Abstract
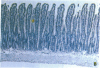
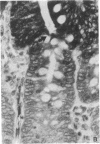
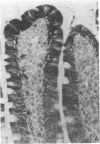
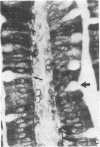
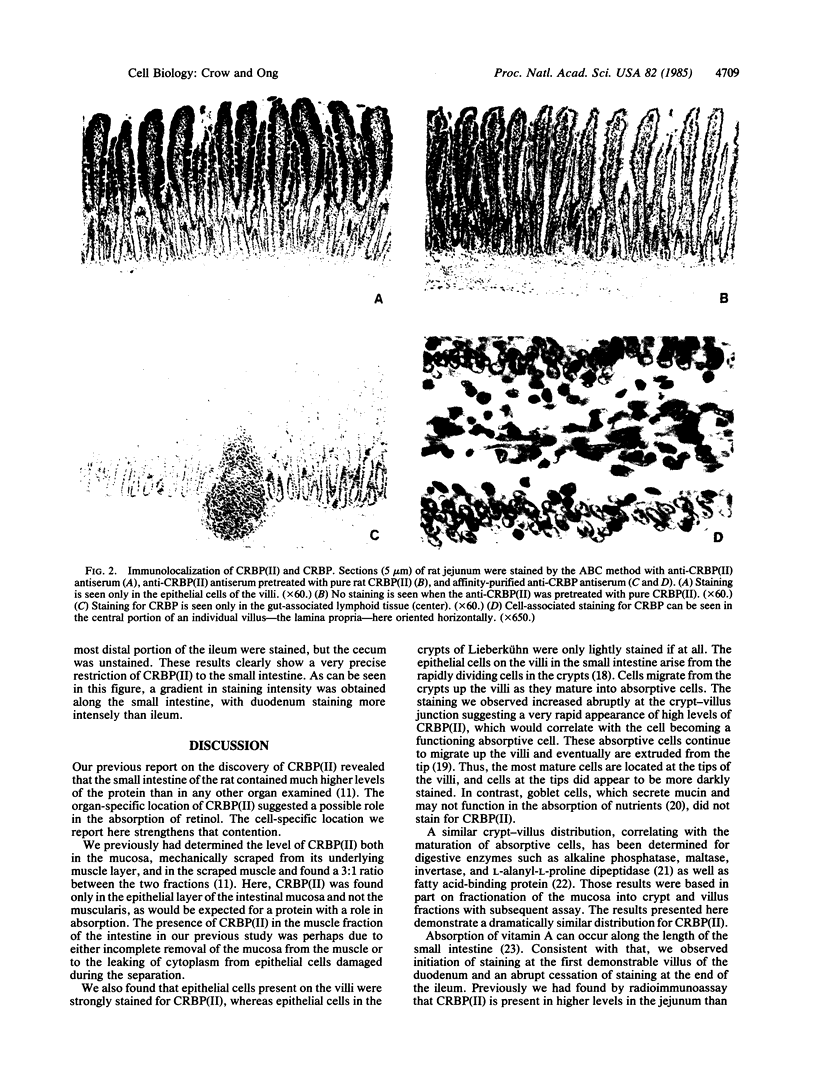
One of us recently has reported the purification of a new retinol-binding protein that is distinctly different from the well-known cellular retinol-binding protein, CRBP. This protein, which we propose to name cellular retinol-binding protein type II [CRBP(II)], was found almost exclusively in the small intestine of the adult rat at levels 1000 times greater than that of CRBP. Here we have determined the cellular location of these two proteins in the small intestine of the rat. By using an immunohistochemical technique, the absorptive cells of the small intestine, from the duodenum to the ileum, were strongly stained when antiserum against CRBP(II) was used. More intense staining was observed in absorptive cells near the tips of the villi than in those located at the base of the villi. However, the proliferative cells in the crypts of Lieberkühn were stained only lightly if at all. In contrast to absorptive cells, goblet cells in the villi did not stain. When tissue sections containing the gastroduodenal junction were examined, no staining was observed in the gastric epithelium, while the epithelium of the most proximal portion of the duodenum showed very strong staining. In tissue sections containing the ileocecal junction, staining terminated abruptly at the end of the distal ileum. No staining was observed in the epithelium of the colon. In contrast, the cellular location of CRBP in the small intestine was quite different from the cellular location of CRBP(II). The epithelial cells of the small intestine showed no staining when affinity-purified anti-CRBP was used. Staining was observed for connective tissue cells in the lamina propria and in cells located within the gut-associated lymphoid tissue. The cell-specific localization pattern determined for these two proteins suggests that CRBP(II), rather than CRBP, is the protein that plays a role in the absorption of retinol.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi N., Smith J. E., Sklan D., Goodman D. S. Radioimmunoassay studies of the tissue distribution and subcellular localization of cellular retinol-binding protein in rats. J Biol Chem. 1981 Sep 25;256(18):9471–9476. [PubMed] [Google Scholar]
- Adler A. J., Martin K. J. Retinol-binding proteins in bovine interphotoreceptor matrix. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1601–1608. doi: 10.1016/s0006-291x(82)80091-0. [DOI] [PubMed] [Google Scholar]
- Bashor M. M., Toft D. O., Chytil F. In vitro binding of retinol to rat-tissue components. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3483–3487. doi: 10.1073/pnas.70.12.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D., Ong D. E., Chytil F. Immunocytochemical localization of cellular retinol binding protein in the rat retina. Invest Ophthalmol Vis Sci. 1984 Aug;25(8):877–883. [PubMed] [Google Scholar]
