Abstract
Summary
Serotonin (5-HT) is a neuromodulator involved in regulating mood, appetite, memory, learning, pain, and establishment of left-right (LR) asymmetry in embryonic development. To explore the role of 5-HT in a variety of physiological contexts, we have created two forms of “caged” 5-HT, BHQ-O-5HT and BHQ-N-5HT. When exposed to 365- or 740-nm light, BHQ-O-5HT releases 5-HT through 1- or 2-photon excitation, respectively. BHQ-O-5HT mediated changes in neural activity in cultured primary sensory neurons from mouse and the trigeminal ganglion and optic tectum of intact zebrafish larvae in the form of high amplitude spiking in response to light. In Xenopus laevis embryos, 5-HT released from BHQ-O-5HT upon exposure to light increased the occurrence of LR patterning defects. Maximal rates of LR defects were observed when 5-HT was released at stage 5 compared to stage 8. These experiments show the potential for BHQ-caged serotonins in studying 5-HT-regulated physiological processes.
Introduction
Serotonin (5-hydroxytyptamine or 5-HT) is an ancient biogenic amine found in wide variety of eukaryotes, including animals, plants, fungi, and pathogenic amoebae (Barnes and Sharp, 1999; Feldberg and Toh, 1953; Hoyer, et al., 1994; Jackson and Yakel, 1995; McGowan, et al., 1983; Roshchina, 2001). In vertebrates, serotonin is a neurotransmitter within both the central and peripheral nervous systems, and also acts as a hormone in diverse tissues (Barnes and Sharp, 1999). In the brain, neurons in the raphe nuclei region produce serotonin (Barnes and Sharp, 1999; Frazer and Hensler, 1999). These neurons project into the cortex and hippocampus and influence an enormous network of excitatory and inhibitory neurotransmission (Frazer and Hensler, 1999) involved in regulating mood, appetite, memory, learning, and other cognitive functions (Barnes and Sharp, 1999; Daubert and Condron, 2010; Feldberg and Toh, 1953; Kang, et al., 2009; McGowan, et al., 1983; Rapport, et al., 1948). Serotonin in the CNS and periphery plays a complex role in mediating pain, both acting as an algesic by exciting the peripheral terminations of primary afferent neurons, and in pain suppression via descending pathways (Bardin, 2011a; Bardin, 2011b; Basbaum and Fields, 1978a; Basbaum and Fields, 1978b). Interestingly, 5-HT is also involved in embryonic development and the establishment of left-right (LR) asymmetry (Levin, et al., 2006; Vandenberg and Levin, 2010). This diversity of function implicates 5-HT in a number of physiological and pathological processes.
In mammals and other vertebrates, 5-HT function is mediated by a large number of different receptors. In mammals, there are 14 structurally and pharmacologically distinct 5-HT receptor subtypes that are grouped into seven major families of 5-HT receptors, designated 5-HT1-7 (Hoyer, et al., 1994). One of these families, the 5-HT3 receptors, comprises ligand-gated ion channels that mediate fast synaptic transmission (Barnes and Sharp, 1999) through a transient inward current that rapidly depolarizes the cell. The remaining six receptor families are members of the G-protein coupled receptor superfamily and mediate a wide range of physiological and pharmacological responses (Barnes and Sharp, 1999). Several classes of antidepressant, antipsychotic, anxiolytic, and antimigraine drugs target these 5-HT signaling systems (Barnes and Sharp, 1999).
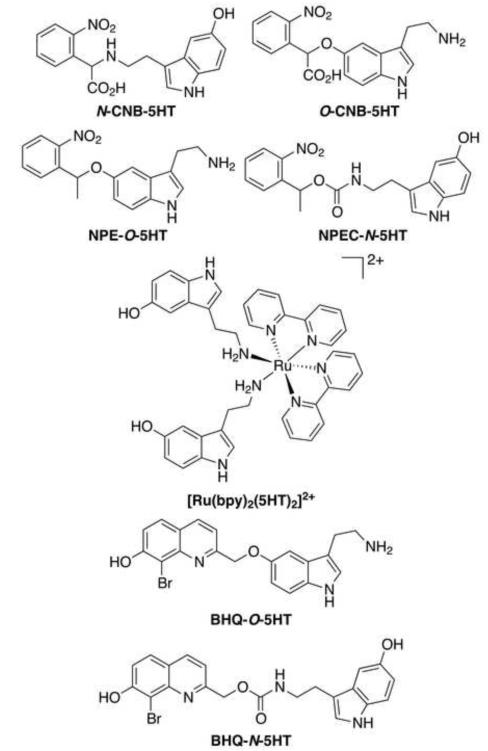
To explore the role of 5-HT in a variety of physiological contexts, a light activated form of it would be useful. This can be achieved by covalently connecting a photoremovable protecting group (PPG) to 5-HT, thereby blocking or “caging” its action (Ellis-Davies, 2007; Klan, et al., 2013; Kramer and Chambers, 2011; Lee, et al., 2009; Mayer and Heckel, 2006; Specht, et al., 2009; Young and Deiters, 2007). Exposure to light releases or “uncages” 5-HT in its active form. Ideally, the caged 5-HT would be highly sensitive to light at wavelengths not detrimental to biological systems, release 5-HT rapidly and in quantitative yield upon light exposure, exhibit no off-target effects, and be completely stable under physiological conditions in the dark. In addition, sensitivity to 5-HT release through 2-photon excitation (2PE) is desirable for localization of release to sub-cellular levels (Bort, et al., 2013; Dore, 2005; Dore and Wilson, 2011; Warther, et al., 2010). Four caged 5-HTs are known in the literature: N-CNB-5HT, O-CNB-5HT, NPEC-N-5HT (Boahen and MacDonald, 2005; Breitinger, et al., 2000), and [Ru(bpy)2(5HT)2]2+ (Zayat, et al., 2006) (Figure 1). Hess and co-workers synthesized N-CNB-5HT and O-CNB-5HT to study the kinetics of the 5-HT3 ligand-gated ion channel (Breitinger, et al., 2000). The rate constant for release of 5-HT from N-CNB-5HT was too slow for that compound to be useful in the study, but O-CNB-5HT had sufficiently rapid release kinetics, albeit low photolysis quantum efficiency and molar absorptivity. The preparation and photolysis of NPE-O-5HT (Boahen and MacDonald, 2005) and [Ru(bpy)2(5HT)2]2+ (Zayat, et al., 2006) were reported by MacDonald and Etchenique, respectively, and Tocris Bioscience sells NPEC-N-5HT commercially (catalog #3991), but the use of these compounds in a study of 5-HT physiology has not yet been reported.
Figure 1.
Caged serotonins.
We report the preparation of two BHQ-protected 5-HT compounds, BHQ-O-5HT and BHQ-N-5HT (Figure 1), and their suitability for spatially and temporally controlling the release of 5-HT through 1PE and 2PE within a biological system. We found that BHQ-O-5HT depolarized sensory neurons when photolyzed in culture and in larval zebrafish comparable to that observed by 5-HT by itself. Light-induced release of 5-HT from BHQ-O-5HT in stage 5 Xenopus embryos significantly increases the rate of LR patterning defects in the frog. Activation of 5-HT at later stages had a less significant effect.
Results and Discussion
We chose BHQ as the caging group for 5-HT because it has good sensitivity to 1PE-mediated photolysis at biologically compatible wavelengths (>350 nm) (Fedoryak and Dore, 2002; Zhu, et al., 2006) and rapid release kinetics (Ma, et al., 2012). High sensitivity to light is important for working in thick or pigmented biological tissues, such as whole larval zebrafish or Xenopus laevis embryos. Rapid release kinetics are critical for studying fast signaling events initiated by neurotransmitters and taking advantage of BHQ’s sensitivity to 2PE (Fedoryak and Dore, 2002; Zhu, et al., 2006), which is better than many groups currently used in biological studies, yet not as sensitive as others (Dore, 2005; Dore and Wilson, 2011; Klan, et al., 2013; Warther, et al., 2010). To take advantage of the tight spatial release that 2PE affords, the release kinetics must be faster than diffusion out of the excitation volume. BHQ’s moderate sensitivity to 2PE and rapid release kinetics could be advantageous for future biological studies.
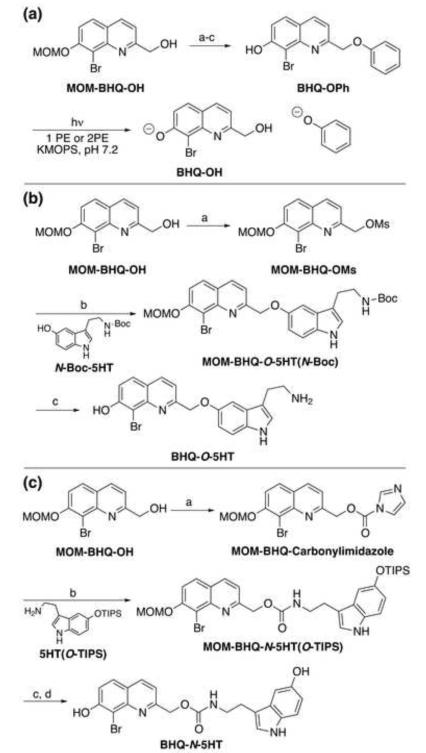
Typically, phenols and alcohols require a carbonate linker for efficient release from the caging group after photoexcitation, but the initially released carbonate must first decarboxylate to yield the free phenol or alcohol. This slow step of the release process (τ = 240-270 μs for phenols) is not optimal (Zhao, et al., 2006). It would be better to release phenol directly. To test this, we synthesized 8-bromo-2-(phenoxymethyl)quinolin-7-ol (BHQ-OPh) from bromo-7-(methoxymethoxy)quinolin-2-yl)methanol (MOM-BHQ-OH, Figure 2a). MOM-BHQ-OH, prepared from 8-bromo-7-hydroxyquinaldine as previously described (Ma, et al., 2012), was converted to the corresponding mesylate, which was subsequently displaced by phenol in good yield to provide the desired phenyl ether. Removal of the methoxymethyl ether (MOM) protecting group with trifluoroacetic acid in methanol afforded BHQ-OPh. BHQ-OPh was reasonably stable under simulated physiological conditions consisting of 100 mM KMOPS buffer at pH 7.2 with a time constant for hydrolysis in the dark τdark = 95 h. BHQ-OPh photolyzes with a quantum efficiency Qu = 0.19 at 365 nm and a 2-photon photolysis uncaging action cross section δu = 0.56 GM at 740 nm in KMOPS buffer (Figure 2a. Figure S1 shows the time course of photolysis of BHQ-OPh by 1PE and 2PE). These results demonstrated that phenols were sufficiently good leaving groups for light mediated release from BHQ and protection and release of the phenol on 5-HT (and other neuromodulators) from BHQ was feasible.
