SUMMARY
γδ T cells rapidly secrete inflammatory cytokines at barrier sites that aid in protection from pathogens, however mechanisms limiting inflammatory damage remain unclear. We found that retinoid-related orphan receptor gamma-t (RORγt) and interleukin (IL)-7 influence γδ T cell homeostasis and function by regulating expression of the inhibitory receptor, B and T Lymphocyte Attenuator (BTLA). The transcription factor RORγt, via its activating function-2 domain, repressed Btla transcription, whereas IL-7 increased BTLA levels on the cell surface. BTLA expression limited γδ T cell numbers and sustained normal γδ T cell subset frequencies by restricting IL-7 responsiveness and expansion of the CD27−RORγt+ population. BTLA also negatively regulated IL-17 and TNF production in CD27− γδ T cells. Consequently, BTLA-deficient mice exhibit enhanced disease in a γδ T cell-dependent model of dermatitis, while BTLA agonism reduced inflammation. Therefore, by coordinating expression of BTLA, RORγt and IL-7 balance suppressive and activation stimuli to regulate γδ T cell homeostasis and inflammatory responses.
INTRODUCTION
Secondary lymphoid organs such as the spleen, lymph nodes and Peyer's patches (PP) promote cellular interactions for efficient adaptive immune responses (Ruddle and Akirav, 2009). Emerging evidence indicates secondary lymphoid organs also provide the critical location for cells mediating early innate defenses (Bekiaris et al., 2008; Junt et al., 2007; Kastenmuller et al., 2012; Schneider et al., 2008). Specialized subsets of innate-like T cells, B cells and innate lymphoid cells (ILCs) reside within the elaborate architecture of lymphoid organs formed by highly differentiated stromal cells and myeloid cells (Junt et al., 2008). A balance of activating and inhibitory signals controls homeostasis of cells within secondary lymphoid organs, however the nature of these cellular circuits and molecular pathways, particularly those involving inhibitory pathways, are incompletely defined. Such knowledge could reveal new opportunities for intervention in pathological immune responses (Germain, 2012).
The differentiation of specific subsets of T cells is promoted by expression of the transcription factor retinoid-related orphan receptor-γ isoform-t (RORγt) (encoded by Rorc) (Jetten, 2009). RORγt is a member of the ROR family of transcription factors that transactivate gene expression by recruiting nuclear repressors or activators containing an LXXLL motif via their activating function-2 (AF2) domain to canonical ROR DNA binding sites through their DNA binding domain (DBD) (Jetten, 2009). In T cells, RORγt binds and activates the Il17 promoter (Zhang et al., 2008) inducing expression of the pro-inflammatory cytokine IL-17, driving the differentiation of conventional CD4+ T helper cells (Th17) and sustaining innate-like gamma-delta (γδ) T cells (Ivanov et al., 2006; Martin et al., 2009; Sutton et al., 2009). Phenotypic profiling of γδ T cells identified two broad subgroups based on the expression of CD27, a member of the tumor necrosis factor receptor superfamily (TNFRSF) (Ribot et al., 2009). The CD27+ subset produces IFNγ, whereas the CD27− subset produces IL-17 (Ribot et al., 2009). During development γδ T cells are largely dependent on IL-7 signaling (He and Malek, 1996; Maki et al., 1996), which regulates the survival of early thymic progenitors (Malissen et al., 1997) and induces V(D)J recombination in the TCR-γ locus (Schlissel et al., 2000). Moreover, IL-7 maintains the homeostasis of γδ T cells (Baccala et al., 2005) and preferentially expands the CD27−IL-17+ subset (Michel et al., 2012). The capacity of γδ T cells to produce IL-17 is acquired during thymic differentiation, independently of TCR signaling (Haas et al., 2012), a feature pointing to their bona fide innate nature. γδ T cells have emerged as potent inflammatory effectors that can be activated through innate as well as antigen receptors, either of which initiate rapid responses to infection (Vantourout and Hayday, 2013; Willcox et al., 2012).
RORγt is also essential for the differentiation of group 3 ILCs, such as lymphoid tissue inducer (LTi) cells, which are required in the embryo for the development of secondary lymphoid organs (Cupedo et al., 2009; Eberl et al., 2004; Mebius et al., 1997), or adult IL-22 secreting ILCs (CD134+IL-22+ ILC) (Kim et al., 2003; Luci et al., 2009; Sanos et al., 2009; Satoh-Takayama et al., 2008), which are important for protection against intestinal infections (Sonnenberg et al., 2012; Tumanov et al., 2011) and induce signals for survival of activated lymphocytes (Bekiaris et al., 2009; Withers et al., 2012). The conservation of the ILC lineage in mice and primates (Sonnenberg et al., 2012) underscores the importance of these cells in the rapid innate defense mechanisms in lymphoid tissues.
The broad expression profile in hematopoietic cells of the inhibitory receptor, B and T lymphocyte attenuator (BTLA) (Han et al., 2004; Hurchla et al., 2005) suggested a potential role in regulation of innate-like T cells and ILCs. BTLA belongs to the immunoglobulin superfamily, contains two immunoreceptor tyrosine-based inhibitory motifs (ITIM) and associates with the Src-homology domain 2 (SH2)-containing protein tyrosine phosphatase (SHP)-1 and SHP-2 (Watanabe et al., 2003). Through ligation with the herpesvirus entry mediator (HVEM, TNFRSF14) (Cheung et al., 2009; Sedy et al., 2005) BTLA maintains the homeostasis of dendritic cells (De Trez et al., 2008) and memory T cells(Krieg et al., 2007), and plays an important role in limiting T cell activation (Sedy et al., 2005; Watanabe et al., 2003). In contrast to other inhibitory receptors that are induced following activation (Odorizzi and Wherry, 2012), BTLA is constitutively expressed in most immune cells (Murphy and Murphy, 2010). However, BTLA expression varies substantially among different lymphoid and myeloid cell types (Hurchla et al., 2005) suggesting regulation of BTLA expression may be an important factor in controlling homeostasis in lymphoid tissues.
In this study we show that RORγt transcriptionally represses Btla accounting for its low expression in CD27−RORγt+ γδ T cells and ILCs. In contrast, IL-7 induces BTLA expression in the majority of γδ T cells and ILCs serving to counter regulate RORγt. Our data further demonstrate that BTLA limits γδ T cell numbers in the thymus and is a negative regulator of γδ T cell subset homeostasis in lymph nodes. The defect in homeostasis in the absence of BTLA can be explained by the hyper-responsiveness of BTLA-deficient CD27− γδ T cells to IL-7. BTLA regulates the production of IL-17 and TNF in a γδ T cell-subset specific manner. Furthermore, BTLA-deficient animals are susceptible to γδ T cell-dependent dermatitis, while BTLA agonism limited disease. This result shows that RORγt and IL-7 form a novel regulatory circuit that impinges on BTLA to control the homeostasis and inflammatory responses of innate-like T cells.
