrants further investigation.
Designer Drugs and Legal Considerations
Mephedrone and MDPV were classified as schedule I drugs within the US under the Synthetic Drug Abuse Prevention Act of 2012 and inclusion of methylone, along with fourteen other synthetic cathinones not discussed here, are planned to be listed as schedule I substances in the Synthetic Cathinones Control Act of 2013. Mephedrone was controlled in the UK and throughout the European Union by December 2010. Scheduling and controlling the synthetic cathinones is warranted given their significant abuse potential, known psychostimulant and hallucinatory effects, and widespread involvement in human toxicity and fatality. Yet classifying the synthetic cathinones as schedule I substances without pre-clinical or human research to support this scheduling is problematic in many ways. The absence of a known and accepted medical use is a major distinction between schedule I (no accepted medical use) and schedule II (accepted medical use) drugs. Accepted medical use will certainly not exist if no research has been performed prior to drug classification, and restriction of research to a limited number of laboratories and institutions with schedule I licenses makes acquiring an accepted medical use difficult. Schedule I classification will reduce synthetic cathinone abuse and consumption, as has already occurred in the UK (Freeman, Morgan, 2012), but at the cost of hindering research into how these drugs exert their effects and how to best treat any dependence or toxicity that may arise from their abuse. Given their comparatively low toxicity to the central monoamine systems when taken alone, synthetic cathinones may be a useful alternative to amphetamines in treating disorders such as attention deficit hyperactivity disorder or treatment-resistant depression. Substantially more behavioral data are required to determine if any abuse liability and long-term behavioral changes associated with synthetic cathinone use outweigh potentially reduced monoamine toxicity. However, pursuing the studies necessary to develop legitimate synthetic cathinone clinical use is made difficult by schedule I status
Nevertheless, the cat-and-mouse game between legislation and clandestine laboratories continues, with new designer stimulants replacing those outlawed almost as soon as legislation passes. Evidence suggests production and consumption of mephedrone has declined since control and scheduling, but new groups of synthetic cathinones that do not fall under current legislation are already being distributed and abused (Brandt et al., 2011).
Conclusions
The synthetic cathinones are the latest in a long line of psychostimulant designer drugs, abused not only for their hedonic and euphoric effects but as a replacement for other tightly regulated stimulants (e.g., cocaine, MDMA, and other amphetamines) that are more expensive, more difficult to obtain and considered less pure. Purchased as bath salts or tablets from dealers, online distributors and head shops worldwide, the synthetic cathinones are insufflated, ingested orally, or injected in binge sessions that can last from several hours to days. Excessive consumption may lead to toxicity with severe neurologic and peripheral symptoms, including death.
The synthetic cathinones are a unique class of psychostimulant designer drugs that affect peripheral and central monoamine systems. Mephedrone and methylone act as transporter substrates, stimulating presynaptic monoamine release and blocking reuptake from the synaptic cleft, whereas MDPV functions as a monoamine reuptake blocker with no apparent effect upon release. The prolonged and increased presence of monoamines in the synaptic cleft likely underlies the stimulant, hedonic and hallucinatory effects of the synthetic cathinones as well as the acute and persistent deficits in the DA and 5-HT systems. Further, these drugs have significant abuse potential, exhibited by human consumption patterns as well as animal self-administration models. The basic pharmacology, toxicology, and central nervous system effects of the synthetic cathinones have been evaluated. In comparison to other stimulants, however, very little is known about the synaptic (i.e., vesicle release, vesicle reuptake, intracellular signals, extracellular receptors), systemic (i.e., changes in patterns of DA and 5-HT activity) or behavioral (i.e., positive reinforcement, negative reinforcement) mechanisms by which synthetic cathinones actually act. Substantially greater research into the consequences and potential benefits of synthetic cathinone use is therefore warranted.
Acknowledgements
This work was supported by grants DA00378, DA00869, DA31883, DA11389, DA13367, and DA19447 from the National Institute on Drug Abuse. We thank Dr. Michelle Baladi and Dr. Mary Pendergast for their critical reading of this manuscript.
