Abstract
Giardial diarrhea in a birth cohort of 452 children in an urban slum in South India was characterized. Of the 155 episodes that occurred in 99 children, 73% were acute diarrhea. Children with better educated mothers and a toilet at home had lower odds of acquiring giardial diarrhea, whereas low socioeconomic status and drinking municipal water were associated with greater risk. Children with co-infections tended to have a slightly longer duration of diarrhea (P = 0.061) and showed significantly more wasting after an episode than children with diarrhea resulting from Giardia alone (P = 0.032). Among the 99 cases, 50 diarrheal and 51 asymptomatic Giardia positive samples were genotyped by polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) at the triose phosphate isomerase gene. Assemblage B was predominant both in giardial diarrhea (80%) and asymptomatic giardiasis (94%). Children with Assemblage A subgroup-II alone or dual infections with both assemblage A and B had diarrhea more frequently (P = 0.07).
Giardia duodenalis, a common intestinal protozoan parasite causes infections that range from asymptomatic cyst passage and acute diarrhea to a syndrome of chronic diarrhea, weight loss, and malabsorption.1 In children in developing countries, giardiasis is associated with stunting and malnutrition and documented to have adverse effects on success at school and cognitive function.2 Molecular characterization of Giardia from humans and animals has been carried out at several loci, including glutamate dehydrogenase (gdh),3 β-giardin,4 small subunit ribosomal RNA (SSU rRNA),5 and the triosephosphate isomerase (tpi or tim)6 genes. These studies have shown that the two major genotypes or assemblages causing human infections are assemblages A and B. In India, reports on the prevalence of giardiasis in children range from 2.67 to 32%.8 In this study, we aimed to describe endemic giardiasis in a birth cohort of children from an urban slum community in Vellore, South India, and identify the prevalent giardial assemblages in the community.
The 452 children in the birth cohort9 were originally recruited for studies on rotaviral10,11 and cryptosporidial diarrhea12 and were followed up twice-weekly up to the age of 3 years. The study setting comprised of three adjacent urban slums in Vellore with an area of around 2.2 km2 and population of ~33,390. Children with diarrhea were assessed clinically, and details of the number of stools passed per day, any associated fever or vomiting, and treatment given was recorded daily until the cessation of diarrhea. Demographic details were recorded at the start of the study. Each stool sample was given a unique identification number and stored in aliquots at −70°C and 4°C. All diarrheal stool samples were screened for enteric parasites, including Giardia cysts and trophozoites by direct wet mount microscopic examination of fresh stool samples. In addition, modified acid fast staining was carried out to identify Cryptosporidium spp. Bacterial enteric pathogens, including Salmonella spp., Shigella spp., Vibrio cholerae, Aeromonas spp., and enteropathogenic E.coli, were identified by stool culture on blood agar and selective media, including sorbitol MacConkey agar, xylose lysine desoxycholate agar, and thiosulphate citrate bile salt sucrose agar. Rotavirus was screened for by enzyme-linked immunosorbent assay (ELISA) (Dako IDEIA, UK) and sapovirus by a hemi-nested polymerase chain reaction (PCR).13 Data generated during this study were double entered using Epi Info 2000 software (CDC, Atlanta, GA) and analyzed using STATA version 9.0 (Stata Corp., College Station, TX). Statistical comparisons were made using Fisher’s exact and χ2 tests for categoric variables and Wilcoxon signed-rank test for continuous variables. A univariate logistic regression was carried out for risk factor analysis and odds ratios (OR) (with 95% confidence interval [CI]) calculated.
For this study, an episode of giardial diarrhea was defined as at least 1 day of diarrhea (three loose stools in a 24 hour period) followed by at least 2 days without diarrhea.14 Acute diarrhea was defined as fewer than 4 days and persistent diarrhea as more than 14 days. Children with diarrhea more than 3 days but fewer than 14 were described as indeterminate. A relapse of giardial diarrhea was defined as a second episode commencing between 2 and 7 days after the conclusion of the original Giardia diarrhea, and a recurrence was defined as infection occurring more than 7 days after the initial Giardia diarrhea.14 The study was approved by the Christian Medical College, Vellore Institutional Review Board, and informed consent was obtained from the parents of these children.