RESULTS
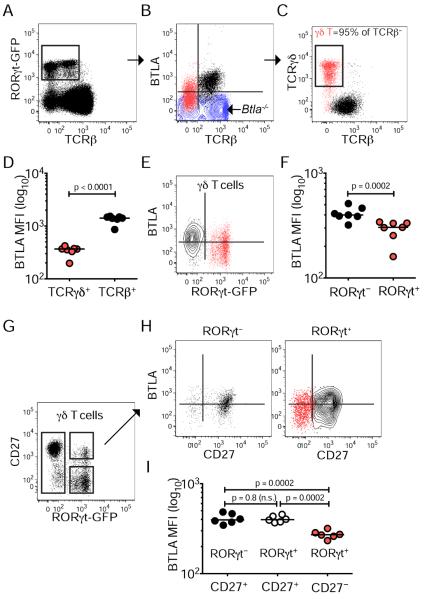
RORγt+ lymphocytes express reduced levels of BTLA
In order to define the expression of BTLA in innate lymphocyte populations we used the Rorc reporter mice expressing GFP (Rorcgfp/+ mice) (Eberl et al., 2004) to discriminate cellular subsets. Within the RORγt+ population of inguinal lymph nodes (iLN), we distinguished BTLA+TCRβ+ and BTLAlowTCRβ− cells (Figure 1A–B). Among RORγt+ cells the BTLAlowTCRβ− population comprised >95% of γδ T cells (Figure 1C) and in general all γδ T cells (RORγt+ and RORγt− alike) expressed lower (4-fold) amounts of BTLA than conventional T cells (Figure 1D). However, there was a difference in surface BTLA between the RORγt+ and RORγt− γδ T cells (1.3-fold) (Figure 1E–F). We identified lower amounts of BTLA in RORγt+ γδ T cells lacking CD27 compared with CD27+ cells, indicating an additional way in which to regulate BTLA expression (Figure 1G–I). Among lymph node RORγt+ cells, the CD27− γδ T cell subset, which is associated with IL-17 production and autoimmune pathology, expressed the lowest amounts of BTLA (Figure 1G–I).
Figure 1. Reduced BTLA expression in CD27− γδ T cells.
Lymphocytes isolated from the iLN of Rorcgfp/+ mice were analyzed by flow cytometry for BTLA expression and the indicated T cell subset surface markers. Expression of RORγt and TCRβ (A), BTLA and TCRβ in RORγt-gated cells (B), TCRγδ and TCRβ in RORγt-gated cells (C), BTLA and RORγt in γδ T cells (E), CD27 and RORγt in γδ T cells (G), and BTLA and CD27 in RORγt− (left) and RORγt+ (right) γδ T cells (H).
(D, F, I) Mean fluorescent intensity (MFI) for BTLA expression in TCRγδ+ and TCRβ+ cells (D), in RORγt− and RORγt+ γδ T cells (F), and in CD27+RORγt−, CD27+RORγt+ and CD27−RORγt+ γδ T cells (I). Each FACS plot is representative of six mice; in graphs, each symbol represents a mouse and lines are medians. See also Figure S1
We observed both BTLAlowTCRβ− and BTLA+TCRβ+ RORγt+ populations in intestinal Peyer's patches (PP) (Figure S1A–B). In contrast to iLN, more than 90% of the BTLAlowTCRβ− lymphocytes were ILCs, defined by the lack of TCRγδ expression (Figure S1C). BTLA expression by ILCs was also reduced compared with conventional TCRβ+ T cells (Figure S1D), and was ~2-fold reduced compared to iLN RORγt+ γδT cells (Figure S1E). Furthermore, we observed the lowest surface BTLA expressed in RORγt+ ILCs compared with RORγt− Thy1.2+CD127+ cells (Figure S1F). In contrast, HVEM levels were nearly the same between TCRβ− and TCRβ+ cells in all lymphoid organs (Figure S1N–O).
In addition, we found that human innate lymphoid cells in blood (CD3−CD117+) expressed substantially lower BTLA levels than conventional T cells (CD3+CD117− cells) (Figure S1G), while IL-22-producing CD117+ ILCs from human tonsils had undetectable surface BTLA (Figure S1H–I). We found similar down-regulation of BTLA in RORγt+ differentiated Th17 compared to unpolarized cells, and in double-positive thymocytes compared to single positive (Figure S1J–M) as previously reported (Han et al., 2004; Hurchla et al., 2005). Thus, overall there was a trend of less BTLA expression in RORγt+ cells in all lymphoid compartments examined in human and mouse indicating a conserved counter-regulatory relationship between these two factors.
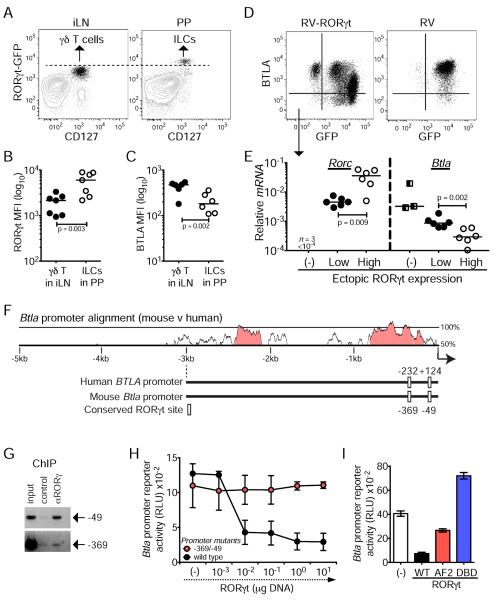
RORγt is a transcriptional repressor of Btla
The selective down modulation of BTLA within RORγt+ innate lymphocytes in mouse and human suggested a potential regulatory interaction. We observed the greatest expression of RORγt in PP-derived ILCs, 5.5-fold more than in γδ T cells from the iLN (Figure 2A–B). Together with the reduced amount of BTLA in ILCs (Figure S1E and Figure 2C), this data suggests and inverse correlation between RORγt and BTLA expression. To test whether RORγt antagonized BTLA expression we ectopically expressed RORγt using an IRES-GFP retrovirus (Ivanov et al., 2006) in a BTLA+ mouse T cell line (Cheung et al., 2009). We found that ectopic expression of high amounts of RORγt resulted in decreased BTLA expression (Figure 2D–E). Furthermore, knock-down of RORγt in these cells restored BTLA expression (Figure S2A) whereas treatment of RORγt-expressing Jurkat cells with the RORγt inhibitor digoxin induced expression of BTLA mRNA and protein (Figure S2B), indicating active RORγt-dependent suppression of BTLA.
Figure 2. RORγt is a transcriptional repressor of Btla.
(A) Expression of RORγt and CD127 in freshly isolated lymphocytes gated on TCRβ− live cells from the iLN (left) and PP (right) of Rorcgfp/+ mice (representative of 8 mice).
(B, C) MFI for RORγt-GFP expression in iLN γδ T cells and PP ILCs (B), and for BTLA expression in RORγt+ γδT cells and ILCs (C).
(D) Expression of BTLA and GFP in cells transduced with pMSCV-IRES-GFP-RORγt retrovirus (RV-RORγt) or with empty retrovirus (RV) (representative of two transduction experiments and six different passages after transduction).