Abbreviations
- METH
Methamphetamine
- MDMA
methylenedioxymethamphetamine
- MDPV
methylenedioxypyrovalerone
- mephedrone
4-methylmethcathinone
- methylone
3,4,-methylenedioxymethcathinone
- DA
dopamine
- 5-HT
serotonin
- DAT
dopamine transporter
- SERT
serotonin transporter
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
All authors declare no conflicts of interest
References
- Anti-Drug Abuse Act of 1986. United States of America. 1986 [Google Scholar]
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Jr, Vandewater SA, Creehan KM, et al. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addiction biology. 2013 doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addiction EMCfDaD. Report on the risk assessment of mephedrone in the framework of the Council Decision on new psychoactive substances. Luxembourg: The Publications Office of the European Union. 2011:193. [Google Scholar]
- Adebamiro A, Perazella MA. Recurrent acute kidney injury following bath salts intoxication. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;59:273–275. doi: 10.1053/j.ajkd.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Angoa-Perez M, Kane MJ, Briggs DI, Francescutti DM, Sykes CE, Shah MM, et al. Mephedrone does not damage dopamine nerve endings of the striatum, but enhances the neurotoxicity of methamphetamine, amphetamine, and MDMA. Journal of neurochemistry. 2012a doi: 10.1111/jnc.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Perez M, Kane MJ, Francescutti DM, Sykes KE, Shah MM, Mohammed AM, et al. Mephedrone, an abused psychoactive component of 'bath salts' and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. Journal of neurochemistry. 2012b;20:1097–1107. doi: 10.1111/j.1471-4159.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromatario M, Bottoni E, Santoni M, Ciallella C. New "lethal highs": a case of a deadly cocktail of GHB and Mephedrone. Forensic science international. 2012;223:e38–e41. doi: 10.1016/j.forsciint.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012a;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful Cocaine-Like Actions of 3,4-Methylenedioxypyrovalerone (MDPV), a Principal Constituent of Psychoactive 'Bath Salts' Products. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012b doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BBC. New 'legal high' arrives in area. British Broadcasting Corporation. 2009 [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annual review of medicine. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of "bath salts" containing 3,4-methylenedioxypyrovalerone. Annals of emergency medicine. 2012;60:103–105. doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Robinson B, Ali S, Schmued LC. Neurotoxic-related changes in tyrosine hydroxylase, microglia, myelin, and the blood-brain barrier in the caudate-putamen from acute methamphetamine exposure. Synapse. 2008;62:193–204. doi: 10.1002/syn.20478. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Freeman S, Sumnall HR, Measham F, Cole J. Analysis of NRG 'legal highs' in the UK: identification and formation of novel cathinones. Drug testing and analysis. 2011;3:569–575. doi: 10.1002/dta.204. [DOI] [PubMed] [Google Scholar]
- Brunt TM, Poortman A, Niesink RJ, van den Brink W. Instability of the ecstasy market and a new kid on the block:mephedrone. Journal of psychopharmacology. 2011;25:1543–1547. doi: 10.1177/0269881110378370. [DOI] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E, Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. British journal of pharmacology. 2012 doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, King LA, Nutt DJ. A web-based survey on mephedrone. Drug and alcohol dependence. 2011;118:19–22. doi: 10.1016/j.drugalcdep.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Centers AAoPC, editor. Centers AAoPC. Bath Salts Data. 2012. [Google Scholar]
- Connor CE, Kuczenski R. Evidence that amphetamine and Na+ gradient reversal increase striatal synaptosomal dopamine synthesis through carrier-mediated efflux of dopamine. Biochemical pharmacology. 1986;35:3123–3130. doi: 10.1016/0006-2952(86)90396-5. [DOI] [PubMed] [Google Scholar]