During the follow-up, almost 2,000 diarrheal episodes were studied between January 2002 and April 2006. There were 155 episodes of giardial diarrhea in 99 children, 53 of whom were male. The mean (SD) age at first episode was 19.34 (7.89) months. The characteristics of giardial diarrhea among children in this slum community have been described in Table 1. Although no child had persistent diarrhea, 1 child had diarrhea for 9 days and 5 children had diarrhea for 8 days. Clinical features, severity of diarrhea, and anthropometric measurements taken after the episode were compared between only Giardia-associated episodes and 36 episodes of Giardia co-infection with other enteric pathogens, including rotavirus (25%), sapovirus (11.1%), Cryptosporidium spp. (16.7%), Vibrio cholerae (11.1%), Shigella spp. (8.3%), Salmonella spp. (8.3%), enteropathogenic E. coli (2.8%), and Aeromonas spp. (30.6%) (Table 2). The World Health Organization (WHO) growth reference curves were used to interpret anthropometric data and calculate Z-scores.15 To identify the risk factors associated with acquiring giardial diarrhea, we also compared sociodemographic data between 99 children with at least one episode of giardial diarrhea with the remaining 353 children in the birth cohort. Although sex, low birth weight, maternal age at birth, family size, number of siblings, and presence of domestic animals or pets in the household were not associated with an increased OR of giardial diarrhea, low socioeconomic status (OR, 95% CI) (1.62, 1.00–2.63) and drinking municipal water (1.69, 0.20–14.24) were associated with giardial diarrhea. Better maternal education (0.57, 0.33–1.01 for grades 1–5 and 0.31, 0.15–0.67 for grades 9 and above) and presence of a toilet at home (0.61, 0.35–1.08) were found to be associated with protection from giardial diarrhea, whereas education of the head of the household, duration of breast feeding, and hand washing were not protective.
Table 1.
Characteristics of Giardial diarrhea among children in a community birth cohort in South India
| Features of Giardial diarrhea | N = 155 episodes |
|---|---|
| Acute (< 4 days) | 113 (72.9%) |
| Indeterminate (4–14 days) | 42 (27.1%) |
| Persistent (> 14 days) | 0 |
| Average duration of diarrhea (IQR) | 2 days (2–4 days) |
| Maximum duration of diarrhea | 9 days |
| N = 99 cases | |
| Multiple episodes | 34 cases |
| Maximum number of episodes | 5 episodes |
| Relapses | 12 (7.7%) |
| Recurrences | 44 (28.4%) |
| Median interval between recurrent episodes (IQR) | 125 days (58–167 days) |
IQR = interquartile range.
Table 2.
Comparison of clinical features in children with Giardia diarrhea with children co-infected Giardia and other enteric pathogens
| Giardia with coinfection N = 36 | Giardia alone N = 119 | P value* | |
|---|---|---|---|
| Median duration of diarrhea (IQR) | 3 (2–4) | 2 (1–3) | 0.061 |
| Acute diarrheal episodes | 22 | 91 | 0.069 |
| Severity of diarrhea (maximum number of stools/day) | |||
| 1–3 episodes/day | 8 | 19 | |
| 4–5 episodes/day | 6 | 24 | 0.645 |
| ≥ 6 episodes/day | 20 | 74 | |
| Malnutrition† | |||
| Stunted | 26 | 70 | 0.173 |
| Wasted | 12 | 19 | 0.032 |
| Underweight | 20 | 49 | 0.180 |
| Associated fever‡ | 6 | 15 | 0.573 |
| Associated vomiting‡ | 4 | 14 | 1.000 |
Fisher’s exact test was used for all analysis, except comparison of the median duration of diarrhea for which the Wilcoxon signed-rank test was used.
Children with height-for-age (HAZ), weight-for-age (WAZ), and weight-for-height (WHZ) scores less than −2 were categorized as stunted, underweight, and wasted, respectively.
Clinical data not available for 2 children with co-infection and for 2 children without co-infection.
IQR = interquartile range.