Figure 2.
(a) Preparation and photolysis of BHQ-OPh. (a) MsCl, DIEA, THF, rt, 2 h, 68%; (b) phenol, 1 M KOH (aq.), THF, 72%; (c) TFA, MeOH. Time courses for the photolysis of BHQ-OPh are shown in Figure S1. (b) Preparation of BHQ-O-5HT. (a) MsCl, DIEA, THF, rt, 2 h, 68%; (b) K2CO3, CH3CN, reflux, 48 h, 78%; (c) TFA, CH2Cl2, rt, 1 h, 57%. (c) Preparation of BHQ-N-5HT. (a) carbonyldiimidazole, THF, rt, 2 h, 63%; (b) DMF, 60 ○C, 12 h, 65%; (c) TBAF, THF, rt 15 min, 85%; (d) conc. HCl (tr), MeOH, rt, 12 h, 55%.
We prepared two versions of the photoactivatable 5-HT: BHQ-O-5HT and BHQ-N-5HT from MOM-BHQ-OH and an appropriately protected 5-HT (Figure 2b and 2c). These compounds are distinguished by the type of linkage between the PPG and 5-HT. The O-version has a phenolic ether linkage, whereas the N-version uses a carbamate linker. The latter would place limitations on the use of BHQ-N-5HT that are discussed below. To synthesize BHQ-O-5HT, MOM-BHQ-OH was converted to the corresponding mesylate and displaced by Boc-protected serotonin (N-Boc-5HT), which was prepared as previously described (Breitinger, et al., 2000), to generate the doubly protected compound MOM-BHQ-O-5HT(N-Boc). Global deprotection with trifluoroacetic acid in dichloromethane provided BHQ-O-5HT (Figure 2b).
BHQ-N-5HT was prepared by activating the primary alcohol with carbonyldiimidazole, then treating the resulting carbamate (MOM-BHQ-Carbonylimidazole) with TIPS-protected serotonin (5HT(O-TIPS), which was prepared as previously described (Ho, et al., 2003). Removal of first the TIPS protecting group with TBAF was followed by the MOM deprotection in acidic methanol to provide BHQ-N-5HT (Figure 2c).
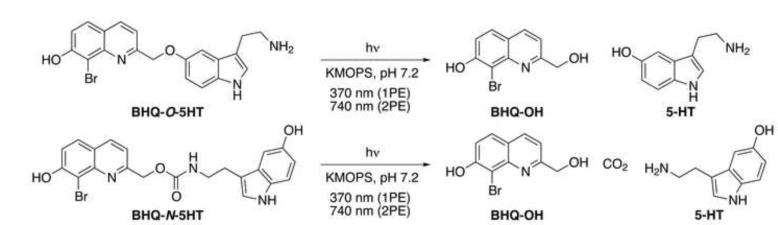
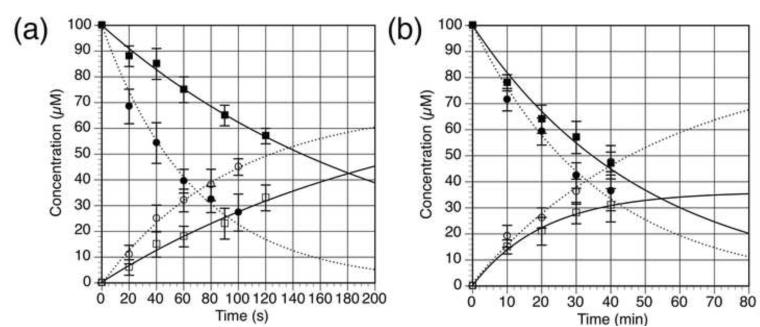
Selected photophysical and photochemical properties of the two forms of caged 5-HT were examined (Table 1) and compared to previously reported caged 5-HTs (Boahen and MacDonald, 2005; Breitinger, et al., 2000; Zayat, et al., 2006). In contrast to the CNB- and NPE-protected 5-HTs, the BHQ-protected 5-HTs have absorbance maxima (λmax) above 350 nm and larger molar absorptivities (ε), but not as high as [Ru(bpy)2(5HT)2]2+, which is more absorbent in the visible region (Table 1). BHQ-O-5HT and BHQ-N-5HT were each photolyzed under simulated physiological conditions (KMOPS buffer, pH 7.2) with 370-nm light from a mercury lamp (1PE) and 740-nm light from a pulsed Ti:sapphire laser (2PE) (Figure 3). The time course of the reaction was monitored by HPLC, measuring the disappearance of the caged compound and appearance of 5-HT (Figure 4). From these data, the 1-photon quantum efficiencies of photolysis (Qu) and the 2PE photolysis action cross-sections (δu) were calculated using previously described methods (Davis, et al., 2009; Fedoryak and Dore, 2002; Furuta, et al., 1999; Lu, et al., 2003; Zhu, et al., 2006). Compared to the CNB- and NPE-protected 5-HTs and [Ru(bpy)2(5HT)2]2+, both BHQ-caged 5-HTs demonstrated superior Qu and sensitivity to light (Qu × ε) at biologically compatible wavelengths. The sensitivity of the NPE-protected 5-HTs were not explicitly reported (Boahen and MacDonald, 2005), but the sensitivity of a related compound, NPE-protected phenylephrine, is 682 at 272 nm (Walker, et al., 1993), a shorter wavelength not well-suited for biological experiments. The absorbance spectrum of NPE-caged ATP drops precipitously as the wavelength increases (Kaplan, et al., 1978), indicating that NPE-protected 5-HTs also have low sensitivity at λ > 300 nm. BHQ-O-5HT was found to be the most sensitive of all seven caged 5-HTs at 368 nm. Both BHQ-protected 5-HTs were stable in the dark in buffered aqueous media. The 2PE cross-sections (δu) were not reported for the CNB-, NPE-, and Ru(bpy)2-protected 5-HTs, but the CNB and NPE groups are not considered sensitive to 2PE (δu ≤ 0.04 GM) (Dore, 2005; Dore and Wilson, 2011; Kiskin, et al., 2002; Warther, et al., 2010) and other conjugates of Ru(bpy)2 are only slightly more sensitive (δu = 0.01-0.14 GM) (Nikolenko, et al., 2005; Salierno, et al., 2010). Two-photon cross-sections (δu) of BHQ-O-5HT and BHQ-N-5HT were at least an order of magnitude larger than CNB- and NPE-protected 5-HT at 740 nm and sufficiently sensitive for use in biological systems. The time course for 2PE is in minutes because the excitation volume is smaller than the sample size and more time is required for a sufficient amount of starting material to be photolyzed to detectable levels.
Table 1.
Selected photophysical and photochemical properties of caged 5-HTs.
| Caged 5-HT | λmax (nm) |
ε (M−1 cm−1) |
Q u | Sensitivity (Qu × ε) |
δu (GM) |
τdark (h) |
|---|---|---|---|---|---|---|
| O-CNB-5HT | 280 | 800 at 337 nm | 0.03 | 24 | N.R. | N.R. |
| [Ru(bpy)2(5HT)2]2+ | 488 | 9,880 | 0.023 | 227 | N.R. | N.R. |
| BHQ-OPh | 369 | 3,200 | 0.19 | 608 | 0.56 | 95 |
| BHQ-O-5HT | 368 | 2,000 | 0.30 | 600 | 0.50 | 260 |
| BHQ-N-5HT | 370 | 2,100 | 0.10 | 210 | 0.42 | 300 |
UV-Vis spectra (Figure S3) and photolysis data on BHQ-OPh (Figure S1), BHQ-O-5HT, andBHQ-N-5HT were acquired in KMOPS buffer, pH 7.2. Absolute values of δu are estimated to beaccurate within a factor of two (Furuta, et al., 1999), but the relative magnitudes of the values areconsistent when measured on the same apparatus as is the case here.
Data for N-CNB-5HT, NPE-O-5HT, and NPEC-N-5HT were not reported.
N.R. (not reported)
GM = 10−50 (cm4 s)/photon
Figure 3.
Photolysis reaction of BHQ-O-5HT and BHQ-N-5HT.
Figure 4.
Time course for photolysis of BHQ-O-5HT (closed circles) and BHQ-N-5HT (closed squares) at (a) 365 nm (1PE) and (b) 740 nm (2 PE) in KMOPS buffer (pH 7.2) and rise of 5-HT from BHQ-O-5HT (open circles) and BHQ-N-5HT (open squares), respectively. The concentration was determined by HPLC using an external standard and is the average of at least 3 runs. Lines are least-squares fits of a single exponential decay or a single exponential rise to max. From the decay curves, Qu and δu were calculated. Error bars represent the standard deviation of the measurement.