- Dargan PI, Albert S, Wood DM. Mephedrone use and associated adverse effects in school and college/university students before the UK legislation change. QJM : monthly journal of the Association of Physicians. 2010;103:875–879. doi: 10.1093/qjmed/hcq134. [DOI] [PubMed] [Google Scholar]
- Davies S, Wood DM, Smith G, Button J, Ramsey J, Archer R, et al. Purchasing 'legal highs' on the Internet--is there consistency in what you get? QJM : monthly journal of the Association of Physicians. 2010;103:489–493. doi: 10.1093/qjmed/hcq056. [DOI] [PubMed] [Google Scholar]
- den Hollander B, Rozov S, Linden AM, Uusi-Oukari M, Ojanpera I, Korpi ER. Long-term cognitive and neurochemical effects of "bath salt" designer drugs methylone and mephedrone. Pharmacology, biochemistry, and behavior. 2013;103:501–509. doi: 10.1016/j.pbb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration DoJ. Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Federal register. 2011;76:65371–65375. [PubMed] [Google Scholar]
- Emerson TS, Cisek JE. Methcathinone: a Russian designer amphetamine infiltrates the rural midwest. Annals of emergency medicine. 1993;22:1897–1903. doi: 10.1016/s0196-0644(05)80419-6. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo Effects of Abused 'Bath Salt' Constituent 3,4-methylenedioxypyrovalerone (MDPV) in Mice: Drug Discrimination, Thermoregulation, and Locomotor Activity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Beyeler ML, Gibb JW, Hanson GR. Oxygen radicals diminish dopamine transporter function in rat striatum. European journal of pharmacology. 1997a;334:111–114. doi: 10.1016/s0014-2999(97)01175-8. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annual review of pharmacology and toxicology. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Wilkins DG, Gibb JW, Hanson GR. Interaction between hyperthermia and oxygen radical formation in the 5-hydroxytryptaminergic response to a single methamphetamine administration. The Journal of pharmacology and experimental therapeutics. 1997b;283:281–285. [PubMed] [Google Scholar]
- Forrester MB. Adolescent synthetic cathinone exposures reported to Texas poison centers. Pediatric emergency care. 2013;29:151–155. doi: 10.1097/PEC.0b013e3182808ae2. [DOI] [PubMed] [Google Scholar]
- Freeman TP, Morgan CJ, Vaughn-Jones J, Hussain N, Karimi K, Curran HV. Cognitive and subjective effects of mephedrone and factors influencing use of a 'new legal high'. Addiction. 2012;107:792–800. doi: 10.1111/j.1360-0443.2011.03719.x. [DOI] [PubMed] [Google Scholar]
- Freudenmann RW, Oxler F, Bernschneider-Reif S. The origin of MDMA (ecstasy) revisited: the true story reconstructed from the original documents. Addiction. 2006;101:1241–1245. doi: 10.1111/j.1360-0443.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- G.m.b.H. BI. 1-(3',4'-methylenedioxy-phenyl)-2-pyrrolidino-alkanones-(1) In: Office USP, editor. United States; 1967. [Google Scholar]
- Gebissa E. Khat in the Horn of Africa: historical perspectives and current trends. Journal of ethnopharmacology. 2010;132:607–614. doi: 10.1016/j.jep.2010.01.063. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, et al. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. The Journal of pharmacology and experimental therapeutics. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GL. Designer drugs: past history and future prospects. Journal of forensic sciences. 1988;33:569–575. [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug and alcohol dependence. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob Peyton III ATS. Novel n-substituted-2-amino-3',4'-methylene-dioxypropiophenones. In: Office EP, editor. 1996. C07D317/66 ed. [Google Scholar]
- James D, Adams RD, Spears R, Cooper G, Lupton DJ, Thompson JP, et al. Clinical characteristics of mephedrone toxicity reported to the U.K. National Poisons Information Service. Emergency medicine journal : EMJ. 2011;28:686–689. doi: 10.1136/emj.2010.096636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, et al. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalix P. Cathinone, an alkaloid from khat leaves with an amphetamine-like releasing effect. Psychopharmacology. 1981;74:269–270. doi: 10.1007/BF00427108. [DOI] [PubMed] [Google Scholar]
