Abstract
Congenital disorders of glycosylation are a group of metabolic disorders with an expansive and highly variable clinical presentation caused by abnormal glycosylation of proteins and lipids. Dolichol kinase (DOLK) catalyzes the final step in biosynthesis of dolichol phosphate (Dol-P), which is the oligosaccharide carrier required for protein N-glycosylation. Human DOLK deficiency, also known as DOLK-CDG or CDG-Im, results in a syndrome that has been reported to manifest with dilated cardiomyopathy of variable severity. A male neonate born to non-consanguineous parents of Palestinian origin presented with dysmorphic features, genital abnormalities, talipes equinovarus, and severe, refractory generalized seizures. Additional multi-systemic manifestations developed including dilated cardiomyopathy, hepatomegaly, severe insulin-resistant hyperglycemia, and renal failure, which were ultimately fatal at age 9 months. Electrospray ionization mass spectrometric (ESI-MS) analysis of transferrin identified a type I congenital disorder of glycosylation; next-generation sequencing demonstrated homozygous p.Q483K DOLK mutations that were confirmed in patient fibroblasts to result in severely reduced substrate binding and catalytic activity. This patient expands the phenotype of DOLK-CDG to include anatomic malformations and multi-systemic dysfunction.
Keywords: Congenital disorder of glycosylation, DOLK-CDG, Dolichol kinase deficiency, Renal failure, Hepatic dysfunction, Insulin-resistant hyperglycemia
1. INTRODUCTION
Congenital disorders of glycosylation (CDGs) are a group of metabolic disorders caused by abnormal protein and lipid glycosylation, a co- and post-translational addition of carbohydrate moieties essential for protein folding, stability, and cell-cell adhesion [1]. N-linked glycosylation involves transfer of pre-assembled oligosaccharides from dolichol-pyrophosphate to an asparagine residue of a protein in the endoplasmic reticulum [1]. Isoelectric focusing (IEF), electrospray ionization mass spectrometry (ESI-MS), high performance liquid chromatography (HPLC), and capillary electrophoresis (CE) are used to search for patterns of underglycosylated serum transferrin seen in N-linked CDGs [2]. Because glycosylation is a fundamental process essential to protein and lipid function, CDGs have pleiotropic effects that often have severe or fatal manifestations in affected individuals. Symptoms generally associated with CDGs include profound global delay, epilepsy, polyneuropathy, ataxia, endocrine abnormalities, ichthyosis, visual and hearing loss, cardiac, liver, renal, and gastrointestinal involvement [3].
In 2007, a CDG involving a defect in dolichol kinase [DOLK, E.C. 2.7.1.108], the enzyme catalyzing the final step in the biosynthesis of dolichol phosphate (Dol-P), was discovered [4]. Dolichol phosphate is essential for N-glycosylation due to its role as the oligosaccharide carrier upon which the lipid-linked oligosaccharide (LLO) precursor is assembled [5]. Dol-P, in the form of Dol-P-Mannose, also acts as a mannose donor for LLO synthesis, O-, C-mannosylation, and glycerol phosphatidylinositol anchor synthesis [5]. The clinical spectrum of previously reported patients with DOLK-CDG (refer to Table 1 for a summary of their symptoms and DOLK mutations), also known as CDG-Im (OMIM #610768), includes seizure disorder and developmental delay [6], progressive dilated cardiomyopathy, severe hypotonia, and ichthyosis [5]. We report a patient with DOLK-CDG sharing symptoms with those patients, but also presenting novel clinical manifestations that expand the DOLK-CDG phenotype.
Table 1.