BHQ-N-5HT might be less useful in applications employing 2PE or activating the ionotrophic 5-HT receptors, because release of 5-HT from BHQ-N-5HT upon light exposure is slow. Initially formed carbamic acid intermediates typically decompose to CO2 and the amine on the 6-7 ms timescale (Papageorgiou and Corrie, 1997), which is slower than the diffusional escape time from the focal volume of 2PE excitation (estimated at 113-900 μs (Kiskin and Ogden, 2002)) and the opening of ionotrophic 5-HT-gated ion channels (5-HT3 receptors open on the order of 1-2 ms timescales and remain open for up to 10 ms (Jackson and Yakel, 1995)). Time-resolved studies on BHQ-caged acetate (Ma, et al., 2012) suggest that BHQ-O-5HT releases 5-HT on nanosecond timescales—orders of magnitude shorter than diffusional timescales.
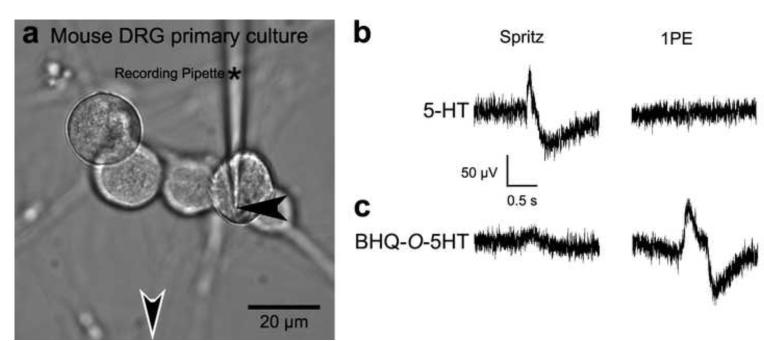
To test the biological effects of BHQ-O-5HT on neural activity, extracellular recordings were obtained from dissociated sensory neurons prepared from mouse dorsal root ganglia (DRG) and the trigeminal ganglion or optic tectum in intact zebrafish larva during exposure to 5-HT, BHQ-O-5HT, or BHQ-OH. Previous studies have shown that 5-HT elicits depolarizing responses in small-diameter trigeminal ganglion neurons in mammals and type A and C primary afferent neurons in the dorsal root ganglia (DRG) of mammals and frogs (Holz and Anderson, 1984; Holz, et al., 1985; Todorovic and Anderson, 1990; Tsutsui, et al., 2008). 5-HT is also known to increase a hyperpolarization-activated cation current in type Aα and Aβ DRG neurons (Cardenas, et al., 1999; Harper and Lawson, 1985a; Harper and Lawson, 1985b; Scroggs, et al., 1994; Villière and McLachlan, 1996). Figure 5 depicts the results for DRG neurons. For these experiments, recordings were obtained from DRG neurons with soma 19±2 μm in diameter (Figure 5a), which are largely nociceptive afferent (C and Aδ) neurons, but can include Aα/Aβ neurons (Harper and Lawson, 1985a; Harper and Lawson, 1985b; Lawson and Waddell, 1991). Therefore, neurons were first tested for a response to 100 μM 5-HT and then the same neurons were exposed to 500 μM BHQ-O-5HT or BHQ-OH (Figure 3), a control to test for the effects of the caging group. All compounds were administered by pressure ejection of 1 nL of solution from a micropipette, the tip of which was located 90-110 μm from the neuron cell body to minimize potential artifacts associated with the pressure ejection. Cells were exposed to a 1-ms pulse of 365-nm light 10 seconds after application of the compound. In all cases, pressure ejection of BHQ-O-5HT induced small changes in baseline activity DRG neurons, which was likely due to small amounts of uncaged 5-HT in the solution; however, activity comparable to uncaged 5-HT was observed only after exposure to a 1-ms pulse of 365-nm light (compare Figure 5b to c). For DRG neurons of this size class, application of 5-HT resulted in a negative extracellular potential (i.e, depolarization) in 26/34 neurons tested and a positive potential (i.e., hyperpolarization-activated cation current) in 8/34 neurons tested. In seventeen cases, where there were multiple cells in the field, biphasic responses were recorded. 5-HT and uncaged BHQ-O-5HT elicited the same effect for any given neuron. Application of the caged compound and uncaging could be performed repeatedly on the same neuron under perfusion conditions. No significant change in neuronal activity was observed after ejection of solvent (Ringers solution with 1% DMSO) or BHQ-OH, or upon exposure to 1PE in the absence of BHQ-O-5HT (Figure 5b and data not shown). Together, these results demonstrated that BHQ-O-5HT could be used to modulate the activity of mammalian neurons in culture. A cell viability assay (Freshney, 1987) showed no statistical difference (t-test, p < 0.01) in the percentage of dying cells between BHQ-O-5HT-treated and untreated control cultures, suggesting that mammalian neurons in culture tolerate BHQ-O-5HT well.
Figure 5.
BHQ-O-5HT acts on mouse DRG neurons in culture. (a) Brightfield image of mouse DRG neurons in culture showing placement of the field recording pipette (black arrowhead) and direction toward the microinjection pipette (open white arrowhead), which was located 100 μm from the cells and is out of the field of view. (b) Pressure injection of 1 nL of a 100 μM buffered 5-HT solution induced activity in the medium sized DRG neuron shown in (a) but no change in activity was observed upon exposure to a 1-ms pulse of 365-nm light (1PE). (c) Although pressure ejection of a 1-mM buffered BHQ-O-5HT solution induced a small change baseline activity in the same neuron as (b), a significant change in activity was observed after exposure to a 1-ms pulse of 365-nm light. The traces comparing 5-HT to BHQ-O-5HT are temporally aligned to facilitate comparison.
Figures 6 depicts the results of using BHQ-O-5HT to control neural activity in vivo. For these experiments, zebrafish larvae were immobilized in agar at 5 dpf and solutions containing 5-HT (100 μM), BHQ-O-5HT (1 mM), or BHQ-OH (1 mM) dissolved in Ringers containing 1% DMSO were microinjected in the vicinity of the maxillary nerve (Figure 6a). Electrical activity was monitored with a field electrode placed under visual guidance on the ventral aspect of the trigeminal ganglion (Figure 6a). This placement also likely detects electrical activity from the anterodorsal lateral line ganglion (Raible and Kruse, 2000). Whereas injection of 5-HT typically induced a characteristic change in the electrographic activity (n = 6/7) injection of BHQ-O-5HT did not significantly affect baseline activity until after photolysis using 365-nm light (n = 6/6). In some experiments, a short-lived electrographic response was observed immediately after injection of BHQ-O-5HT but before exposure to light, suggesting that a small amount of 5-HT was released during handling or injection; however, in these cases, exposure to light also resulted in a significant electrographic response, indicating that the majority of the compound had remained in a caged state. In these experiments, repeated exposure to light resulted in repeated induction of neural activity (Figure 6c) for up to 10 trials; however, as expected, the amount of activity began to diminish with time and the number of exposures to light. This suggests that enough of caged compound remained after illumination to permit repeated stimulation experiments in intact preparations. No change in baseline activity was observed after injection with BHQ-OH or by exposing the larva to flashes of light in the absence of BHQ-O-5HT. Comparable results were obtained for experiments directed toward the optic tectum (Figure S2). We observed no mortality resulting from injection or an obvious increase in cell death in the vicinity of the injection site (n = 47) during the course of the experiments, indicating that the zebrafish tolerate BHQ-O-5HT.
Figure 6.
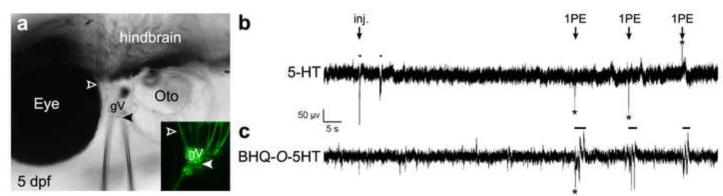
BHQ-O-5HT excites trigeminal neurons in intact zebrafish larva. (a) Lateral brightfield view of a zebrafish larva at 5 dpf showing placements of the field recording (black arrowhead) and microinjection (open white arrowhead) pipettes relative to the trigeminal ganglion (gV). Dorsal is up and anterior is left, with the eye, hindbrain and ear (oto, otocyst) indicated for reference. The inset is a confocal fluorescence image of the trigeminal ganglion obtained from a comparable experiment using a 5 dpf larva expressing the cameleon calcium indicator in all neurons. Placements of the recording (arrowhead) and injection (open arrowhead) pipets are indicated. (b-c) In vivo extracellular field recordings of 5-HT induced changes in trigeminal ganglion activity. (b) Baseline recordings from the ventral aspect of the trigeminal ganglion show low amplitude neural activity. Microinjection of 0.5 nL of a 1 mM buffered 5-HT solution in the region of the putative maxillary nerve elicited a brief bust of high amplitude spiking; in some cases, this initial discharge was followed by a second burst within a few seconds of the injection. Bars above traces denote significant changes from baseline activity. No change in activity was observed upon exposure to three 1-ms pulses of 365-nm light (1PE) spaced ~15 s apart. Electronic spikes associated with the lamp discharge are denoted with asterisks; these occur about 60% of the time. (c) Micro-injection of 1 nL of a 500-mM buffered BHQ-O-5HT solution did not alter baseline activity. Photolysis of BHQ-O-5HT by exposure to 1-ms pulses of 365-nm light elicited high amplitude spiking that typically lasted a few seconds (bars). The traces comparing 5-HT to BHQ-O-5HT are temporally aligned to facilitate comparison. A similar experiment was carried out in the optic tectum of 7 dpf zebrafish larva (Figure S2).