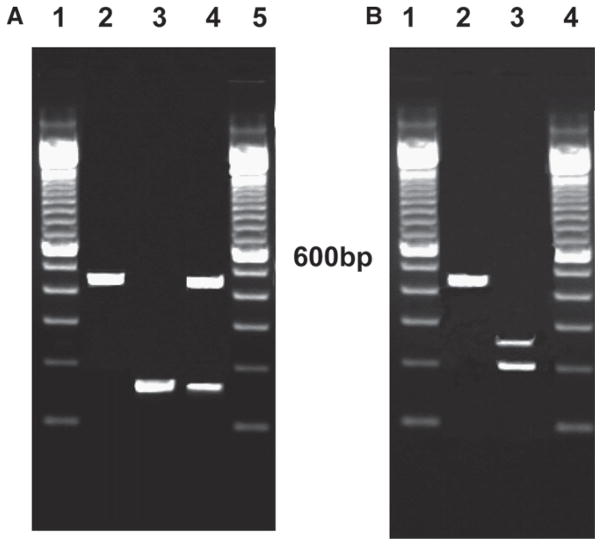
To identify the prevalent assemblages in the community, DNA was extracted from 50 randomly selected cases with giardial diarrhea with the QIAamp stool DNA minikit (Qiagen Inc., Valencia, CA). Previously published primers and protocols were used for detection of assemblages A and B at the tpi locus by a nested PCR6 with a minor modification of adding 5% DMSO to the PCR reaction as described by other workers for this GC-rich gene.16 The nested tpi PCR showed a 100% correlation with microscopy. This was followed by restriction fragment length polymorphism (RFLP) with RsaI to identify assemblage A subgroups (Figure 1). As suggested by other workers,3 the PCR-RFLP was validated by sequence analysis of one fecal sample classified as assemblage A. There was > 98% sequence identity with the Genbank accession number U57897, assemblage A group II.17 We also included as controls, 51 children who had asymptomatic giardial infections diagnosed by stool microscopy. These children lived in the same urban slum area as the children with giardial diarrhea, but only 5 belonged to the birth cohort. The remaining 46 samples were collected from among children attending the primary health center in the study area for illnesses not related to the gastrointestinal tract between March and July 2008. Informed consent was obtained from the parent.
Figure 1.
A, Nested PCR at the tpi locus for identification of Giardia assemblages (Lane 1 and 5—molecular weight marker; Lane 2—assemblage A; Lane 3—assemblage B; Lane 4—coinfection of assemblage A and B). B, TpiA RFLP with RsaI for assemblage A (Lane 1 and 4—molecular weight marker; Lane 2—assemblage A–I; Lane 3—assemblage A–II).
A majority of infections among the diarrheal and asymptomatic samples genotyped were a result of assemblage B (80% and 94%), corresponding to the findings of previous studies in the region, including India,16 Bangladesh,18 Philippines,19 and also from Europe3,20 and Brazil.21 Among the remaining diarrheal samples, 5 were assemblage A and by RFLP analysis, belonged to subgroup II, an anthroponotic subgroup and 5 had a dual infection with assemblages A and B as previously reported elsewhere.6 Among the dual infections, only one assemblage A isolate could be genotyped by RFLP, and this also belonged to group II. In the asymptomatic children, only 2/51 children were infected with assemblage A-II and 1 with both assemblages B- and A-II. When both single and dual infections were considered, children with Assemblage A infection (N = 13) tended to have diarrhea more frequently (N = 10) than not (N = 3) (P = 0.074), but the association was not as strong as demonstrated by Haque and others.18 There was no difference in features of diarrhea like severity (measured as maximum number of stools per day), dehydration, duration or malnutrition after diarrhea or associated symptoms, such as vomiting and fever between children with assemblage A and B infection. Previous reports on association of a particular genotype with diarrhea have been conflicting, with some reports suggesting an increased odds of diarrhea for assemblage A,18,22–24 whereas others have shown a correlation of symptoms,25,26 increased oocyst shedding,21 and persistence of infection with assemblage B.25 Two previous studies have however, reported no association of genotype with symptoms.21,27
This pilot study provides information on the characteristics of endemic giardial diarrhea in children in the community in South India, and is the first to document the common assemblages causing symptomatic and asymptomatic infections in the community. The prevalence of assemblage B and the occurrence of only subgroup A II indicate an anthroponotic transmission cycle as has been seen in other countries in Asia19 and the Indian subcontinent.18 However, the role of domestic animals and livestock as a potential source of infection for humans in the community also needs to be researched. Larger studies with asymptomatic and symptomatic cases followed up longitudinally along with glutamate dehydrogenase (gdh) gene analysis to subtype assemblage B isolates could lead to better knowledge of the association of these assemblages with diarrhea in children.