(E) mRNA levels (relative to L32) of Rorc and Btla in FACS-sorted RORγt-GFP−, RORγt-GFPLow and RORγt-GFPHigh cells that were transduced with RV-RORγt (each symbol represents a different passage of the indicated FACS-sorted cell populations). In (B–C) each symbol represents a mouse and in (B) lines are medians.
(F) VISTA plot of sequence similarity (>70%, 100 bp, pink) between the 5kb promoter regions of the human and mouse BTLA coding genes and graphical representation of the conserved RORγt binding sites and their positions relative to the transcription start (indicated by arrow).
(G) PCR analysis using primers specific for the RORγt binding sites −49 and −369 following a ChIP assay with anti-RORγ or control IgG in RV-RORγt transduced cells (representative of two experiments).
(H) Btla promoter reporter activity in Jurkat cells co-transfected with wild type or mutated promoter, and the indicated amounts of RORγt expressing plasmid (mean±sem of two experiments with two replicates each).
(I) Btla promoter reporter activity in Jurkat cells co-transfected with wild-type promoter in the presence or absence of wild-type (wt) or Activation Function domain 2 (AF2)-mutant or DNA Binding Domain (DBD)-mutant RORγt (mean±sem of two experiments with two replicates each). See also Figure S2.
In order to determine whether RORγt directly repressed BTLA transcription we analyzed the Btla and BTLA promoters for conserved regulatory regions. We found two conserved canonical RORγt binding sites (Jetten, 2009) located at −232 and +124 in the human and at −369 and −49 in the mouse genes encoding BTLA (Figure 2F & Figure S2E). We next used chromatin immunoprecipitation (ChIP) and PCR amplification to determine whether ectopically expressed RORγt bound to the Btla locus (Figure 2D). Both conserved sites in the mouse promoter were precipitated with anti-RORγt Ab (Figure 2G), although the −49 site amplified a stronger signal in transfected cells, and in primary mouse thymocytes in which we probed for endogenous RORγt (Figure S2C). We next cloned the proximal 0.5 kb Btla promoter into a luciferase reporter to determine how RORγt regulates promoter activity. In this regard, titrating quantities of RORγt suppressed activity of the wild-type promoter, but not a promoter with mutations at positions −369 and −49 (Figure 2H), directly demonstrating that RORγt can function as a transcriptional repressor for BTLA (Figure 2H). While the −369 site contributes more to BTLA suppression, mutation of both sites is required for optimal BTLA promoter activity (Figure S2D). We further sought to determine how RORγt mediates repressive activity by truncating either its DBD, or its AF2 domain, which may recruit transcriptional repressors (Figure S2F–H). Btla promoter activity was partially restored when the RORγt AF2 region was truncated, and enhanced when the DBD regions were truncated (Figure 2I). Thus, regulation of Btla requires RORγt binding to the promoter as well as interactions with a transcriptional co-repressor.
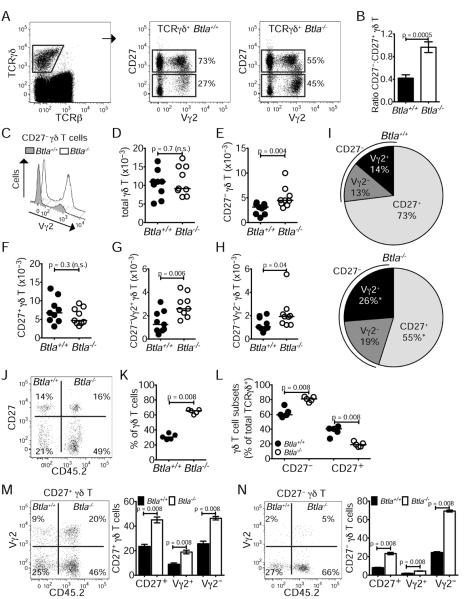
BTLA negatively regulates homeostasis of γδ T cells in lymph nodes
We next sought to determine whether the regulation of BTLA by RORγt resulted in altered distribution of γδ T cell subsets in the iLN. Among γδ T cells we observed an increase in the frequency and numbers of CD27− cells in BTLA-deficient iLN compared to wild-type (Figure 3A–B, D–H), with a more pronounced skewing towards Vγ2-expressing cells, possibly reflecting unrestricted embryonic development of these cells in the absence of BTLA (Figure 3C) (Haas et al., 2012). CD27+ γδ T cells trended towards lower numbers in iLN and total γδ T cell numbers were not different between wild-type and Btla−/− mice (Figure 3D–H). Together these data indicate that BTLA acts via a cell-intrinsic mechanism to repress expansion of CD27− cells within the γδ T cell niche. In the absence of BTLA, the increase in CD27− cell numbers resulted in a redistribution of the major γδ T cell subsets (Figure 3I).
Figure 3. BTLA negatively regulates the homeostasis of γδ T cells in lymph nodes.
Lymphocytes isolated from the iLN of Btla+/+ and Btla−/− mice were analyzed for TCRγδ, TCRβ, CD27 and Vγ2 expression by flow cytometry.
(A) Expression of CD27 and Vγ2 in TCRγδ+TCRβ− gated cells from Btla+/+ and Btla−/− mice.
(B) Ratio of CD27−:CD27+ γδ T cells in Btla+/+ and Btla−/− mice.
(C) Offset overlayed histograms indicate the increase in CD27−Vγ2+ cells in Btla−/− mice.
(D–H) Numbers of γδ T cells. (D) total, (E) CD27−, (F) CD27+, (G) CD27−Vγ2−, (H) CD27−Vγ2+.
(I) Pie charts showing the distribution of CD27+, CD27−Vγ2+ and CD27−Vγ2− γδ T cells in Btla+/+ (top) and Btla−/− (bottom) mice (* denotes a p value < 0.05). Each FACS plot is representative of nine mice from three experiments; in the graphs, each symbol represents a mouse and lines are medians.
(J–N) Mice were lethally irradiated and reconstituted with a 1:1 mixture of Btla+/+ (CD45.2−) and Btla−/− (CD45.2+) bone marrow, and blood at three weeks (J–L) or iLN at eight weeks (M–N) following reconstitution were analyzed. (J) FACS plot indicates frequencies of γδ T cell subsets in TCRγδ+TCRβ− gated cells. (K, L) Percentage (%) of CD45.2+ Btla−/− and CD45.2− Btla+/+ total γδ T cells (K), or of CD45.2+ Btla−/− and CD45.2− Btla+/+ γδ T cell subsets (L) in blood. (M, N) Frequencies of CD45.2+ Btla−/− and CD45.2− Btla+/+ CD27+ γδ T cell subsets (M) or of CD45.2+ Btla−/− and CD45.2− Btla+/+ CD27− γδ T cell subsets (N) in iLN. Graphs show percentages of CD45.2+ Btla−/− and CD45.2− Btla+/+ within CD27+ (M) or CD27− (N) subsets (left bars), and within Vγ2+/− fractions (right bars). Data are representative of five mice; in scatter graphs, each symbol represents a mouse and lines are medians; bar graphs show mean±sem of five mice. See also Figure S3.