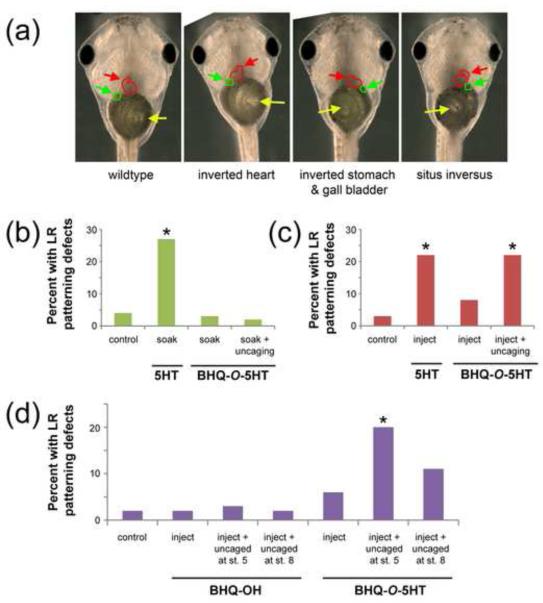
Because 5-HT signaling is required for LR patterning in Xenopus laevis embryos (Fukumoto, et al., 2005a; Fukumoto, et al., 2005b; Vandenberg, et al., 2013), we used this endpoint and animal model to assay the physiological action of caged serotonin molecules. To test the effects of BHQ-O-5HT on LR patterning, embryos were soaked in BHQ-O-5HT (1 mM) from 1-cell through 32-cell (stage 5), washed, and then both the top and bottom of the embryos were exposed to light for 1 h using a broad-spectrum lamp to uncage 5-HT. LR patterning was assessed at stage 45 via inspection of three asymmetric organs: the heart, stomach, and gall bladder (Figure 7a). In contrast to embryos soaked in 5-HT, no LR patterning defects were observed, indicating that BHQ-O-5HT does not penetrate the cell membrane of the Xenopus embryo and is not taken up via the serotonin transporter (Figure 7b). Additional embryos were injected with BHQ-O-5HT at 1-cell and 5-HT was uncaged starting at the 16-cell stage using the broad-spectrum lamp. LR defects including situs inversus and heterotaxia were observed in BHQ-O-5HT injected embryos following uncaging with a similar percent of affected embryos as those observed when 5-HT is injected (Figure 7c). A low rate of LR defects was also observed in embryos injected with BHQ-O-5HT that were maintained in the dark throughout the experiment, suggesting that a small amount of 5-HT is released during handling, injection, or incubation. To verify temporal control of BHQ-O-5HT uncaging, embryos were injected at 1-cell and then maintained in the dark until two later stages of development: 32-cell (stage 5) and early blastula (stage 8). Significant increases in LR patterning defects were observed only when uncaging occurred at stage 5 (Figure 7d).
Figure 7.
BHQ-O-5HT disrupts LR patterning in Xenopus laevis embryos. (a) Position of three organs (heart, red arrow; stomach, yellow arrow; gall bladder, green arrow) in wild type tadpoles and tadpoles with LR patterning defects. Single-cell embryos were soaked (b) or injected (c) with 5-HT or BHQ-O-5HT and LR defects were assessed following uncaging. (d) Temporal assessment of BHQ-O-5HT uncaging indicates a critical period for the effect of 5-HT on LR patterning. Treatment with BHQ-OH ruled out effects of the PPG and the uncaging light treatment in LR defects. * p < 0.01 relative to controls, chi-square test.
Under all conditions, BHQ-O-5HT produced very low levels of toxicity, including few dead or deformed embryos or tadpoles. In experiments where embryos were soaked in BHQ-O-5HT, toxicity rates were 5-6% either when maintained in the dark or uncaged, similar to the rates observed in untreated controls (7%, p > 0.05). Similarly, when BHQ-O-5HT was injected, low toxicity (4-12%) was observed whether maintained in the dark or uncaged, similar to rates observed in controls (5-7%, p > 0.05). Injections with BHQ-OH produced few LR patterning defects (2-3%, see Figure 7d) and little toxicity (4-6% compared to 5% in untreated controls, p > 0.05). Soaking and injection experiments were also conducted with BHQ-N-5HT, but uncaging this molecule did not disrupt LR patterning in any experiment, regardless of which treatment route was utilized (data not shown). This might be due to the inability of light to penetrate the pigment in early cleavage stage embryos with enough intensity to release 5-HT from BHQ-N-5HT.
Significance
Based on the ability of the BHQ protecting group to photochemically release phenol through both 1- and 2-photon excitation, the two forms of caged 5-HT prepared, BHQ-O-5HT and BHQ-N-5HT, have higher quantum efficiencies (Qu) and 2-photon uncaging action cross-sections (δu) than previously reported caged serotonins. BHQ-O-5HT was found to be more sensitive to light at 368 and 740 nm than BHQ-N-5HT, and based on the behavior of similar compounds (Ma, et al., 2012), its release kinetics are expected to be faster than diffusion rates and the opening of 5-HT3 receptors. BHQ-O-5HT mediated the light activation of 5-HT, subsequently depolarizing mammalian neurons in culture or in the nervous system of intact larval zebrafish (5-7 dpf). In the developing Xenopus embryo, light induced release of 5-HT disrupted LR patterning maximally at stage 5 of development.
Taken together, these studies demonstrate the potential of BHQ-caged 5-HT to enable the advanced study of serotonin’s physiological role in a variety of biological contexts, whole animal studies in particular. For example, BHQ-caged 5-HT could enable the exploration of mechanisms involved in the propagation of coherent neural activity (i.e., seizures) in the brain, potentially impacting our understanding of epilepsy and other seizure disorders. More broadly, BHQ-caged 5-HT could be used to explore the role of 5-HT in modulating mood, appetite, memory, learning, and other cognitive functions. Additionally, 5-HT has been shown to play important roles in early developmental patterning events outside of neural tissue, such as LR patterning and melanocyte differentiation. Thus, BHQ-caged 5-HT, which can be manipulated both spatially and temporally, provides significant experimental power to dissect and understand the mechanisms behind 5-HT-mediated signaling in the developing embryo. The BHQ-caged serotonins are relatively non-toxic, have relatively little leakage, and can be released using standard laboratory equipment, enabling biologists to better probe the role of 5-HT signaling pathways in the brain and in early developmental processes.
Experimental Procedures
Preparation of BHQ-OPh
(8-Bromo-7-(methoxymethoxy)quinolin-2-yl)methyl methanesulfonate (MOM-BHQ-OMs)
(8-Bromo-7-(methoxymethoxy)quinolin-2-yl)methanol (MOM-BHQ-OH, 0.526 g, 1.76 mmol) was dissolved in THF. Diisopropyl ethyl amine (0.61 mL, 3.52 mmol) and methanesulfonyl chloride (0.20 mL, 2.64 mmol) were successively added dropwise followed by stirring at rt for 2 h. The reaction was concentrated and the residue purified over silica gel with a gradient from 100% hexanes to 2:3 EtOAc/hexanes, collecting the product as a white solid (0.446 g, 68%): 1H NMR (400 MHz, CDCl3) δ 8.19 (d, 1H), 7.79 (d, 1H), 7.55 (d, 1H), 7.52 (d, 1H), 5.57 (s, 2H), 5.43 (s, 2H), 3.58 (s, 3H), 3.23 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 155.8, 155.7, 146.1, 137.9, 128.2, 124.9, 118.7, 118.0, 112.5; 95.6, 72.4, 56.9, 38.7; HRMS-ESI (m/z) calcd for [M+H]+ 375.9849, 377.9828; found 375.9846, 377.9825.
8-Bromo-7-(methoxymethoxy)-2-(phenoxymethyl)quinoline (MOM-BHQ-OPh)
(8-Bromo-7-(methoxymethoxy)quinolin-2-yl)methyl methanesulfonate (MOM-BHQ-OMs, 0.035 g, 0.093 mmol) was dissolved in THF (2 mL). Phenol (0.016 g, 0.17 mmol) was added followed by 1 M potassium hydroxide solution (170 μL) and the reaction was stirred at room temperature for 12 h. The solvent was removed on a rotary evaporator and the remaining residue was taken up in EtOAc, which was washed successively with water and brine. The EtOAc was removed on a rotary evaporator, and the remaining residue purified by column chromatography with 9:1 hexanes/EtOAc. Fractions were collected and concentrated to yield a residue on the flask wall (0.025 g, 0.067 mmol, 72%): 1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 8.2 Hz, 1H), 7.75 (d, J = 9.0 Hz, 1H), 7.65 (d, J = 8.2 Hz, 1H), 7.50 (d, J = 9.0 Hz, 1H), 7.30 (m, 2H), 7.05 (d, J = 7.8 Hz, 2H), 6.97 (t, J = 7.4 Hz, 1H), 5.46 (s, 2H), 5.42 (s, 2H), 3.59 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 160.0, 158.6, 155.4, 146.0, 137.3, 129.8, 128.2, 124.7, 121.4, 118.3, 117.4, 115.110,112.3, 95.6, 71.4, 56.9; HRMS-ESI (m/z) calcd for [M+H]+ 374.0386, 376.0366; found 374.0401, 376.0382.