Acknowledgments
We thank Corrine Amar at the Health Protection Agency Centre for Infections, Colindale, UK and Stephanie Johnston at the Division of Parasitic Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, for generously providing DNA samples and protocols during standardization of the tpi PCR-RFLP.
Financial Support: This work was supported by the Fogarty International Research Cooperative Agreement, National Institutes of Health grant FIC R03TW2711, the Global Infectious Disease Research training award D43 TW007392, and the Fogarty International Clinical Research Scholars Programs.
References
- 1.Ali SA, Hill DR. Giardia intestinalis. Curr Opin Infect Dis. 2003;16:453–460. doi: 10.1097/00001432-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mekhlafi MS, Azlin M, Nor Aini U, Shaik A, Sa’iah A, Fatmah MS, Ismail MG, Ahmad Firdaus MS, Aisah MY, Rozlida AR, Norhayati M. Giardiasis as a predictor of childhood malnutrition in Orang Asli children in Malaysia. Trans R Soc Trop Med Hyg. 2005;99:686–691. doi: 10.1016/j.trstmh.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Bertrand I, Albertini L, Schwartzbrod J. Comparison of two target genes for detection and genotyping of Giardia lamblia in human feces by PCR and PCR-restriction fragment length polymorphism. J Clin Microbiol. 2005;43:5940–5944. doi: 10.1128/JCM.43.12.5940-5944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lalle M, Pozio E, Capelli G, Bruschi F, Crotti D, Caccio SM. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardiaduodenalis and identification of potentially zoonotic subgenotypes. Int J Parasitol. 2005;35:207–213. doi: 10.1016/j.ijpara.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Traub R, Wade S, Read C, Thompson A, Mohammed H. Molecular characterization of potentially zoonotic isolates of Giardia duodenalis in horses. Vet Parasitol. 2005;130:317–321. doi: 10.1016/j.vetpar.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Amar CF, Dear PH, Pedraza-Diaz S, Looker N, Linnane E, McLauchlin J. Sensitive PCR-restriction fragment length polymorphism assay for detection and genotyping of Giardia duodenalis in human feces. J Clin Microbiol. 2002;40:446–452. doi: 10.1128/JCM.40.2.446-452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee BD, Thawani G, Sanyal SN. Etiology of acute childhood diarrhoea in Calcutta. Trop Gastroenterol. 1989;10:158–166. [PubMed] [Google Scholar]
- 8.Awasthi S, Pande VK. Prevalence of malnutrition and intestinal parasites in preschool slum children in Lucknow. Indian Pediatr. 1997;34:599–605. [PubMed] [Google Scholar]
- 9.Gladstone BP, Muliyil J, Jaffar S, Wheeler JG, Lefevre AM, Iturriza-Gomara M, Gray JJ, Bose A, Estes MK, Brown DW, Kang G. Infant morbidity in an Indian slum birth cohort. Arch Dis Child. 2007;00:479–484. doi: 10.1136/adc.2006.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee I, Ramani S, Primrose B, Moses P, Iturriza-Gomara M, Gray JJ, Jaffar S, Monica B, Muliyil JP, Brown DW, Estes MK, Kang G. Comparative study of the epidemiology of rota-virus in children from a community-based birth cohort and a hospital in South India. J Clin Microbiol. 2006;44:2468–2474. doi: 10.1128/JCM.01882-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee I, Iturriza-Gomara M, Rajendran P, Primrose B, Ramani S, Gray JJ, Brown DW, Kang G. Molecular characterization of G11P[25] and G3P[3] human rotavirus strains associated with asymptomatic infection in South India. J Med Virol. 2007;79:1768–1774. doi: 10.1002/jmv.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajjampur SS, Gladstone BP, Selvapandian D, Muliyil JP, Ward H, Kang G. Molecular and spatial epidemiology of cryptosporidiosis in children in a semiurban community in South India. J Clin Microbiol. 