Summary of clinical manifestations, mutations, and outcomes of reported DOLK-CDG patients. (NA: Data not available)
| Original # | Our patient | 4-month old [6] | 10-year old [6] | I/2 [3] | I/5 [3] | I/6 [3] | II/2 [3] | II/3 [3] | III/1 [3] |
| Gender | M | M | F | M | F | M | M | M | F |
| Ethnic Background | Palestinian | Syrian-Turkish | Syrian-Turkish | Druze | Druze | Druze | Druze | Druze | Bedouin |
| Consanguinity | −(to best of parental knowledge) | + | + | + | + | + | + | + | + |
| DOLK mutations | Homo. c. 1447C > A; (p.Q483 K) | Homo. c.2T>C; (p.M1I) | Homo. c.2T>C; (p.M1I) | Homo c.1222C > G; (p. H408D) | Homo c.1222C > G; (p. H408D) | Homo c.1222C > G; (p. H408D) | Homo c.1222C > G; (p. H408D) | Homo c.1222C > G; (p. H408D) | Homo c.912G > T; (p.W304 C) |
| Onset of Symptomsa | Early | Early | Early | Late | Late | Late | Late | Late | Late |
| Neurologic Abnormalities | Refractory Seizures, Cortical Atrophy | Generalized tonic-clonic seizures, Multifocal seizures | Generalized tonic-clonic seizures, Multifocal seizures | − | − | − | − | − | − |
| Cognitive Impairment | Profound global delay | Intellectual disability, Developmental delay | Intellectual disability, Autism spectrum disorder | − | − | − | Mild developmental delay | − | − |
| Dilated Cardio myopathy | + | − | − | + | + | + | + | + | + |
| Hepatic Involvement | Hepatomegaly with ↑ Transaminases, Ascites | − | − | NA | ↑ Transaminases | ↑ Transaminases | ↑ Transaminases | ↑ Transaminases | − |
| Abnor | − | NA | NA | NA | + | + | + | + | + |
| mal Coagulation Factors | |||||||||
| Renal Involvement | Hyperkalemia, Anuria | − | − | NA | NA | NA | NA | NA | NA |
| Endocrine Involvement | Insulin-Resistant Hyperglycemia | − | − | NA | − | − | Low cortisol | − | Low cortisol/ACTH |
| Muscular Hypotonia | + | − | − | − | − | + | − | − | − |
| Creatine Kinase Elevation | − | NA | NA | NA | − | − | − | − | − |
| Opthalmological Findings | Nystagmus | − | Strabismus | − | − | − | − | − | − |
| Ichthyosis/dry skin/erythroderma | − | − | − | NA | − | + | − | − | − |
| Dysmorphic Features | Clubbed Feet, Penoscrotal fusion | − | − | − | − | Short stature | − | − | − |
| Death | 4 months | − | − | 9 years | − | 16.5 years | − | − | − |
| 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| III/2 [3] | III/3 [3] | III/4 [3] | IV/2 [10] | IV/3 [10] | GH [4] | NB [4] | ASB [4] | AYB [4] | Frequency of Symptom in DOLK-CDG patients |
| M | M | M | F | M | M | M | M | F | − |
| Bedouin | Bedouin | Bedouin | Indian | Indian | German | German | Turkish | Turkish | − |
| + | + | + | − | − | + | + | + | + | 83% (15/18) |
| Homo c.912G > T; (p.W304 C) | Homo c.912G > T; (p.W304 C) | Homo c.912G > T; (p.W304 C) | Homo c.3G>A; (p.M1C) | Homo c.3G>A; (p.M1C) | Homo c.295T > A; (p.C99S) | Homo c.295T > A; (p.C99S) | Homo c.295T > A; (p.C99S) | Homo c.295T > A; (p.C99S) | − |
| Late | Late | Late | Late | Late | Early | Early | Early | Early | − |
| − | − | − | − | − | + | + | + | + | 39% (7/18) |
| − | − | − | Mild developmental delay | Mild developmental delay | NA | NA | NA | NA | 43% (6/14) |
| + | + | + | + | − | − | + | + | + | 83% (15/18) |
| NA | − | − | NA | NA | − | − | − | NA | 38% (5/18) |
| NA | + | + | + | + | NA | NA | NA | NA | 90% (9/10) |
| NA | NA | NA | NA | NA | NA | NA | NA | NA | Our patient |
| NA | − | − | NA | NA | NA | Hypoketotic Hypoglycemia | NA | NA | 36% (4/11) |
| − | − | − | + | − | + | + | + | + | 39% (7/18) |
| NA | − | − | + | + | NA | NA | NA | − | 22% (2/11) |
| − | − | − | − | − | Nystagmus | − | NA | NA | 19% (3/16) |
| + | + | + | + | − | + | + | + | + | 53% (9/17) |
| − | − | − | − | − | − | − | Hair loss, Sparse hair | − | 17% (3/18) |
| 9 years | − | − | 11 years | − | 8.5 months | 6 months | 7 months | 4 months | Earlya 28% (5/18) Latea 22% (4/18) |
Early = before the age of 1. Late = after the age of 1.