8-Bromo-2-(phenoxymethyl)quinolin-7-ol (BHQ-OPh)
8-Bromo-7-(methoxymethoxy)-2-(phenoxymethyl)quinoline (MOM-BHQ-OPh, 0.025 g, 0.067 mmol) was dissolved in MeOH (1 mL). TFA (0.5 mL) was added and the reaction was stirred for 30 min. The solvent was evaporated and the remaining residue was taken up in EtOAc, washed successively with water and brine, and concentrated. The remaining residue was purified by column chromatography with 8:2 hexanes/EtOAc. Fractions were collected and concentrated to yield a residue on the flask wall: 1H NMR (400 MHz, CDCl3) δ 8.11 (d, J = 8.6 Hz, 1H), 7.71 (d, J = 8.6 Hz, 1H), 7.61 (d, J = 8.6 Hz, 1H), 7.31 (m, 3H), 7.04 (d, J = 7.8 Hz, 2H), 6.98 (t, J = 7.4 Hz, 1H), 5.35 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 159.8, 158.6, 154.4, 145.5, 137.4, 129.8, 128.6, 123.9, 121.4, 117.9, 117.7, 115.1, 108.0, 71.3; HRMS-ESI (m/z) calcd for [M+H]+ 330.0124, 332.0104; found 330.0136, 332.0123.
Preparation of BHQ-N-5HT
(8-Bromo-7-(methoxymethoxy)quinolin-2-yl)methyl 1H-imidazole-1-carboxylate (MOM-BHQ-Carbonylimidazole)
MOM-BHQ-OH (0.100 g, 0.34 mmol) was dissolved in THF. Carbonyldiimidazole (0.082 g, 0.50 mmol) was added, and the reaction stirred at rt for 2 h. The reaction was concentrated and the residue dissolved in EtOAc, washed successively with water and brine, dried over MgSO4, filtered, and concentrated in vacuo. The crude product was purifed by column chromatography with silica gel, eluting with a gradient from 1:1 EtOAc/Hexanes to 100% EtOAc, yielding a white solid (0.084 g, 63%): 1H NMR (400 MHz, CDCl3) δ 8.28 (s, 1H), 8.14 (d, J = 8.4 Hz, 1H), 7.74 (d, J = 9.0 Hz, 1H), 7.55 (s, 1H), 7.50 (d, J = 9.0 Hz, 1H), 7.39 (d, J = 8.4 Hz, 1H), 7.11 (s, 1H), 5.75 (s, 2H), 5.39 (s, 2H), 3.56 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 155.8, 155.4, 148.9, 146.1, 137.6, 137.6, 131.0, 128.0, 124.7, 118.0, 117.7, 117.6, 112.6, 95.6, 69.9, 56.8; HRMS-ESI (m/z) calcd for [M+H]+ 392.0246, 394.0225; found 392.0262, 394.0244.
(8-Bromo-7-(methoxymethoxy)quinolin-2-yl)methyl (2-(5-((triisopropylsilyl)oxy)-1H-indol-3-yl)ethyl)carbamate (MOM-BHQ-N-5HT(O-TIPS))
2-(5-((Triisopropylsilyl)oxy)-1H-indol-3-yl)ethanamine (5HT(O-TIPS)), 0.067 g, 0.020 mmol) was dissolved in a small amount of DMF. MOM-BHQ-Carbonylimidazole (0.100 g, 0.25 mmol) was added and the reaction heated to 60 °C and stirred overnight. The solvent was removed in vacuo and the residue partitioned between EtOAc and water. The EtOAc layer was dried over MgSO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography with silica gel, eluting with a gradient from 100% hexanes to 1:1 EtOAc/hexanes, yielding the product as a solid (0.0859 g, 65%): 1H NMR (400 MHz, CDCl3) δ 8.07 (d, J = 8.4 Hz, 1H), 7.95 (s, 1H), 7.72 (d, J = 9.0 Hz, 1H), 7.48 (d, J = 9.0 Hz, 1H), 7.36 (d, J = 8.4 Hz, 1H), 7.18 (d, J = 8.7 Hz, 1H), 7.04 (s, 1H), 7.00 (s, 1H), 6.81 (d, J = 8.7 Hz, 1H), 5.44 (s, 2H), 5.40 (s, 2H), 5.02 (br, 1H), 3.58 (t, J = 6.6 Hz, 2H), 3.57 (s, 3H), 2.95 (t, J = 6.6 Hz, 2H), 1.26 (m, J = 7.3 Hz, 3H), 1.11 (d, J = 7.3 Hz, 18H); 13C NMR (126 MHz, CDCl3) δ 158.8, 156.2, 155.2, 149.7, 145.8, 137.0, 131.8, 127.9, 127.8, 124.4, 122.9, 118.1, 117.2, 116.3, 112.3, 111.4, 107.8, 95.4, 77.2, 67.5, 56.6, 41.2, 25.7, 18.1, 12.7; HRMS-ESI (m/z) calcd for [M+H]+ 656.2155, 658.2135; found 656.2171, 658.2154.
(8-Bromo-7-(methoxymethoxy)quinolin-2-yl)methyl (2-(5-hydroxy-1H-indol-3-yl)ethyl)carbamate (MOM-BHQ-N-5HT)
MOM-BHQ-N-5HT(O-TIPS) (85.9 mg, 0.13 mmol) was dissolved in a small amount of THF. TBAF (0.2 mL, 1.0 M in THF) was added slowly and the reaction stirred at rt for 15 min. The reaction was concentrated and the residue partitioned between EtOAc and water. The organic layer was washed with water and brine, dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography with silica gel, eluting with a gradient from 100% hexanes to 1:1 EtOAc/hexanes, yielding the product as a solid (55 mg, 85%): 1H NMR (400 MHz, CDCl3) δ 8.06 (s, 1H), 8.02 (d, J = 8.3 Hz, 1H), 7.97 (d, J = 9.1 Hz, 1H), 7.67 (d, J = 9.0 Hz, 1H), 7.45 (d, J = 9.1 Hz, 1H), 7.31 (d, J = 8.5 Hz, 1H), 7.13 (d, J = 8.6 Hz, 1H), 6.96 (d, J = 13.2 Hz, 1H), 6.74 (dd, J = 9.1, 14.4 Hz, 1H), 5.41 (s, 2H), 5.38 (s, 2H), 5.18 (br, 1H), 3.56 (s, 3H), 3.48 (q, J = 6.6 Hz, 2H), 2.92 (d, J = 26.8 Hz, 1H), 2.84 (t, J = 6.8 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 158.8, 156.7, 155.5, 149.8, 145.9, 137.4, 131.8, 128.1,126.2, 124.6, 123.4, 118.3, 117.5, 115.8, 112.3, 112.1, 103.2, 103.3, 95.6, 67.7, 56.9, 41.6, 26.0; HRMS-ESI (m/z) calcd for [M+H]+ 500.0821, 502.0801; found 500.0823, 502.0810.
(8-Bromo-7-hydroxyquinolin-2-yl)methyl (2-(5-hydroxy-1H-indol-3-yl)ethyl)carbamate (BHQ-N-5HT)
MOM-BHQ-N-5HT (45 mg, 0.090 mmol) was dissolved in methanol. A small amount of conc. HCl was added and the reaction stirred overnight. The reaction was diluted with EtOAc and washed successively with sat. NaHCO3 and brine, dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The crude product was purified by HPLC with 50% CH3CN/50% H2O (w/ 0.1% TFA) and the first peak (ret. time 4.5 min) was collected and concentrated (22.5 mg, 55%): 1H NMR (500 MHz, (CD3)2CO) δ 9.63 (br, 1H), 8.11 (d, J = 8.3 Hz, 1H), 7.68 (d, J = 8.8 Hz, 1H), 7.26 (t, J = 8.3 Hz, 2H), 7.23 (d, J = 8.8 Hz, 1H), 7.07 (d, J = 8.6 Hz, 1H), 6.98 (s, 1H), 6.88 (s, 1H), 6.57 (d, J = 8.6 Hz, 1H), 6.48 (br, 1H), 5.22 (s, 2H), 3.34 (t, J = 7.3 Hz, 2H), 2.79 (t, J = 7.3 Hz, 2H); 13C NMR (101 MHz, (CD3)2CO) δ 159.1, 156.2, 155.8, 150.7, 145.9, 137.1, 131.6, 128.5, 128.1, 127.6, 123.2, 118.6, 116.8, 111.6, 111.5, 106.9, 102.6, 66.8, 41.6, 29.7, 25.9; HRMS-ESI (m/z) calcd for [M+H]+ 456.0559, 458.0538; found 456.0574, 458.0567.
Preparation of BHQ-O-5HT
tert-Butyl (2-(5-((8-bromo-7-(methoxymethoxy)quinolin-2-yl)methoxy)-1H-indol-3-yl)ethyl)carbamate (MOM-BHQ-O-5HT(N-Boc))
tert-Butyl (2-(5-hydroxy-1H-indol-3-yl)ethyl)carbamate (N-Boc-5HT, 97 mg, 0.35 mmol) was dissolved in acetonitrile and potassium carbonate (86 mg, 0.62 mmol) was added. MOM-BHQ-OMs (188 mg, 0.50 mmol) was added in one portion and the reaction stirred at reflux for 2 d. The reaction was allowed to cool, then filtered and concentrated. The residue was purified by column chromatography with silica gel, eluting with a gradient from 100% hexanes to 3:1 EtOAc/hexanes, yielding a yellow oil (151 mg, 78%): 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 8.4 Hz, 1H), 7.97 (s, 1H), 7.76 (d, J = 9.0 Hz, 1H), 7.74 (d, J = 8.4 Hz, 1H), 7.50 (d, J = 9.0 Hz, 1H), 7.23 (s, 1H), 7.02 (m, 2H), 5.50 (s, 2H), 5.42 (s, 2H), 3.59 (s, 3H), 3.42 (t, J = 6.5 Hz, 2H), 2.90 (t, J = 6.5 Hz, 2H), 1.43 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 170.7, 161.2, 161.1, 160.6, 156.1, 155.3, 153.0, 146.0, 137.2, 131.9, 128.2, 124.7, 118.5, 117.3, 112.8, 112.1, 103.5, 102.6, 95.6, 77.4, 72.2, 56.9, 40.8, 28.7, 26.0; HRMS-ESI (m/z) calcd for [M+H]+ 556.1447, 558.1427; found 556.1432, 558.1420.