2007;45:915–920. doi: 10.1128/JCM.01590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monica B, Ramani S, Banerjee I, Primrose B, Iturriza-Gomara M, Gallimore CI, Brown DW, Fathima M, Moses PD, Gray JJ, Kang G. Human caliciviruses in symptomatic and asymptomatic infections in children in Vellore, South India. J Med Virol. 2007;79:544–551. doi: 10.1002/jmv.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman RD, Moore SR, Lima AA, Nataro JP, Guerrant RL, Sears CL. A longitudinal study of Giardia lamblia infection in north-east Brazilian children. Trop Med Int Health. 2001;6:624–634. doi: 10.1046/j.1365-3156.2001.00757.x. [DOI] [PubMed] [Google Scholar]
- 15.WHO. Use and interpretation of anthropometric indicators of nutritional status. Report of a WHO working group. Bull World Health Organ. 1986;64:929–941. [PMC free article] [PubMed] [Google Scholar]
- 16.Sulaiman IM, Fayer R, Bern C, Gilman RH, Trout JM, Schantz PM, Das P, Lal AA, Xiao L. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg Infect Dis. 2003;9:1444–1452. doi: 10.3201/eid0911.030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baruch AC, Isaac-Renton J, Adam RD. The molecular epidemiology of Giardia lamblia: a sequence-based approach. J Infect Dis. 1996;174:233–236. doi: 10.1093/infdis/174.1.233. [DOI] [PubMed] [Google Scholar]
- 18.Haque R, Roy S, Kabir M, Stroup SE, Mondal D, Houpt ER. Giardia assemblage A infection and diarrhea in Bangladesh. J Infect Dis. 2005;192:2171–2173. doi: 10.1086/498169. [DOI] [PubMed] [Google Scholar]
- 19.Yason JA, Rivera WL. Genotyping of Giardia duodenalis isolates among residents of slum area in Manila, Philippines. Parasitol Res. 2007;101:681–687. doi: 10.1007/s00436-007-0533-8. [DOI] [PubMed] [Google Scholar]
- 20.Amar CF, Dear PH, McLauchlin J. Detection and genotyping by real-time PCR/RFLP analyses of Giardia duodenalis from human feces. J Med Microbiol. 2003;52:681–683. doi: 10.1099/jmm.0.05193-0. [DOI] [PubMed] [Google Scholar]
- 21.Kohli A, Bushen OY, Pinkerton RC, Houpt E, Newman RD, Sears CL, Lima AA, Guerrant RL. Giardia duodenalis assemblage, clinical presentation and markers of intestinal inflammation in Brazilian children. Trans R Soc Trop Med Hyg. 2008;102:718–725. doi: 10.1016/j.trstmh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aydin AF, Besirbellioglu BA, Avci IY, Tanyuksel M, Araz E, Pahsa A. Classification of Giardia duodenalis parasites in Turkey into groups A and B using restriction fragment length polymorphism. Diagn Microbiol Infect Dis. 2004;50:147–151. doi: 10.1016/j.diagmicrobio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Read C, Walters J, Robertson ID, Thompson RC. Correlation between genotype of Giardia duodenalis and diarrhoea. Int J Parasitol. 2002;32:229–231. doi: 10.1016/s0020-7519(01)00340-x. [DOI] [PubMed] [Google Scholar]
- 24.Sahagun J, Clavel A, Goni P, Seral C, Llorente MT, Castillo FJ, Capilla S, Arias A, Gomez-Lus R. Correlation between the presence of symptoms and the Giardia duodenalis genotype. Eur J Clin Microbiol Infect Dis. 2008;27:81–83. doi: 10.1007/s10096-007-0404-3. [DOI] [PubMed] [Google Scholar]
- 25.Homan WL, Mank TG. Human giardiasis: genotype linked differences in clinical symptomatology. Int J Parasitol. 2001;31:822–826. doi: 10.1016/s0020-7519(01)00183-7. [DOI] [PubMed] [Google Scholar]
- 26.Gelanew T, Lalle M, Hailu A, Pozio E, Caccio SM. Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Trop. 2007;102:92–99. doi: 10.1016/j.actatropica.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Eligio-Garcia L, Cortes-Campos A, Jimenez-Cardoso E. Genotype of Giardia intestinalis isolates from children and dogs and its relationship to host origin. Parasitol Res. 2005;97:1–6. doi: 10.1007/s00436-005-1368-9. [DOI] [PubMed] [Google Scholar]