In order to determine whether expansion of CD27− γδ T cells in the periphery of BTLA-deficient animals originated during development, we assessed the expression of BTLA in thymic γδ T cell subsets. In this regard, the CD27− subset showed lower BTLA expression than the CD27+ subset, similar to that in lymph nodes (Figure S3A–C). Thymic TCRγδ+ cell numbers were increased in BTLA-deficient mice independent of whether they expressed CD27 and there was no change in the distribution of γδ T cell subsets (Figure S3D–I). Thus, while BTLA restricts the expansion of all thymic γδ T cells, this does not explain the specific expansion of CD27− γδ T cells in the periphery.
We further examined whether BTLA-deficiency conferred a competitive advantage to γδ T cells undergoing homeostatic expansion following bone marrow reconstitution of irradiated animals. We observed an increased proportion of BTLA-deficient γδ T cells among circulating lymphocytes at three or eight weeks post-transfer, demonstrating that BTLA-deficient progenitors outcompeted their wild-type counterparts within these niches (Figure 3J–L). In addition, Btla−/− γδ T cell subsets outcompeted their wild-type counterparts within the lymph nodes of reconstituted animals (Figure 3M–N). However, within chimeras we observed reduced numbers of Vγ2+CD27− γδ T cells from either donor, which may reflect suboptimal development in the absence of an embryonic environment (Haas et al., 2012).
In addition, the number of ILCs resident within gut-associated lymphoid tissues did not differ between wild-type and BTLA-deficient mice (Figure S3J). However, within mixed bone marrow chimeric mice, we observed increased numbers of Btla−/− ILCs in the spleens of recipient mice (Btla−/−:Btla+/+ ratio of 1.8) (Figure S3K–L), indicating that BTLA deficiency provided a competitive growth advantage compared to their wild-type counterparts.
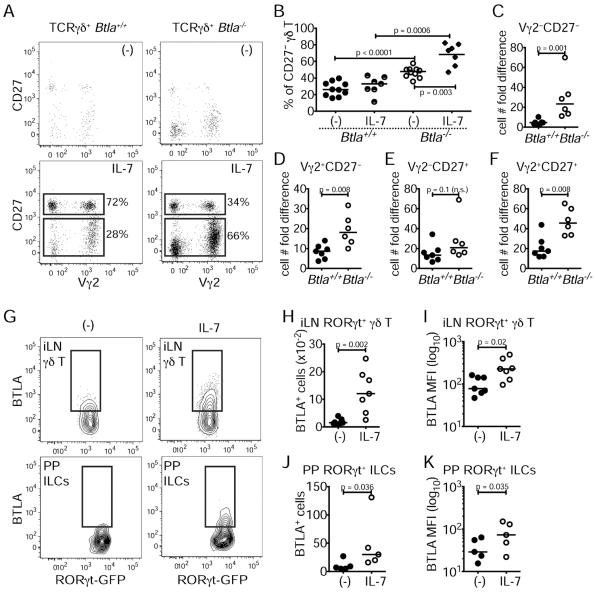
IL-7 and BTLA form a negative feedback loop
We reasoned that expansion of CD27− γδ T cells in BTLA-deficient mice in peripheral lymphoid organs may be due to unrestricted IL-7 receptor signaling as IL-7 is critical for γδ T cell homeostasis (Baccala et al., 2005) and preferentially affects the CD27− subset (Michel et al., 2012). IL-7 treatment sustained γδ T cell viability in cultures of lymph node-derived lymphocytes as compared to untreated cultures (Figure 4A). Furthermore, BTLA-deficient γδ T cells, and in particular the CD27− subset, were hyper-responsive to IL-7 treatment compared to wild-type controls, as measured by an increased cell frequency and persistence in culture upon IL-7 stimulation (Figure 4B–F), which was not due to differences in the amount of CD127 expression (Figure S4A). Thus, BTLA restricts γδ T cell responsiveness to IL-7.
Figure 4. IL-7 and BTLA form a negative feedback loop.
Equal numbers of lymphocytes from the iLN of Btla+/+ and Btla−/− mice were cultured for four days with or without 10 ng/ml IL-7.
(A) Expression of CD27 and Vγ2 with or without IL-7 in TCRγδ+TCRβ− gated cells.
(B) Percentage (%) of Btla+/+ and Btla−/− CD27− γδ T cells with or without IL-7.
(C–F) The fold difference in γδ T cell cellularity as defined by the ratio of cell number with or without IL-7. (C) Vγ2−CD27− γδ T cells, (D) Vγ2+CD27− γδ T cells, (E) Vγ2−CD27+ γδ T cells, (F) Vγ2+CD27+ γδ T cells (FACS plots are representative of four experiments; in graphs each symbol represents an experiment and lines are medians).
(G–K) Lymphocytes from the iLN and PP of Rorcgfp/+ mice were enriched for ILCs and CD27− γδ T cells and cultured with or without IL-7 for two days (cells are RORγt+TCRβ− gated). (G) Expression of BTLA and RORγt in iLN γδ T cells (top) and PP ILCs (bottom) with or without IL-7. (H) Numbers of BTLA+ iLN γδ T cells. (I) MFI for BTLA expression in iLN γδ T cells. (J) Numbers of BTLA+ PP ILCs. (K) MFI for BTLA expression in PP ILCs. FACS plots are representative of seven (iLN) or five (PP) independent experiments. In graphs each symbol represents an experiment and lines are medians. See also Figure S4.
We and others have shown activation-induced regulation of BTLA expression in a cell-specific manner (Han et al., 2004; Hurchla et al., 2005). We assessed whether IL-7 stimulation itself altered BTLA expression in γδ T cells or ILCs by culturing iLN and PP lymphocytes from Rorcgfp/+ mice in the presence of IL-7 and analyzing BTLA expression two days later. Compared to untreated controls, IL-7 induced a higher number of BTLA+ cells and increased surface BTLA expression in both γδ T cells and ILCs (Figure 4G–K). Additionally, we found that BTLA expression in γδ T cells was induced with IL-2, and that IL-7 up-regulation of BTLA depends upon Signal Transducer and Activator of Transcription (STAT)-5 (Figure S4B–C). In contrast, IL-23 and IL-1β, two potent γδ T cell activators (Sutton et al., 2009), had either no or only a minimal effect in BTLA induction (Figure S4B). Thus, IL-7 signaling induces BTLA expression, which in turn limits IL-7-dependent responses.