2-(((3-(2-Aminoethyl)-1H-indol-5-yl)oxy)methyl)-8-bromoquinolin-7-ol (BHQ-O-5HT)
MOM-BHQ-O-5HT(N-Boc) (0.047 g, 0.085 mmol) was dissolved in methylene chloride. Trifluoroacetic acid was added and the reaction stirred at rt for 1 h. The solvent was removed in vacuo and the residue purified by HPLC with 50% CH3CN/50% H2O (w/ 0.1% TFA). Fractions containing only one peak were combined and concentrated (0.020 g, 57%): 1H NMR (600 MHz, (CD3OD) δ 8.29 (d, J = 8.4 Hz, 1H), 7.80 (d, J = 8.8 Hz, 1H), 7.67 (d, J = 8.3 Hz, 1H), 7.31 (d, J = 7.6 Hz, 1H) , 7.30 (d, J = 8.8 Hz, 1H), 7.24 (d, J = 2.1 Hz, 1H), 7.15 (s, 1H), 6.98 (dd, J = 8.8, 2.2 Hz, 1H), 5.46 (s, 2H), 3.20 (m, 2H), 3.06 (t, J = 7.3 Hz, 2H); 13C NMR (150 MHz, (CD3OD) δ 162.2, 161.8, 151.6, 147.5, 138.9, 133.3, 131.5, 129.0, 125.3, 125.2, 122.2, 117.6, 113.1, 112.9, 109.6, 103.3, 98.9, 61.6, 41.2, 24.7; HRMS-ESI (m/z) calcd for [M+H]+ 412.0661, 414.0640; found 412.0651, 414.0626.
1H NMR spectra for all new compounds and an HPLC chromatogram for BHQ-O-5HT demonstrating purity are provided in the supplemental information.
Photochemistry
Determination of the Molar Extinction Coefficient (ε)
A weighed portion of BHQ-O-5HT was dissolved in methanol. A measured aliquot of this solution was withdrawn and placed in KMOPS buffer (3.0 mL) and mixed thoroughly to generate a 100-μM solution of BHQ-O-5HT. The absorbance A of this solution at λmax = 368 nm was measured. This method was repeated twice with different masses of BHQ-O-5HT. The three absorbance values obtained were averaged and the molar extinction coefficient at λmax = 368 nm was calculated to be 2,000 M-1cm-1 using the equation A = εlc, where A is the absorbance, l is the path length of the cuvette, and c is the concentration of the solution. The ε of BHQ-OPh and BHQ-N-5HT was measured similarly. See Figure S3 for representative UV-vis spectra of BHQ-OPh, BHQ-N-5HT , and BHQ-O-5HT.
Determination of the Time Constant for Dark Hydrolysis (τdark)
Three 100-μM solutions of BHQ-O-5HT in KMOPS were created and stored in the dark. Aliquots (20 μL) were removed periodically from each solution and analyzed by HPLC. The concentration of BHQ-O-5HT (measured by external standard) for each time point for each solution was averaged and plotted versus time. A simple single exponential decay curve provided the best fit and was used to determine the time constant for dark hydrolysis (τdark). The τdark of BHQ-OPh and BHQ-N-5HT was measured similarly.
Determination of the Uncaging Quantum Efficiency (Qu)
As previously described (Adams, et al., 1988; Davis, et al., 2009; Fedoryak and Dore, 2002; Furuta, et al., 1999; Lu, et al., 2003; Zhu, et al., 2006), quantum efficiency was calculated using the equation Qu = (Iσt90%)-1, where I is the irradiation intensity in einstein·cm−2·s−1, σ is the decadic extinction coefficient (1,000 times ε), and t90% is the time in seconds required for the conversion of 90% of the starting material to product. To find t90%, a solution of BHQ-O-5HT in KMOPS was prepared and placed in a cuvette along with a small stir bar. While stirring, the solution was irradiated with UV light from a mercury lamp (Spectroline SB-100P, Spectronics Corporation) equipped with two glass filters (CS0-52, CS7-60, Ace Glass) so that the wavelength was restricted to 365 ± 15 nm. Periodically, 20-μL aliquots were removed and analyzed by HPLC. The time points collected were as follows: 0, 20, 40, 60, 90, and 120 s. The concentration of BHQ-O-5HT remaining (measured by external standard) was plotted versus time of photolysis. A simple single exponential decay curve provided the best fit for the data and was used to extrapolate t90%. The lamp’s UV intensity I was measured using potassium ferrioxalate actinometry (Hatchard and Parker, 1956). Initially, 6 mM potassium ferrioxalate solution (3 mL) was irradiated with the mercury lamp for 60 s. A portion of this solution (2 mL) was combined with aqueous buffer (3 mL), 0.1% phenanthroline solution (3 mL), and 2 M KF solution (1 mL) in a 25-mL volumetric flask. Deionized water was added to generate a 25 mL solution. A blank solution was also prepared using the same method, but the potassium ferrioxalate used in the blank was not irradiated. Both solutions rested for one hour and the blank was then used as a baseline against which the absorbance of the irradiated solution was measured at 510 nm. The following equation was used to calculate lamp intensity:
where V3 is the volume of dilution (25 mL), V2 is the volume of irradiated potassium ferrioxalate solution taken for analysis (2 mL), ΔD510 is the absorption of the solution at 510 nm, ε510 is the actinometry extinction coefficient (1.11 × 104 M-1cm-1), ΦFe is the quantum yield for production of ferrous ions from potassium ferrioxalate at 365 nm, and t represents the time of irradiation. The ΔD510 value used for calculations is the average of two measurements taken before and after irradiation of BHQ-O-5HT. The Qu of BHQ-OPh and BHQ-N-5HT was measured similarly.
Determination of Two-photon Action Cross-Sections (δu)
The 2PE photolysis action cross-sections (δu) were measured using previously described methods (Davis, et al., 2009; Fedoryak and Dore, 2002; Furuta, et al., 1999; Lu, et al., 2003; Zhu, et al., 2006) using fluorescein as an external standard to estimate the pulse parameters of the laser. A portion of BHQ-O-5HT was dissolved in KMOPS buffer and the concentration of the solution was found using UV-Vis absorption in conjunction with Beer’s law. Aliquots (25 μL) of this solution were placed in a microcuvette (10×1×1 mm illuminated dimensions) and irradiated with a fs-pulsed and mode-locked Ti:Sapphire laser (Chameleon Ultra II, Coherent) with 740-nm light at an average power of 300 mW. Three samples were irradiated for each of the following time periods: 0, 10, 20, 30, and 40 min. The samples (20-μL aliquots) were analyzed by HPLC as in the Qu measurement to determine the extent of photolysis at each time point. A solution of fluorescein at pH 9.0 was prepared to act as a standard because of its well-characterized 2PE cross-section (δaF = 30 GM at 740 nm) and quantum yield (QF2 = 0.9). UV-Vis absorption at 488 nm was used to determine the fluorescein concentration. Aliquots (25 μL) of fluorescein solution were placed in the microcuvette and irradiated by the laser in the same apparatus used for the BHQ-O-5HT photolysis. The fluorescence emission from the solution was measured with a radiometer (SED033 detector on an IL-1700, International Light) before and after the BHQ-O-5HT samples were irradiated and the two values were averaged. The following equation was used to calculate the two-photon action cross-section for BHQ-O-5HT:
where Np is the number of product molecules formed per second (determined by HPLC), Φ is the collection efficiency of the detector on the radiometer used to measure the fluorescence of fluorescein passing through the cuvette window and through a 535/545 nm bandpass filter at a right angle to the laser’s beam, CF is the concentration of fluorescein, <F(t)> is the time averaged fluorescent photon flux (photons/s) of fluorescein measured by the radiometer, and CS is the initial concentration of the caged compound. The δu of BHQ-OPh and BHQ-N-5HT was measured similarly.
Electrophysiological Recordings
DRG Neurons
Dissociated primary sensory neurons were prepared from mouse DRGs as previously described (Malin, et al., 2007), and plated on lysine/laminin coated coverslips. For extracellular recordings, the slips with cells were mounted in a horizontal perfusion chamber (PC-H; Siskiyou, Inc.) with a chloride-coated silver reference electrode attached, and placed on the stage of an upright microscope (Zeiss Examiner.Z1). Solutions of 5-HT (100 μM), BHQ-O-5HT (500 μM), or BHQ-OH (500 μM) in normal Ringer's solution containing 1% DMSO were delivered focally to the desired cell by pressure ejection (Picospritzer II, Parker Hannifin) of 1 nL volumes from a fine tipped glass micropipette, the tip of which was placed 100±10 μm from the cell of interest. Flash photolysis was achieved using a Cairn Flash Photolysis System and OptoSource xenon and mercury/xenon mixed-gas arc light source (Cairn Research) equipped with a 365/10 nm bandpass filter (Chroma) and coupled by a fiber optic cable to the Zeiss Examiner microscope through the external port of a Colibri illumination system. Light was focused on the cells of interest using the microscope optics and a water immersion lenses (20×, 40×, or 63×) with ultraviolet transmission properties. Extracellular recordings were made using glass microelectrodes (15–20 MΩ impedance) loaded with normal Ringer’s solution (116 mM NaCl, 2.9 mM KCl, 1.8 mM CaCl2, 5.0 mM HEPES, pH 7.2.) that were gently affixed to the soma by suction. Electrical activity was recorded using an Axoclamp 900a amplifier (Axon Instruments, Union City, Ca, USA). The amplified voltage was passed through a Hum Bug Noise Eliminator (AutoMate Scientific, Berkley, CA, USA), band-pass filtered from 1 Hz–0.1 kHz, and digitized at 10 kHz using a Digidata 1440 interface and stored on a PC using pClamp software (version 10.3, Axon). For all experiments, the set-up procedure was carried out in dark room conditions at room temperature (23○C).