2. CASE REPORT
2.1
The male patient was born following an uncomplicated pregnancy except for an instance of first trimester bleeding, to a 23-year-old primagravida and a 26-year-old father of nonconsanguineous Palestinian origin. He was delivered full-term by Caesarean due to nonreassuring fetal heart rate and meconium-stained amniotic fluid. Physical examination showed systolic heart murmur, hypotonia, bilateral talipes equinovarus, sacral dimple with hairy tuft, localized lower extremity hypertrichosis, and penoscrotal fusion. No other skin abnormalities or optic atrophy were noted. Echocardiogram showed no evidence of cardiac dilatation or abnormal function. He had good initial respiratory effort; however, shortly thereafter manifested repeated episodes of apnea, cyanosis, and bradycardia. The events were found to be caused by partial or generalized seizures, as an electroencephalogram revealed non-specific encephalopathy and generalized multifocal seizures. The apneic episodes occurred so frequently that he was intubated and mechanically ventilated. Brain imaging demonstrated diffuse cortical atrophy and delayed myelination, particularly in the cerebellar hemispheres. He had gastroesophageal reflux and dysphagia, failed to thrive, and eventually required a gastrostomy tube for enteral feeding. N-linked CDG was suspected when ESI-MS of transferrin (Mayo Clinic, Rochester, MN) demonstrated elevated mono-oligosaccharide:di-oligosaccharide transferrin ratio of 0.707 (reference range < 0.100) and a-oligosaccharide:di-oligosaccharide transferrin ratio of 0.216 (reference range < 0.050). These ratios are indicative of Type I CDG, resulting in impaired synthesis or transfer of the LLO precursor that subsequently generates proteins with unoccupied glycosylation sites [2]. This is in comparison to Type II CDGs, which are caused by impaired processing, such as trimming and remodeling, of the protein-bound oligosaccharide, creating proteins that have fully occupied glycosylation sites but with abnormal glycans [2].
2.2
At age 3 months, the patient developed sinus tachycardia, hypertension, unexplained hypokalemia (K+ 3.0 mEq/L, reference range 3.6–6.0 mEq/L), and hyperglycemia (glucose 461 mg/dL, reference range 65–110 mg/dL) despite low glucose infusion rate of 2 mg/kg/minute. While potassium levels eventually stabilized, his glucose remained markedly elevated without ketoacidosis. Even high continuous insulin infusion (1.3 units/kg/hour) did not stabilize the glucose. He developed hepatomegaly with elevated aspartate aminotransferase (174 units/L, reference range 22–58 units/L), alanine aminotransferase (121 units/L, reference range 11–39 units/L), and alkaline phosphatase (1,310 units/L, reference range 100–302 units/L). Prothrombin international normalized ratio was 1.1 (reference range 0.8 – 1.2 seconds) and partial thromboplastin time was 30.3 (reference range 23 – 40 seconds). Creatine phosphokinase was not elevated at 66 (reference range 41 – 277 units/L). Clinical deterioration was evidenced by absent response to stimulation or primitive reflexes, sluggish pupils, hypotonia, and bilateral ankle clonus.