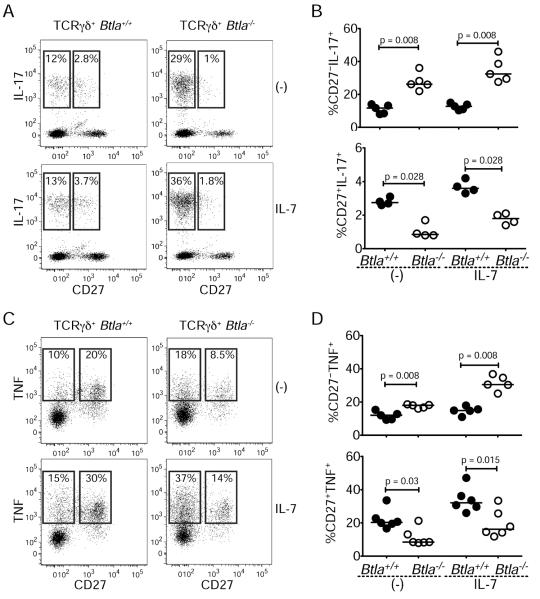
BTLA inhibits γδ T cell production of IL-17 and TNF
Mature γδ T cells contribute to inflammatory responses through the secretion of cytokines, IL-17 and TNF. We asked whether BTLA inhibited the ability of γδ T cells stimulated with IL-7 (Michel et al., 2012) to produce IL-17 and TNF. As expected, among the γδ T cell subsets a greater frequency of CD27− cells expressed IL-17, while more CD27+ cells expressed TNF (Figure 5). IL-7 treatment enhanced the frequency of both IL-17- and TNF-expressing cells in all subsets (Figure 5). However, more CD27− γδ T cells from BTLA-deficient mice produced IL-17 (Figure 5A–B) or TNF (Figure 5C–D) compared to CD27− wild-type cells, irrespective of exogenous IL-7. Moreover, there was a higher frequency of IL-17-producing Btla−/− γδ T cells irrespective of Vγ2 expression (Figure S5). Thus, BTLA negatively regulates the homeostatic, pre-programed capacity of the CD27− subset of γδ T cells to produce IL-17 and TNF. In contrast, within the CD27+ subset of BTLA-deficient γδ T cells an overall reduction in the frequency of TNF-expressing cells was observed after IL-7 treatment (Figure 5). Together, these results indicate that BTLA regulates cytokine production in γδ T cell subsets in a cell-specific manner independent of its effects on homeostasis.
Figure 5. BTLA regulates γδ T cell production of IL-17 and TNF.
Lymph node lymphocytes from Btla+/+ and Btla−/− mice were cultured for 18 hours with or without IL-7 before stimulating with PMA and ionomycin for 3.5 hours and then analyzed for cytokine production by flow cytometry.
(A, C) Expression of IL-17 (A) or TNF (C) and CD27 with (bottom) or without (top) IL-7.
(B, D) Percentage of IL-17- (B) or TNF- (D) expressing CD27− (top) or CD27+ (bottom) Btla+/+ and Btla−/− γδ T cells with or without IL-7. Each FACS plot is representative of four experiments; graphs are percent cytokine positive cells within subset gate, each symbol represents an experiment and lines are medians. See also Figure S5.
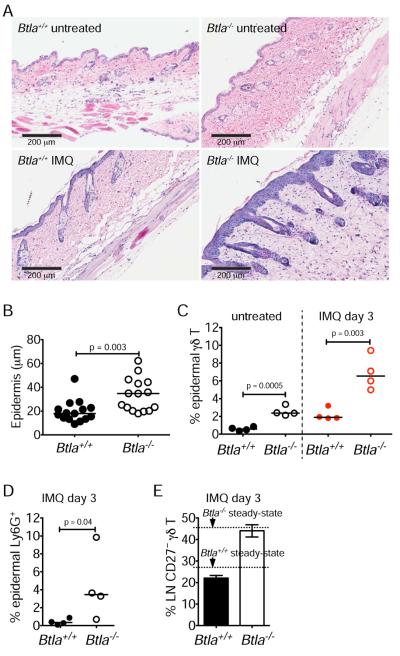
BTLA inhibits γδ T cell-dependent dermatitis
γδ T cells play critical roles in establishing skin inflammation (Vantourout and Hayday, 2013). In mice, IL-17-producing γδ T cells have been shown to be the key initiators of imiquimod (IMQ)-activated psoriasis (Pantelyushin et al., 2012), and we reasoned that BTLA-deficiency might confer susceptibility in this model of disease. Using an acute dermatitis induction model, we observed substantial inflammation in the skin of BTLA-deficient animals compared to minimally affected wild-type animals three days after a single dermal application of IMQ. Within the skin of IMQ-treated Btla−/− mice there was extensive erythema and more epidermal hyperplasia (Figure 6A–B and Figure S6A). In addition, the number of infiltrating γδ T cells that were CD27−Vγ3− (non-DETC) and expressed Vγ2, (Figure S6B) concordantly increased in Btla−/− animals, as did the number of infiltrating Ly6G+ granulocytes (Figure 6C–D). Btla−/− mice showed increased skin γδ T cells at steady state (Figure 6C and Figure S6B) suggesting that the absence of BTLA pre-disposes mice to skin inflammation. There was no preferential expansion of CD27− γδ T cells in lymph nodes (Figure 6E) suggesting that the BTLA-dependent γδ T cell response was localized and occurred in the absence of a systemic response. As previously shown, the response to IMQ was independent of CD4+ αβ T cells (Figure S6C–E) (Pantelyushin et al., 2012).
Figure 6. Btla−/− animals are susceptible to dermatitis.
Btla+/+ and Btla−/− mice were treated once with 50 mg Aldara (IMQ) cream and were analyzed three days later (all data are representative of two experiments).
(A) H&E staining of skin sections from untreated or IMQ-treated Btla+/+ and Btla−/− mice.
(B) Thickness of the epidermis in IMQ-treated mice (each symbol represents an epidermal region within the tissue sections; data are pooled of three mice per group).
(C–E) Percentage (%) of epidermal γδ T cells (TCRγδ+Vg3−) in untreated or IMQ-treated mice (C), of epidermal Ly6G+ cells in IMQ-treated Btla+/+ and Btla−/− mice (D), and of lymph node (LN) CD27− γδ T cells in IMQ-treated Btla+/+ and Btla−/− mice (E). In (C–D) each symbol represents a mouse and lines are medians. (E) shows mean±sem of four mice. See also Figure S6.
Upon repeated application of IMQ (5 days), an overwhelming inflammation occurred in both wild-type and Btla−/− mice obscuring any significant differences in epidermal thickness or infiltration of Ly6G-expressing granulocytes, although γδ T cell numbers remained increased in the skin of Btla−/− mice (Figure S6H–I). In addition, we observed enlarged lymph nodes in BTLA-deficient mice compared to wild-type mice and a higher frequency of CD27− cells (Figure S6F–G), which constituted over 80% of the γδ T cell population in either genetic background. This latter result indicates that the longer duration of IMQ treatment resulted in a systemic response causing a greater expansion of BTLA-deficient γδ T cells.