- Eriksson U., Das K., Busch C., Nordlinder H., Rask L., Sundelin J., Sällström J., Peterson P. A. Cellular retinol-binding protein. Quantitation and distribution. J Biol Chem. 1984 Nov 10;259(21):13464–13470. [PubMed] [Google Scholar]
- Futterman S., Saari J. C., Blair S. Occurrence of a binding protein for 11-cis-retinal in retina. J Biol Chem. 1977 May 25;252(10):3267–3271. [PubMed] [Google Scholar]
- Hollander D. Intestinal absorption of vitamins A, E, D, and K. J Lab Clin Med. 1981 Apr;97(4):449–462. [PubMed] [Google Scholar]
- Hollander D., Muralidhara K. S. Vitamin A1 intestinal absorption in vivo: influence of luminal factors on transport. Am J Physiol. 1977 May;232(5):E471–E477. doi: 10.1152/ajpendo.1977.232.5.E471. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kanai M., Raz A., Goodman D. S. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968 Sep;47(9):2025–2044. doi: 10.1172/JCI105889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBLOND C. P., MESSIER B. Renewal of chief cells and goblet cells in the small intestine as shown by radioautography after injection of thymidine-H3 into mice. Anat Rec. 1958 Nov;132(3):247–259. doi: 10.1002/ar.1091320303. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai Y. L., Wiggert B., Liu Y. P., Chader G. J. Interphotoreceptor retinol-binding proteins: possible transport vehicles between compartments of the retina. Nature. 1982 Aug 26;298(5877):848–849. doi: 10.1038/298848a0. [DOI] [PubMed] [Google Scholar]
- Liou G. I., Bridges C. D., Fong S. L., Alvarez R. A., Gonzalez-Fernandez F. Vitamin A transport between retina and pigment epithelium--an interstitial protein carrying endogenous retinol (interstitial retinol-binding protein). Vision Res. 1982;22(12):1457–1467. doi: 10.1016/0042-6989(82)90210-3. [DOI] [PubMed] [Google Scholar]
- Nordström C., Dahlqvist A., Josefsson L. Quantitative determination of enzymes in different parts of the villi and crypts of rat small intestine. Comparison of alkaline phosphatase, disaccharidases and dipepeptidases. J Histochem Cytochem. 1967 Dec;15(12):713–721. doi: 10.1177/15.12.713. [DOI] [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A. Fatty acid-binding protein in small intestine. Identification, isolation, and evidence for its role in cellular fatty acid transport. J Clin Invest. 1974 Aug;54(2):326–338. doi: 10.1172/JCI107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong D. E. A novel retinol-binding protein from rat. Purification and partial characterization. J Biol Chem. 1984 Feb 10;259(3):1476–1482. [PubMed] [Google Scholar]
- Ong D. E., Chytil F. Retinoic acid-binding protein in rat tissue. Partial purification and comparison to rat tissue retinol-binding protein. J Biol Chem. 1975 Aug 10;250(15):6113–6117. [PubMed] [Google Scholar]
- Ong D. E., Crow J. A., Chytil F. Radioimmunochemical determination of cellular retinol- and cellular retinoic acid-binding proteins in cytosols of rat tissues. J Biol Chem. 1982 Nov 25;257(22):13385–13389. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Smith J. E., Goodman D. S. Retinol-binding protein and the regulation of vitamin A transport. Fed Proc. 1979 Oct;38(11):2504–2509. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]