2.3
During his last four days of life, he developed wide-complex tachycardia with atrioventricular dissociation, and his cardiac function rapidly deteriorated with decline in contractile function (20.3%, reference range > 28%) and ejection fraction (43.6%, reference range > 55%). Borderline cardiac dilatation, concentric left ventricular hypertrophy, and decreased left ventricular function were noted. He developed renal failure and marked abdominal distension secondary to hepatomegaly and ascites (albumin 2.5 g/dL, reference range 2.7–4.8 g/dL). He was noted to have dysconjugate gaze, sluggishly reactive pupillary light reflex, and became non-responsive and hypotonic with occasional spontaneous four-extremity clonus. He expired at 4 months from combined cardiac, renal, and liver failure.
3. METHODS
3.1 Human subjects
3.1.1
The study was granted exempt status by the institutional review board of Children’s Hospital of Orange County as IRB study #130657.
3.2 Identification of DOLK mutations
3.2.1
Patient DNA was analyzed using a targeted 47 gene next generation sequencing panel specific for disorders of glycosylation [7]. This panel utilized RainDance Technologies™ microdroplet enrichment of the targeted region, which included all exons for each of the 47 genes and at least 25 nucleotides upstream and downstream of each exon. After enrichment, samples were run on a SOLiD™ 3 Plus system (LifeTechnologies, Carlsbad, CA), and the average coverage for this sample was 1200x with 87% of the nucleotides having 100x coverage or greater. Bioinformatic filtering for the known SNPs assisted with the identification of changes that were most likely novel and therefore warranted further consideration and confirmation by Sanger sequencing.
3.3 Preparation of microsomal fractions from cultured human cells
3.3.1
Human cells from primary cultures were incubated in PBS containing 10 mM EDTA, collected by centrifugation (1,000×g, 10 min), resuspended in ice-cold 10 mM HEPES-NaOH, pH 7.4, 0.25 M sucrose, 1 mM dithiothreitol, and lysed by probe sonication with a Kontes Micro Ultrasonic Cell Disruptor (40% full power, 15 sec, 2 pulses). Homogenates were sedimented at 1,000×g, 10 min, to remove unbroken cells, and microsomes were recovered from the supernatant by centrifugation at 100,000×g, 20 min in a Beckman TL-100 Ultracentrifuge. Microsomes were washed with lysis buffer one time, resuspended to ~10 mg/ml protein in lysis buffer and stored at −20°C until analysis.
3.4 In vitro analysis of dolichol kinase activity
3.4.1
Reaction mixtures for dolichol kinase activity contained 50 millimolar (mM) Tris-Cl (pH 8.0), 10 mM CaCl2, 5 mM NaF, 1 mM sodium orthovanadate, 5 mM dithiothreitol, 10 mM UTP, 0.025% Nonidet P-40, 100 micromolar (µM) dolichol (dispersed in 0.5% Nonidet P-40 by probe sonication), microsomal fraction from cultured CDG cells (50 micrograms of membrane protein, prepared as described previously), 0.125 molar sucrose and the indicated concentration of [γ-32P]CTP (5 to 40 µM, 745 counts per minute/picomole) in a total volume of 0.01 mL. Following incubation at 37°C for 10 min, the enzymatic products were extracted into CHCl3/CH3OH and assayed as described previously [8].