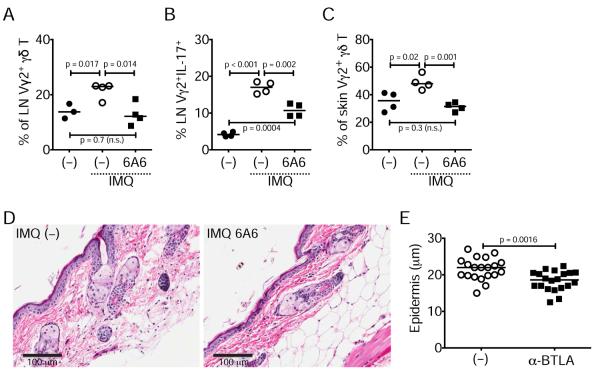
In order to directly test whether BTLA could inhibit dermatitis and inflammatory γδ T cells we used an agonistic anti-BTLA antibody (clone 6A6) (Hurchla et al., 2005) in wild-type animals treated with IMQ on days 1, 3, and 5, and analyzed Vγ2+CD27− γδ T cell expansion and IL-17 production within lymph nodes and skin. We found that BTLA activation inhibited the IMQ-dependent increase of γδ T cells in lymph nodes and skin and reduced their capacity to produce IL-17 (Figure 7A–C). In parallel, we observed significantly reduced epidermal thickening in animals treated with anti-BTLA compared to control animals (Figure 7D–E). Thus, BTLA directly limits IMQ-induced skin inflammation.
Figure 7. Treatment with agonistic anti-BTLA inhibits γδ T cells and restricts dermatitis.
Normal wild-type mice were treated three times with IMQ alone or with IMQ and anti-BTLA (6A6) antibody and at day 6 lymph nodes and skin were analyzed (in graphs each symbol represents a mouse and line is median).
(A–C) Percentage (%) of lymph node Vγ2+ CD27− γδ T cells in naïve (–) and IMQ- or IMQ+6A6- treated animals (A), of lymph node Vγ2+CD27−IL-17+ γδ T cells in naïve (–) and IMQ- or IMQ+6A6-treated animals (B), and of skin Vγ2+ CD27− γδ T cells in naïve (–) and IMQ- or IMQ+6A6-treated animals (C).
(D) H&E staining of skin sections from IMQ- or IMQ+6A6-treated animals
(E) Thickness of the epidermis in IMQ- or IMQ+6A6-treated animals (each symbol represents an epidermal region within the tissue sections; data are pooled of four mice per group).
DISCUSSION
In the present study we demonstrate that BTLA regulates the homeostasis of γδ T cells and ILCs in lymphoid tissues. RORγt intrinsically suppresses BTLA mRNA transcription, limiting BTLA translation and membrane expression, whereas IL-7 increases BTLA membrane expression to counterbalance RORγt. Our observations define BTLA as a key component in homeostasis that controls the number of innate lymphocytes in secondary lymphoid tissues. BTLA also controls IL-7-dependent proliferation and production of IL-17 and TNF in mature lymph node γδT cells. Thus, in response to inflammtory stimuli, BTLA provides a brake to autoimmune pathology that is revealed in BTLA-deficient animals, which contain a dysregulated proportion of inflammatory γδ T cells, correlating with increased susceptibility to dermatitis.
Our data links both the DNA and cofactor binding domains of RORγt to its repressive activity on Btla transcription. While RORγt is not known to interact with corepressors, it does interact with the coactivator Runx1 to drive IL-17 expression (Zhang et al., 2008). However, related ROR family members RORα, RORβ, and RORγ, which regulate a variety of developmental, circadian and metabolic processes, are known to interact with the corepressors NCOR1, NCOR2, RIP140, and neuronal interacting factor in a ligand-independent fashion to repress specific gene expression (Horlein et al., 1995; Jetten, 2009; Solt et al., 2011). Thus, it is not unreasonable to suggest that RORγt may interact with corepressors, which act to limit the expression of additional RORγt targets. While it remains unclear how specific promoter sequences are activated or repressed by RORγt binding, this is likely a result of promoter specific cofactor recruitment. We therefore propose that one of the mechanisms that regulate homeostasis of CD27− γδ T cells is via RORγt-dependent transcriptional down-modulation of BTLA.
Our results showing BTLA regulates IL-7-dependent homeostasis of γδ T cells and ILCs are consistent with previous reports that BTLA regulates homeostasis of CD8 memory T cells and splenic dendritic cell subsets (De Trez et al., 2008; Krieg et al., 2007). Although BTLA has not been reported to specifically regulate cytokine-induced signaling or cellular activation, it was previously suggested that BTLA could regulate responses to IL-2 or other cytokines (Krieg et al., 2007). The BTLA-binding phosphatase SHP-1 has been found to inhibit signaling initiated by IL-2 and IL-4, and likely binds directly to the cytokine receptors themselves to destabilize Janus kinase STAT signaling complexes (Pao et al., 2007). It is unclear whether BTLA similarly inhibits IL-7 receptor signaling, however a growing range of pathways appears susceptible to BTLA-associated tyrosine phosphatase activity including Toll-like receptor signaling (Kobayashi et al., 2013). Together, these observations that IL-7 up-regulates cell surface BTLA, which then limits IL-7 receptor signaling in γδ T cells, exemplifies a negative-feedback loop.
BTLA surface expression differs among polarized T helper subsets, indicating that T cell expression of BTLA may be determined by a combination of activating cytokines within the differentiating milieu. For example, in Th1 cells BTLA is highly expressed as compared to Th2 cells (Watanabe et al., 2003), implicating the GATA3, STAT and T-box families of polarizing transcription factors may be active in engaging their respective binding sites within the conserved regions of the Btla promoter (Loots et al., 2002). Consistent with RORγt-dependent regulation of Btla transcription, we found that Th17 cells express substantially less (>2-fold) surface BTLA than non-polarized CD4+ T cells. In addition to TCR signals that drive the γδ T cell fate choice, CD27−RORγt+ γδ T cells likely develop in response to IL-1 and IL-23 signaling (Sutton et al., 2009). It is unclear why specific lymphocyte subsets show different responses to BTLA activity, but one mechanism may involve subset-specific intrinsic complexes between BTLA and HVEM, or other cosignaling molecules.
γδ T cells have been implicated in a variety of inflammatory diseases, supported by experimental evidence utilizing mouse autoimmune models of psoriasis, multiple sclerosis, and diabetes (Vantourout and Hayday, 2013). Our findings using a γδ T cell-dependent model of inflammatory dermatitis contribute to previous data that BTLA-deficient animals are prone to the induction of autoimmune disease that in wild-type animals results in subclinical outcomes (Watanabe et al., 2003). In addition, BTLA control of early tissue-specific γδ T cell associated pathology highlights the role of BTLA in regulation of innate-like cells. These results find additional relevance in recent human genome-wide association studies showing significant linkage of HVEM to autoimmune diseases including multiple sclerosis (Sawcer et al., 2011), celiac disease (Dubois et al., 2010), sclerosing cholangitis (Folseraas et al., 2012) and rheumatoid arthritis (Coenen et al., 2009; Kurreeman et al., 2012).
The susceptibility of γδ T cells to inhibitory signaling makes BTLA an attractive target for selective biologics. In this regard, a monoclonal antibody to BTLA (6A6) that competitively inhibits the HVEM-BTLA interaction (Cheung et al., 2009) suppressed γδ T cell expansion and IL-17 production within lymph nodes and skin following IMQ induced inflammation.