- Kasick DP, McKnight CA, Klisovic E. "Bath salt" ingestion leading to severe intoxication delirium: two cases and a brief review of the emergence of mephedrone use. The American journal of drug and alcohol abuse. 2012;38:176–180. doi: 10.3109/00952990.2011.643999. [DOI] [PubMed] [Google Scholar]
- Kavanagh P, O'Brien J, Power JD, Talbot B, McDermott SD. 'Smoking' mephedrone: The identification of the pyrolysis products of 4-methylmethcathinone hydrochloride. Drug testing and analysis. 2012 doi: 10.1002/dta.1373. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, et al. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. British journal of pharmacology. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinker F, Hasan K, Paulus W, Nitsche MA, Liebetanz D. Pharmacological blockade and genetic absence of the dopamine D2 receptor specifically modulate voluntary locomotor activity in mice. Behavioural brain research. 2013;242C:117–124. doi: 10.1016/j.bbr.2012.12.038. [DOI] [PubMed] [Google Scholar]
- Kram TC, Cooper DA, Allen AC. Behind the identification of China White. Analytical chemistry. 1981;53:1379A–1386A. doi: 10.1021/ac00235a003. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Levitan R, Skolnik A. Compartment Syndrome After "Bath Salts" Use: A Case Series. Annals of emergency medicine. 2013 doi: 10.1016/j.annemergmed.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, et al. Mephedrone ('bath salt') elicits conditioned place preference and dopamine-sensitive motor activation. Drug and alcohol dependence. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. British journal of pharmacology. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti LJ, Antonides HM. Analysis of Synthetic Cathinones Commonly Found in Bath Salts in Human Performance and Postmortem Toxicology: Method Development, Drug Distribution and Interpretation of Results. Journal of analytical toxicology. 2013 doi: 10.1093/jat/bks136. [DOI] [PubMed] [Google Scholar]
- Martinez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2012;22:231–236. doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in "bath salts" on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas-Morey P, Visser MH, Winkelmolen L, Touw DJ. Clinical Toxicology and Management of Intoxications With Synthetic Cathinones ("Bath Salts") Journal of pharmacy practice. 2012 doi: 10.1177/0897190012465949. [DOI] [PubMed] [Google Scholar]
- Maskell PD, De Paoli G, Seneviratne C, Pounder DJ. Mephedrone (4-methylmethcathinone)-related deaths. Journal of analytical toxicology. 2011;35:188–191. doi: 10.1093/anatox/35.3.188. [DOI] [PubMed] [Google Scholar]
- Meng H, Cao J, Kang J, Ying X, Ji J, Reynolds W, et al. Mephedrone, a new designer drug of abuse, produces acute hemodynamic effects in the rat. Toxicology letters. 2012;208:62–68. doi: 10.1016/j.toxlet.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Miller ML, Creehan KM, Angrish D, Barlow DJ, Houseknecht KL, Dickerson TJ, et al. Changes in ambient temperature differentially alter the thermoregulatory, cardiac and locomotor stimulant effects of 4-methylmethcathinone (mephedrone) Drug and alcohol dependence. 2013;127:248–253. doi: 10.1016/j.drugalcdep.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H. Mephedrone: The Phantom Menace. Vice. 2010:98–100. [Google Scholar]
- Motbey CP, Karanges E, Li KM, Wilkinson S, Winstock AR, Ramsay J, et al. Mephedrone in adolescent rats: residual memory impairment and acute but not lasting 5-HT depletion. PloS one. 2012;7:e45473. doi: 10.1371/journal.pone.0045473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JE, Agoglia AE, Fish EW, Krouse MC, Malanga CJ. Mephedrone (4-methylmethcathinone) and intracranial self-stimulation in C57BL/6J mice: comparison to cocaine. Behavioural brain research. 2012;234:76–81. doi: 10.1016/j.bbr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA. Psychoactive "bath salts" intoxication with methylenedioxypyrovalerone. The American journal of medicine. 2012;125:854–858. doi: 10.1016/j.amjmed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Sanchez SdB. Sur un homologue de l'ephedrine. Bulletin de la Société Chimique de France. 1929 [Google Scholar]
- Schifano F, Corkery J, Ghodse AH. Suspected and confirmed fatalities associated with mephedrone (4-methylmethcathinone, "meow meow") in the United Kingdom. Journal of clinical psychopharmacology. 2012;32:710–714. doi: 10.1097/JCP.0b013e318266c70c. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE. Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacology series. 1988;4:161–175. doi: 10.1007/978-3-642-73223-2_13. [DOI] [PubMed] [Google Scholar]