Assessment of Toxicity
DRG neurons in culture were exposed to BHQ-O-5HT (1 mM) followed by incubation in the dark for 8 to 12 h. Viable cell counts were made using Trypan Blue, a dye that is excluded from living cells (Freshney, 1987). The percentage of dead cells was determined by dividing the number of Trypan Blue-stained cells by the total number of cells on a 22 × 22-mm coverslip.
Larval Zebrafish
Larval zebrafish (Danio rerio) of the WIK strain were obtained from animals maintained in the University of Georgia Zebrafish Facility following standard procedures (Westerfield, 2007). Embryos and larvae were staged using standard staging criteria (Kimmel, et al., 1995; Westerfield, 2007). All experiments conformed to the guidelines on the ethical use of animals. All experimental procedures were conducted according to National Institutes of Health guidelines under protocols approved by the University of Georgia Institutional Animal Care and Use Committee and were designed to minimize animal suffering.
Larval zebrafish, 5 days post-fertilization (5 dpf) of age, were immobilized by exposure to alpha bungarotoxin (Trapani and Nicolson, 2010) and mounted in 1.2% agarose made with normal Ringer’s in a 35-mm petri dish. A sharp glass microelectrode (15–20 MΩ impedance), loaded with normal Ringer’s solution, was placed under visual guidance on the ventral aspect of the trigeminal ganglion and the chloride-coated silver reference wire was placed was placed touching a dorsal region of the tail. For drug delivery, a second sharp glass pipet was inserted in the vicinity of the maxillary nerve. After a 2-minute baseline was recorded, 0.5 nl 5-HT (100 μM), BHQ-O-5HT (1 mM), or BHQ-OH (1 mM) dissolved in Ringers containing 1% DMSO were pressure injected (Picospritzer II, Parker Hannifin) and the neurological responses recorded. Flash photolysis was achieved and electrical activity recorded as described above. As with the DRG experiments, these experiments were carried out under dark room conditions and at room temperature.
Assessment of Toxicity
The mortality of the zebrafish larvae after injection of BHQ-O-5HT into the trigeminal nerve or the optic tectum was used as a measure of the toxicity of BHQ-O-5HT. Cell death at the injection site was assessed by differential interference contrast (DIC) microscopy and/or acridine orange staining.
In vivo Assay of LR Patterning in Xenopus laevis Embryos
Animals
Xenopus embryos were collected and fertilized according to standard protocols (Sive, et al., 2000) in 0.1X Modified Marc’s Ringers (MMR) pH 7.8 containing 0.1% gentamicin and staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by Tufts University’s Institutional Animal Care and Use Committee (#M2011-70).
Microinjection of Xenopus Embryos
Single-cell embryos were placed in 3% Ficoll in 1X MMR and injected in the animal pole using standard methods (50-100 ms pulses with borosilicate glass needles calibrated for a bubble pressure of 50-70 kPa in water). Injections occurred under a red lamp to prevent spurious uncaging of the molecules and were otherwise protected from light. For 5-HT, 30 ng was injected; for BHQ-O-5HT, 50 ng was injected; for BHQ-OH, 40 ng was injected. Following injections, embryos were washed and incubated at 18 ○C in the dark.
Embryo Soaking
Single-cell embryos were placed in 1X MMR containing 5-HT (5 mM) or BHQ-O-5HT (1 mM). Embryos were washed at stage 5 or stage 8 and returned to 0.1X MMR. Following treatment, embryos were washed and kept in 0.1X MMR at 18 ○C in the dark.
Uncaging 5-HT from BHQ-O-5HT
Following soaking or injection with BHQ-O-5HT, embryos were placed on a platform and subjected to high intensity broad spectrum light from two light sources: one was located below the embryos and one was located above the embryos. Light treatment progressed for one hour and embryos were then washed and returned to a dark 18 ○C incubator.
Laterality Assay
At stage 45, Xenopus embryos were analyzed for position of the heart (looping to the left), stomach (coiling to the left), and gall bladder (positioned on the right). Heterotaxia was defined as the reversal in position of one or more organs. Only embryos with normal dorsoanterior patterning were scored. Percent with LR patterning defects was calculated as the absolute number of heterotaxic embryos divided by the total number of scorable embryos. A chi-square test with Pearson correction for increased stringency was used to compare absolute counts of heterotaxic embryos.
Assessment of Toxicity
The number of embryos and tadpoles that died or were otherwise malformed (abnormal dorsoanterior patterning, edema, spina bifida, etc.) was counted for each treatment group. The toxicity rate was calculated as the absolute number of dead and malformed embryos divided by the total number of embryos. A chi-square test with Pearson correction for increased stringency was used to compare toxicity rates between treated and untreated groups.
Supplementary Material
Highlights.
BHQ-caged serotonins release 5-HT efficiently through 1- and 2-photon excitation.
Light activation of 5-HT induces serotonergic signaling in neurons and zebrafish.
BHQ-caged serotonin enables temporal control of left-right patterning in Xenopus.
Acknowledgements
We thank Matthew J. O’Connor for technical assistance. Grants to J.D.L and T.M.D. (NIH R01 NS070159), M.L. (AHA 0740088N, NIH GM077425), and T.M.D. (NSF CHE-1012412 and CHE-1317760) supported the work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams SR, Kao JPY, Grynkiewicz G, Minta A, Tsien RY. Biologically useful chelators that release Ca2+ upon illumination. J. Am. Chem. Soc. 1988;110:3212–3220. [Google Scholar]
- Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol. 2011a;22:390–404. doi: 10.1097/FBP.0b013e328349aae4. [DOI] [PubMed] [Google Scholar]
- Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behavioural pharmacology. 2011b;22:390–404. doi: 10.1097/FBP.0b013e328349aae4. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann. Neurol. 1978a;4:451–459. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978b;4:451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- Boahen YO, MacDonald GM. A concise approach to caged serotonin for Fourier transform infrared (FT-IR) difference photolysis studies. J. Ghana Sci. Assoc. 2005;7:54–59. [Google Scholar]
- Bort G, Gallavardin T, Ogden D, Dalko PI. From One-Photon to Two-Photon Probes: "Caged" Compounds, Actuators, and Photoswitches. Angew. Chem., Int. Ed. 2013;52:4526–4537. doi: 10.1002/anie.201204203. [DOI] [PubMed] [Google Scholar]
- Breitinger H-GA, Wieboldt R, Ramesh D, Carpenter BK, Hess GP. Synthesis and Characterization of Photolabile Derivatives of Serotonin for Chemical Kinetic Investigations of the Serotonin 5-HT3 Receptor. Biochemistry. 2000;39:5500–5508. doi: 10.1021/bi992781q. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Del Mar LP, Vysokanov AV, Arnold PB, Cardenas LM, Surmeier DJ, Scroggs RS. Serotonergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. J. Physiol. 1999;518:507–523. doi: 10.1111/j.1469-7793.1999.0507p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert EA, Condron BG. Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci. 2010;33:424–434. doi: 10.1016/j.tins.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Kragor CH, Reddie KG, Wilson HC, Zhu Y, Dore TM. Substituent Effects on the Sensitivity of a Quinoline Photoremovable Protecting Group to One- and Two-Photon Excitation. J. Org. Chem. 2009;74:1721–1729. doi: 10.1021/jo802658a. [DOI] [PubMed] [Google Scholar]
- Dore TM. Multiphoton Phototriggers for Exploring Cell Physiology. In: Goeldner M, Givens RS, editors. In Dynamic Studies in Biology: Phototriggers, Photoswitches, and Caged Biomolecules. Wiley-VCH; Weinheim, Germany: 2005. pp. 435–459. [Google Scholar]
- Dore TM, Wilson HC. Chromophores for the Delivery of Bioactive Molecules with Two-Photon Excitation. In: Chambers JJ, Kramer RH, editors. In Photosensitive Molecules for Controlling Biological Function. Humana Press; New York: 2011. pp. 57–92. [Google Scholar]
- Ellis-Davies GCR. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat. Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoryak OD, Dore TM. Brominated hydroxyquinoline as a photolabile protecting group with sensitivity to multiphoton excitation. Org. Lett. 2002;4:3419–3422. doi: 10.1021/ol026524g. [DOI] [PubMed] [Google Scholar]
- Feldberg W, Toh CC. Distribution of 5-hydroxytryptamine (serotonin, enteramine) in the wall of the digestive tract. J. Physiol. 1953;119:352–362. doi: 10.1113/jphysiol.1953.sp004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A, Hensler JG. Understanding the neuroanatomical organization of serotonergic cells in brain provides insight into the functions of this neurotransmitter. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. In Basic Neurochemistry. 6th Lippincott-Raven; Philadelphia, PA: 1999. pp. 264–268. [Google Scholar]
- Freshney RI. Culture of Animal Cells: A Manual of Basic Technique. 2nd A. R. Liss; New York: 1987. [Google Scholar]
- Fukumoto T, Blakely R, Levin M. Serotonin Transporter Function Is an Early Step in Left-Right Patterning in Chick and Frog Embryos. Dev. Neurosci. 2005a;27:349–363. doi: 10.1159/000088451. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kema IP, Levin M. Serotonin Signaling Is a Very Early Step in Patterning of the Left-Right Axis in Chick and Frog Embryos. Curr. Biol. 2005b;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Furuta T, Wang SSH, Dantzker JL, Dore TM, Bybee WJ, Callaway EM, Denk W, Tsien RY. Brominated 7-hydroxycoumarin-4-ylmethyls: photolabile protecting groups with biologically useful cross-sections for two photon photolysis. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1193–1200. doi: 10.1073/pnas.96.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J. Physiol. 1985a;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J. Physiol. 1985b;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchard CG, Parker CA. A new sensitive chemical actinometer. II. Potassium ferrioxalate as a standard chemical actinometer. Proc. R. Soc. London. 1956;235:518–536. Ser. A. [Google Scholar]
- Ho K.-k., Baldwin JJ, Bohnstedt AC, Kultgen SG, McDonald E, Guo T, Morphy JR, Rankovic Z, Horlick R, Appell KC. Preparation of 2-(aminomethyl) arylamide analgesics. 2003. U.S. Patent Application 10364039.