Indeed, in several disease models, BTLA specific antibodies can alter disease progression (Murphy and Murphy, 2010). We determined BTLA is also required for optimal inflammatory cytokine production from the CD27+ γδ T cell subset, potentially serving to integrate signals that sustain survival during effector and memory cell differentiation as occurs in conventional αβ T cells (Steinberg et al., 2008), and perhaps in conjunction with CD27 or other costimulatory TNF receptor members. These data suggest that selective activation of BTLA may restore the balance of these pre-programmed γδ T cell subsets and/or the repertoire of γδ T cell specificity in order to control autoimmune pathogenesis. Collectively, we demonstrate a novel molecular pathway in which RORγt and IL-7 coordinate the expression of BTLA and thus balance suppressive and activation stimuli to regulate the homeostasis and inflammatory responses of γδ T cells.
EXPERIMENTAL PROCEDURES
Mice and mouse cell preparations
All experiments were approved by the Sanford|Burnham IACUC. Mice were bred in a C57BL/6 background and housed in the SBMRI animal facility. Rorcgfp/+, Btla−/−, and B6.SJL-PtprcaPep3/BoyJ mice were from Jackson. Rag2−/−Il2rg−/− mice were from Taconic. Transfer of BM cell (2×106/mouse) cells was performed by retro-orbital injection. iLN, PP or spleen cells were passed through a 70 μm strainer. ILCs and CD27− γδT cells were enriched using the BD IMag™ system (BD Biosciences, Palo Alto, CA). BM cells were flushed from femurs with PBS. Small intestinal lamina propria lymphocytes were prepared as previously described (Steinberg et al., 2008).
Flow cytometry
Surface staining was performed on ice for 20–30 min. Intracellular staining was performed using the BD Cytofix/Cytoperm™ Kit according the manufacturer's instructions (BD Biosciences). Antibodies and data acquisition in SI.
Imiquimod treatment and histology
50 mg of commercially available Aldara (5% imiquimod) cream was applied on the shaved backs of mice once or thrice every other day. Three days later animals were sacrificed and epidermal lymphocytes were prepared by incubating the tissue in 0.25% Trypsin-EDTA solution for two hours. Alternatively, mice were treated daily for five consecutive days before analysis. Anti-BTLA (6A6 clone, BioXCell, West Lebanon, NH) injections were performed i.p. with 100 μg antibody per mouse one day before IMQ and then a day after each IMQ application. Formalin fixed tissue was embedded in paraffin and H&E stained sections were scanned using ScanScope® XT system at 20×.
Promoter analysis
The human and mouse promoter regions were aligned with VISTA (genome.lbl.gov/vista) using a region 15kb upstream and 1kb downstream of the transcription start of each gene, with the additional identification of the common RORα-RORγt binding site (Jetten, 2009). Chromatin immuno-precipitation was performed from the RORγt-transfected PE16 T cell line with 2 μg αRORγ (Santa Cruz Biotechnology, Inc., Dallas, TX) or 2 μg control rabbit Ig using the SimpleChip® Kit (Cell Signaling Technology®, Danvers, MA).
In vitro cultures
For IL-7 induced expansion iLN lymphocytes were cultured in RF10 media (RPMI+10%FBS,p/s,L-Glutamine) at 107/ml in 12-well plates for four days. For IL-7 induced BTLA expression iLN or PP lymphocytes were enriched for ILCs and CD27− γδ T cells and cultured in RF10 media in 24-well plates containing feeder adherent cells for two days. Following enrichment, cells from 3 animals were cultured in 1ml in a single well. IL-7 (R&D Systems, Minneapolis, MN) was added at 10 ng/ml. For IL-17 and TNF production whole lymph node cells were cultured with or without 10 ng/ml IL-7 for 18 hours and then re-stimulated with PMA (50 ng/ml), ionomycin (750 ng/ml) and Golgi Stop™ (BD Biosciences) for 3.5 hours. TH17 differentiation was performed by isolating CD4+ T cells from spleens and culturing in anti-CD3 (5 μg/ml) coated 24-well plates at 1.35×106/ml with anti-CD28 (2 μg/ml), IL-6 (20 ng/ml) and TGFβ1 (2 ng/ml). At day 5, cells were washed and re-stimulated with PMA+ionomycin and Golgi Stop™ (BD Biosciences) for 3.5 hours. For induction of human IL-22, tonsil lymphocytes were cultured in RF10 media at 1.5×106/ml in 24-well plates for 6 hours in the presence of 40 ng/ml IL-23 (eBiosciences) and 10 ng/ml IL-1β (R&D Systems). Golgi Stop™ (BD Biosciences) was added during the final 3 hours.
Statistical analysis and software
All FACS data were acquired and compensated using BD FACSDiva v6.2 software and analyzed using FlowJo v9.5.2. Graphs were plotted using Prism 6.0c. Statistical analysis using 2-way ANOVA in R software was used to correct for variations in the mean fluorescent intensities (MFI) between FACS experiments and uses the following formula: lm.X = lm(Response~y1+y2, data=A), where X = a given set of data, Response = the MFI, y1 = variable 1 as defined in a given FACS experiment with specific cytometer settings, y2 = variable 2 defined as the two non-numerical treatment groups to be compared (e.g. γδT versus αβT cells). All other statistical analyses (comparisons of cell numbers or cell expansion) were performed using the Mann-Whitney U-test or t-test in Prism.
Supplementary Material
IL-7 induces BTLA expression and RORγt represses BTLA transcription
BTLA restricts γδ T cell expansion during homeostasis and inflammation
BTLA regulates γδ T cell production of IL-17 and TNF
BTLA-deficient animals are susceptible to γδ T cell-dependent dermatitis
ACKNOWLEDGMENTS
We thank Amy Cortez and Yoav Altman for assistance with Flow Cytometry, Stephanie Villareal and Buddy Charbono for assistance with animal work, and Robbin Newlin and Guillermina Garcia for assistance with histology. This study was supported by grants from the US National Institutes of Health (R37AI33068; AI48073, AI067890, CA164679 to CFW) and a gift from the Jean Perkins Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST The authors declare no conflict of interest.