- Shalev A, Munitz H. The neuroleptic malignant syndrome: agent and host interaction. Acta psychiatrica Scandinavica. 1986;73:337–347. doi: 10.1111/j.1600-0447.1986.tb02694.x. [DOI] [PubMed] [Google Scholar]
- Shishido T, Watanabe Y, Kato K, Horikoshi R, Niwa SI. Effects of dopamine, NMDA, opiate, and serotonin-related agents on acute methamphetamine-induced self-injurious behavior in mice. Pharmacology, biochemistry, and behavior. 2000;66:579–583. doi: 10.1016/s0091-3057(00)00219-7. [DOI] [PubMed] [Google Scholar]
- Shortall SE, Macerola AE, Swaby RT, Jayson R, Korsah C, Pillidge KE, et al. Behavioural and neurochemical comparison of chronic intermittent cathinone, mephedrone and MDMA administration to the rat. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2012 doi: 10.1016/j.euroneuro.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of "bath salts" and "legal highs" (synthetic cathinones) in the United States. Clinical toxicology. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Stoica MV, Felthous AR. Acute Psychosis Induced by Bath Salts: A Case Report with Clinical and Forensic Implications. Journal of forensic sciences. 2013 doi: 10.1111/1556-4029.12038. [DOI] [PubMed] [Google Scholar]
- Services NH, editor. Statistics on Drug Misuse: England. England: 2011. The NHS Information Centre LS. 2011. [Google Scholar]
- Thornton SL, Gerona RR, Tomaszewski CA. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. Journal of medical toxicology : official journal of the American College of Medical Toxicology. 2012;8:310–313. doi: 10.1007/s13181-012-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Drug Enforcement Administration OoDC. Administration USDE, editor. National Forensic Laboratory Information System Special Report: Synthetic Cannabinoids and Synthetic Cathinones Reported in NFLIS, 2009–2010. 2011
- U.S. Drug Enforcement Administration OoDC. National Forensic Laboratory Information System: Year 2011 Annual Report. In: Administration USDE, editor. Springfield, VA: 2012. [Google Scholar]
- Wagner GC, Avena N, Kita T, Nakashima T, Fisher H, Halladay AK. Risperidone reduction of amphetamine-induced self-injurious behavior in mice. Neuropharmacology. 2004;46:700–708. doi: 10.1016/j.neuropharm.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, et al. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addiction biology. 2012 doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand TJ, Wax PM, Schwartz T, Finkelstein Y, Gorodetsky R, Brent J, et al. The Toxicology Investigators Consortium Case Registry--the 2011 experience. Journal of medical toxicology : official journal of the American College of Medical Toxicology. 2012;8:360–377. doi: 10.1007/s13181-012-0264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction. 2011;106:1991–1996. doi: 10.1111/j.1360-0443.2011.03502.x. [DOI] [PubMed] [Google Scholar]
- Wood DM, Hunter L, Measham F, Dargan PI. Limited use of novel psychoactive substances in South London nightclubs. QJM : monthly journal of the Association of Physicians. 2012;105:959–964. doi: 10.1093/qjmed/hcs107. [DOI] [PubMed] [Google Scholar]
- Wood DM, Stribley V, Dargan PI, Davies S, Holt DW, Ramsey J. Variability in the 3,4-methylenedioxymethamphetamine content of 'ecstasy' tablets in the UK. Emergency medicine journal : EMJ. 2011;28:764–765. doi: 10.1136/emj.2010.092270. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, et al. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PloS one. 2012a;7:e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Jr, Vandewater SA, Angrish D, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) and d-methamphetamine improve visuospatial associative memory, but not spatial working memory, in rhesus macaques. British journal of pharmacology. 2012b;167:1342–1352. doi: 10.1111/j.1476-5381.2012.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziporyn T. A growing industry and menace: makeshift laboratory's designer drugs. JAMA : the journal of the American Medical Association. 1986;256:3061–3063. [PubMed] [Google Scholar]