4. RESULTS
4.1
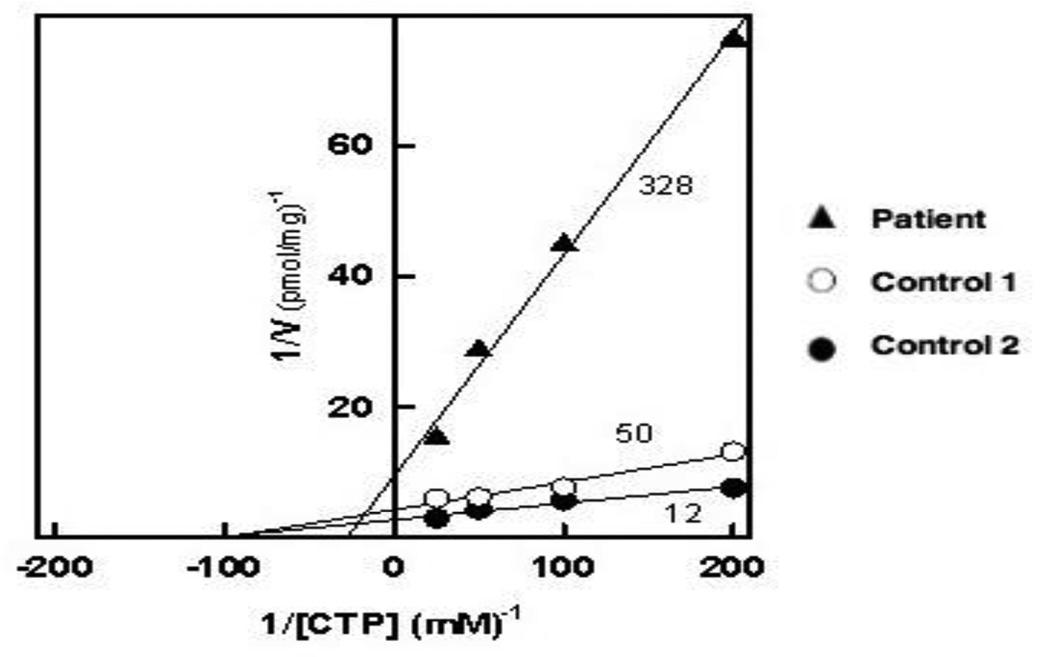
Next-generation sequencing identified homozygous c.1447C>A (p.Q483K) DOLK mutations; both parents were heterozygous carriers. Functional studies showed that the mutant DOLK enzyme demonstrated reduced Vmax (100 pmol/min, controls 238 and 417 pmol/min) and increased Km (36 µM, controls 10.2 and 10.4 µM), confirming that the DOLK p.Q483K mutation encodes a form of dolichol kinase with severely deficient enzymatic activity (Figure 1).
Figure 1. Kinetic analysis of dolichol kinase activity in membrane fractions from cultured human cells.
Microsomal fractions from human primary cultures from control 1 (○), control 2 (●) and our patient (▲) were assayed for dolichol kinase at the indicated concentrations of CTP as described in ‘Methods.’ The data is displayed as a Lineweaver-Burk plot with regression lines calculated by the regression analysis function available in Sigma Plot 12.0 and are representative of three separate analyses.
5. DISCUSSION
Our case adds to the existing clinical phenotype of DOLK-CDG patients with a different spectrum of multisystemic involvement compared to other cases. Previously reported DOLKCDG patients who died suffered mainly from dilated cardiomyopathy [5] or seizure disorder [6]. While our patient developed heart failure and seizures, he also had multiple major malformations such as talipes equinovarus and penoscrotal fusion, which have not yet been reported in DOLK-CDG patients. In addition, he had severe insulin-resistant hyperglycemia, which is different from the hyperinsulinemic hypoglycemia typically seen in the type I CDGs [2]. He also developed renal failure and hepatic synthetic dysfunction as evidenced by electrolyte disturbances, anuria, hepatomegaly, elevated transaminases, and ascites.
In comparison to the 17 other DOLK-CDG patients listed in Table 1, our patient presents with traits that are both common and novel to the DOLK-CDG phenotype. Common DOLK-CDG symptoms that were seen in our patient include dilated cardiomyopathy (seen in 15 of 18 DOLKCDG patients), seizures (seen in 7 of 18 DOLK-CDG patients), and muscular hypotonia (seen in 7 of 18 DOLK-CDG patients) mostly without creatine kinase elevation. Symptoms that our patient had that are novel to the DOLK-CDG phenotype include renal failure, insulin-resistant hyperglycemia, and dysmorphic features.