REFERENCES
- Baccala R, Witherden D, Gonzalez-Quintial R, Dummer W, Surh CD, Havran WL, Theofilopoulos AN. Gamma delta T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J Immunol. 2005;174:4606–4612. doi: 10.4049/jimmunol.174.8.4606. [DOI] [PubMed] [Google Scholar]
- Bekiaris V, Gaspal F, McConnell FM, Kim MY, Withers DR, Sweet C, Anderson G, Lane PJ. NK cells protect secondary lymphoid tissue from cytomegalovirus via a CD30-dependent mechanism. Eur J Immunol. 2009;39:2800–2808. doi: 10.1002/eji.200939508. [DOI] [PubMed] [Google Scholar]
- Bekiaris V, Timoshenko O, Hou TZ, Toellner K, Shakib S, Gaspal F, McConnell FM, Parnell SM, Withers D, Buckley CD, et al. Ly49H+ NK cells migrate to and protect splenic white pulp stroma from murine cytomegalovirus infection. J Immunol. 2008;180:6768–6776. doi: 10.4049/jimmunol.180.10.6768. [DOI] [PubMed] [Google Scholar]
- Cheung TC, Oborne LM, Steinberg MW, Macauley MG, Fukuyama S, Sanjo H, D'Souza C, Norris PS, Pfeffer K, Murphy KM, et al. T cell intrinsic heterodimeric complexes between HVEM and BTLA determine receptivity to the surrounding microenvironment. J Immunol. 2009;183:7286–7296. doi: 10.4049/jimmunol.0902490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen MJ, Trynka G, Heskamp S, Franke B, van Diemen CC, Smolonska J, van Leeuwen M, Brouwer E, Boezen MH, Postma DS, et al. Common and different genetic background for rheumatoid arthritis and coeliac disease. Hum Mol Genet. 2009;18:4195–4203. doi: 10.1093/hmg/ddp365. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- De Trez C, Schneider K, Potter K, Droin N, Fulton J, Norris PS, Ha SW, Fu YX, Murphy T, Murphy KM, et al. The inhibitory HVEM-BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J Immunol. 2008;180:238–248. doi: 10.4049/jimmunol.180.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois PC, Trynka G, Franke L, Hunt KA, Romanos J, Curtotti A, Zhernakova A, Heap GA, Adany R, Aromaa A, et al. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet. 2010;42:295–302. doi: 10.1038/ng.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Folseraas T, Melum E, Rausch P, Juran BD, Ellinghaus E, Shiryaev A, Laerdahl JK, Ellinghaus D, Schramm C, Weismuller TJ, et al. Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J Hepatol. 2012;57:366–375. doi: 10.1016/j.jhep.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain RN. Maintaining system homeostasis: the third law of Newtonian immunology. Nat Immunol. 2012;13:902–906. doi: 10.1038/ni.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JD, Ravens S, Duber S, Sandrock I, Oberdorfer L, Kashani E, Chennupati V, Fohse L, Naumann R, Weiss S, et al. Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Han P, Goularte OD, Rufner K, Wilkinson B, Kaye J. An inhibitory Ig superfamily protein expressed by lymphocytes and APCs is also an early marker of thymocyte positive selection. J Immunol. 2004;172:5931–5939. doi: 10.4049/jimmunol.172.10.5931. [DOI] [PubMed] [Google Scholar]
- He YW, Malek TR. Interleukin-7 receptor alpha is essential for the development of gamma delta + T cells, but not natural killer cells. The Journal of experimental medicine. 1996;184:289–293. doi: 10.1084/jem.184.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass CK, et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- Hurchla MA, Sedy JR, Gavrieli M, Drake CG, Murphy TL, Murphy KM. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. J Immunol. 2005;174:3377–3385. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol. 2008;8:764–775. doi: 10.1038/nri2414. [DOI] [PubMed] [Google Scholar]
- Kastenmuller W, Torabi-Parizi P, Subramanian N, Lammermann T, Germain RN. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150:1235–1248. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, Raykundalia C, Walker LS, Goodall MD, Lane PJ. CD4(+)CD3(−) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–654. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Iwata A, Suzuki K, Suto A, Kawashima S, Saito Y, Owada T, Kobayashi M, Watanabe N, Nakajima H. B and T lymphocyte attenuator inhibits LPS-induced endotoxic shock by suppressing Toll-like receptor 4 signaling in innate immune cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5121–5126. doi: 10.1073/pnas.1222093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg C, Boyman O, Fu YX, Kaye J. B and T lymphocyte attenuator regulates CD8+ T cell-intrinsic homeostasis and memory cell generation. Nature immunology. 2007;8:162–171. doi: 10.1038/ni1418. [DOI] [PubMed] [Google Scholar]
- Kurreeman FA, Stahl EA, Okada Y, Liao K, Diogo D, Raychaudhuri S, Freudenberg J, Kochi Y, Patsopoulos NA, Gupta N, et al. Use of a multiethnic approach to identify rheumatoid- arthritis-susceptibility loci, 1p36 and 17q12. Am J Hum Genet. 2012;90:524–532. doi: 10.1016/j.ajhg.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots GG, Ovcharenko I, Pachter L, Dubchak I, Rubin EM. rVista for comparative sequence-based discovery of functional transcription factor binding sites. Genome research. 2002;12:832–839. doi: 10.1101/gr.225502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- Maki K, Sunaga S, Komagata Y, Kodaira Y, Mabuchi A, Karasuyama H, Yokomuro K, Miyazaki JI, Ikuta K. Interleukin 7 receptor-deficient mice lack gammadelta T cells. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7172–7177. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M, Pereira P, Gerber DJ, Malissen B, DiSanto JP. The common cytokine receptor gamma chain controls survival of gamma/delta T cells. The Journal of experimental medicine. 1997;186:1277–1285. doi: 10.1084/jem.186.8.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- Michel ML, Pang DJ, Haque SF, Potocnik AJ, Pennington DJ, Hayday AC. Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing gammadelta cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17549–17554. doi: 10.1073/pnas.1204327109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- Odorizzi PM, Wherry EJ. Inhibitory receptors on lymphocytes: insights from infections. J Immunol. 2012;188:2957–2965. doi: 10.4049/jimmunol.1100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, Becher B. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. The Journal of clinical investigation. 2012;122:2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao LI, Badour K, Siminovitch KA, Neel BG. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annual review of immunology. 2007;25:473–523. doi: 10.1146/annurev.immunol.23.021704.115647. [DOI] [PubMed] [Google Scholar]
- Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nature immunology. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle NH, Akirav EM. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol. 2009;183:2205–2212. doi: 10.4049/jimmunol.0804324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nature immunology. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel MS, Durum SD, Muegge K. The interleukin 7 receptor is required for T cell receptor gamma locus accessibility to the V(D)J recombinase. The Journal of experimental medicine. 2000;191:1045–1050. doi: 10.1084/jem.191.6.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K, Loewendorf A, De Trez C, Fulton J, Rhode A, Shumway H, Ha S, Patterson G, Pfeffer K, Nedospasov SA, et al. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell host & microbe. 2008;3:67–76. doi: 10.1016/j.chom.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, Murphy KM. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidovic D, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MW, Turovskaya O, Shaikh RB, Kim G, McCole DF, Pfeffer K, Murphy KM, Ware CF, Kronenberg M. A crucial role for HVEM and BTLA in preventing intestinal inflammation. J Exp Med. 2008;205:1463–1476. doi: 10.1084/jem.20071160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Tumanov AV, Koroleva EP, Guo X, Wang Y, Kruglov A, Nedospasov S, Fu YX. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell host & microbe. 2011;10:44–53. doi: 10.1016/j.chom.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nature reviews. Immunology. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, Moreau JF, Hayday AC, Willcox BE, Dechanet-Merville J. Cytomegalovirus and tumor stress surveillance by binding of a human gammadelta T cell antigen receptor to endothelial protein C receptor. Nature immunology. 2012;13:872–879. doi: 10.1038/ni.2394. [DOI] [PubMed] [Google Scholar]
- Withers DR, Gaspal FM, Mackley EC, Marriott CL, Ross EA, Desanti GE, Roberts NA, White AJ, Flores-Langarica A, McConnell FM, et al. Cutting edge: lymphoid tissue inducer cells maintain memory CD4 T cells within secondary lymphoid tissue. J Immunol. 2012;189:2094–2098. doi: 10.4049/jimmunol.1201639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.