- Holz GGI, Anderson EG. The actions of serotonin on frog primary afferent terminals and cell bodies. Comp. Biochem. Physiol., C: Comp. Pharmacol. Toxicol. 1984;77C:13–21. doi: 10.1016/0742-8413(84)90124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GGI, Shefner SA, Anderson EG. Serotonin depolarizes type A and C primary afferents: an intracellular study in bullfrog dorsal root ganglion. Brain Res. 1985;327:71–79. doi: 10.1016/0006-8993(85)91500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Jackson MB, Yakel JL. The 5-HT3 receptor channel. Annu. Rev. Physiol. 1995;57:447–468. doi: 10.1146/annurev.ph.57.030195.002311. [DOI] [PubMed] [Google Scholar]
- Kang K, Park S, Kim YS, Lee S, Back K. Biosynthesis and biotechnological production of serotonin derivatives. Appl. Microbiol. Biotechnol. 2009;83:27–34. doi: 10.1007/s00253-009-1956-1. [DOI] [PubMed] [Google Scholar]
- Kaplan JH, Forbush B, III, Hoffman JF. Rapid photolytic release of adenosine 5′-triphosphate from a protected analogue: Utilization by the Na:K pump of human red blood cell ghosts. Biochemistry. 1978;17:1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kiskin NI, Chillingworth R, McCray JA, Piston D, Ogden D. The efficiency of two-photon photolysis of a "caged" fluorophore, o-1-(2-nitrophenyl)ethylpyranine, in relation to photodamage of synaptic terminals. Eur. BioPhys. J. 2002;30:588–604. doi: 10.1007/s00249-001-0187-x. [DOI] [PubMed] [Google Scholar]
- Kiskin NI, Ogden D. Two-photon excitation and photolysis by pulsed laser illumination modelled by spatially non-uniform reactions with simultaneous diffusion. Eur. BioPhys. J. 2002;30:571–587. doi: 10.1007/s00249-001-0186-y. [DOI] [PubMed] [Google Scholar]
- Klan P, Solomek T, Bochet CG, Blanc A, Givens R, Rubina M, Popik V, Kostikov A, Wirz J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013;113:119–191. doi: 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer RH. In: Photosensitive Molecules for Controlling Biological Function. Chambers JJ, editor. Humana Press; New York: 2011. [Google Scholar]
- Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J. Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-M, Larson DR, Lawrence DS. Illuminating the Chemistry of Life: Design, Synthesis, and Applications of "Caged" and Related Photoresponsive Compounds. ACS Chem. Biol. 2009;4:409–427. doi: 10.1021/cb900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Buznikov GA, Lauder JM. Of Minds and Embryos: Left-Right Asymmetry and the Serotonergic Controls of Pre-Neural Morphogenesis. Dev. Neurosci. 2006;28:171–185. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- Lu M, Fedoryak OD, Moister BR, Dore TM. Bhc-diol as a photolabile protecting group for aldehydes and ketones. Org. Lett. 2003;5:2119–2122. doi: 10.1021/ol034536b. [DOI] [PubMed] [Google Scholar]
- Ma J, Rea AC, An H, Ma C, Guan X, Li M-D, Su T, Yeung CS, Harris KT, Zhu Y, Nganga JL, Fedoryak OD, Dore TM, Phillips DL. Unraveling the Mechanism of the Photodeprotection Reaction of 8-Bromo- and 8-Chloro-7-hydroxyquinoline Caged Acetates. Chem.—Eur. J. 2012;18:6854–6865. doi: 10.1002/chem.201200366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2:152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- Mayer G, Heckel A. Biologically active molecules with a "light switch". Angew. Chem., Int. Ed. 2006;45:4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- McGowan K, Kane A, Asarkof N, Wicks J, Guerina V, Kellum J, Baron S, Gintzler AR, Donowitz M. Entamoeba histolytica causes intestinal secretion: role of serotonin. Science. 1983;221:762–764. doi: 10.1126/science.6308760. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) North-Holland Publishing Company; Amsterdam: 1967. [Google Scholar]
- Nikolenko V, Yuste R, Zayat L, Baraldo LM, Etchenique R. Two-photon uncaging of neurochemicals using inorganic metal complexes. Chem. Commun. 2005:1752–1754. doi: 10.1039/b418572b. [DOI] [PubMed] [Google Scholar]
- Papageorgiou G, Corrie JET. Synthesis and properties of carbamoyl derivatives of photolabile benzoins. Tetrahedron. 1997;53:3917–3932. [Google Scholar]
- Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421:189–198. [PubMed] [Google Scholar]
- Rapport MM, Green AA, Page IH. Serum vasoconstrictor (serotonin). IV. Isolation and characterization. J. Biol. Chem. 1948;176:1243–1251. [PubMed] [Google Scholar]
- Roshchina VV. Neurotransmitters in Plant Life. Science Publishers; Enfield, NH: 2001. [Google Scholar]
- Salierno M, Marceca E, Peterka DS, Yuste R, Etchenique R. A fast ruthenium polypyridine cage complex photoreleases glutamate with visible or IR light in one and two photon regimes. J. Inorg. Biochem. 2010;104:418–422. doi: 10.1016/j.jinorgbio.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroggs RS, Todorovic SM, Anderson EG, Fox AP. Variation in IH, IIR, and ILEAK between acutely isolated adult rat dorsal root ganglion neurons of different size. J. Neurophysiol. 1994;71:271–279. doi: 10.1152/jn.1994.71.1.271. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. [Google Scholar]
- Specht A, Bolze F, Omran Z, Nicoud J-F, Goeldner M. Photochemical tools to study dynamic biological processes. HFSP J. 2009;3:255–264. doi: 10.2976/1.3132954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic S, Anderson EG. 5-HT2 and 5-HT3 receptors mediate two distinct depolarizing responses in rat dorsal root ganglion neurons. Brain Res. 1990;511:71–79. doi: 10.1016/0006-8993(90)90226-2. [DOI] [PubMed] [Google Scholar]
- Trapani JG, Nicolson T. Physiological recordings from zebrafish lateral-line hair cells and afferent neurons. Methods Cell Biol. 2010;100:219–231. doi: 10.1016/B978-0-12-384892-5.00008-6. [DOI] [PubMed] [Google Scholar]
- Tsutsui Y, Ikeda M, Takeda M, Matsumoto S. Excitability of small-diameter trigeminal ganglion neurons by 5-HT is mediated by enhancement of the tetrodotoxin-resistant sodium current due to the activation of 5-HT4 receptors and/or by the inhibition of the transient potassium current. Neuroscience. 2008;157:683–696. doi: 10.1016/j.neuroscience.2008.09.024. [DOI] [PubMed] [Google Scholar]
- Vandenberg, L,N, Levin M. Far from solved: a perspective on what we know about early mechanisms of left-right asymmetry. Dev. Dyn. 2010;239:3131–3146. doi: 10.1002/dvdy.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Lemire JM, Levin M. Serotonin has early, cilia-independent roles in Xenopus left-right patterning. Dis. Models Mech. 2013;6:261–268. doi: 10.1242/dmm.010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villière V, McLachlan EM. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. J. Neurophysiol. 1996;76:1924–1941. doi: 10.1152/jn.1996.76.3.1924. [DOI] [PubMed] [Google Scholar]
- Walker JW, Martin H, Schmitt FR, Barsotti RJ. Rapid release of an α-adrenergic receptor ligand from photolabile analogs. Biochemistry. 1993;32:1338–1345. doi: 10.1021/bi00056a020. [DOI] [PubMed] [Google Scholar]
- Warther D, Gug S, Specht A, Bolze F, Nicoud JF, Mourot A, Goeldner M. Two-photon uncaging: New prospects in neuroscience and cellular biology. Bioorg. Med. Chem. 2010;18:7753–7758. doi: 10.1016/j.bmc.2010.04.084. [DOI] [PubMed] [Google Scholar]
- Westerfield M, editor. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) 5th University of Oregon Press; Eugene, OR: 2007. [Google Scholar]
- Young DD, Deiters A. Photochemical control of biological processes. Org. Biomol. Chem. 2007;5:999–1005. doi: 10.1039/b616410m. [DOI] [PubMed] [Google Scholar]
- Zayat L, Salierno M, Etchenique R. Ruthenium(II) Bipyridyl Complexes as Photolabile Caging Groups for Amines. Inorg. Chem. 2006;45:1728–1731. doi: 10.1021/ic0512983. [DOI] [PubMed] [Google Scholar]
- Zhao J, Gover TD, Muralidharan S, Auston DA, Weinreich D, Kao JPY. Caged Vanilloid Ligands for Activation of TRPV1 Receptors by 1- and 2-Photon Excitation. Biochemistry. 2006;45:4915–4926. doi: 10.1021/bi052082f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Pavlos CM, Toscano JP, Dore TM. 8-Bromo-7-hydroxyquinoline as a Photoremovable Protecting Group for Physiological Use: Mechanism and Scope. J. Am. Chem. Soc. 2006;128:4267–4276. doi: 10.1021/ja0555320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.