It is worthwhile to consider the symptoms of DOLK-CDG in comparison to the CDGs involving the enzymatic steps immediately prior to, and following, dolichol phosphorylation: SRD5A3-CDG and the three DPM-CDGs, respectively. These dolichol-phosphate-associated type I CDGs have distinct presentations that overlap considerably. Mutations in the steroid 5- alpha reductase gene SRD5A3 result in deficient reduction of polyprenol to dolichol. The clinical presentation of patients with SRD5A3-CDG (OMIM#612379) includes facial dysmorphism, cerebellar vermis hypoplasia, iris and retina colobomas, optic atrophy, nystagmus, visual loss, transient microcytic anemia, elevated transaminases, coagulation abnormalities, and ichthyosis [5]. One male from the index SRD5A3-CDG kindred had micropenis and cryptorchidism due to hypopituitarism [9]. Dol-P-Mannose is synthesized from Dol-P and mannose by the DPMsynthase complex, a heterotrimer of dolichyl-phosphate mannosyltransferase polypeptides 1, 2, and 3 encoded by the corresponding DPM1-3 genes [5]. Patients with deficiencies of the DPM complex (OMIM#s608799, 615042, and 612937) present with symptoms of both type I N-linked CDG and deficient O-mannosylation of α-dystroglycan. With the exception of the single DPM3- CDG patient with normal cognition, adult-onset dilated cardiomyopathy and stroke-like episode [11], other reported DPM-CDGs presented early in life with hypotonia, congenital contractures, severe delays in development, refractory seizure disorder, and reduced or absent vision [12,13]. Many also demonstrated elevations in creatine kinase due to α-dystroglycan-related muscular dystrophy, and reduced activity of proteins C, S, and antithrombin III. Comparing DOLK-CDG to SRD5A3 and DPM-CDGs, developmental and ophthalmologic abnormalities, transaminase elevation, abnormal coagulation factors, and anemia have been reported in all. Cardiomyopathy, absent in SRD5A3-CDG due to a presumed alternative dolichol synthetic pathway [5], has been linked to abnormal O-mannosylation of α-dystroglycan in DOLK- and DPM-CDGs [10–13], yet 78% of DOLK-CDG patients had normal creatine kinase. This pattern of severe cardiac and milder skeletal involvement has been observed in FKRP-related congenital muscular dystrophy, another α-dystroglycanopathy [14]. With no known secondary pathway of Dol-P synthesis [5], such discordance is possibly explained by a higher tolerance for α-dystroglycan hypoglycosylation in skeletal muscle compared to cardiac muscle. However, given the finding of camptodactyly in a DPM1-CDG patient [12], our patient’s equinovarus deformity may be a consequence of fetal hypokinesia arising from muscular dystrophy.
Our patient’s severe, neonatal-onset constellation of symptoms may be explained by the relatively high levels of DOLK expression in the brain, heart, liver, and kidney [10] and the severe effects of the p.Q483K DOLK mutation upon enzymatic function, verifying in silico predictions of this mutation as “damaging” by SIFT (score 0.003) and “probably damaging” by Polyphen-2 (score 0.975) [15,16]. However, in silico analysis should be verified by expression, evidenced by the “damaging” predictions made by SIFT and Polyphen-2 for the DOLK p.W304C and p.H408D, in contrast with the ability of both mutant enzymes to partially rescue glycosylation in a DOLK-ortholog deficient yeast strain, and the later-onset dilated cardiomyopathy human phenotypes observed for both mutations [10]. This DOLK-CDG case emphasizes the importance of testing with transferrin IEF, ESI-MS, HPLC, or CE methods to evaluate patients with dysmorphic features, multiple organ system dysfunction, or combined symptoms of deficient N-linked and O-linked glycosylation for CDGs, especially those involving dolichol metabolism.
HIGHLIGHTS.
We report a patient with novel traits that expand the DOLK-CDG phenotype.
The p.Q483K DOLK mutation encodes a severely impaired dolichol kinase enzyme.
The patient had dysmorphic features, refractory seizures, and insulin resistance.
Cardiomyopathy, hepatomegaly, and anuria developed prior to death at 4 months.
ACKNOWLEDGEMENTS
This work was supported by The Rocket Fund and by R01 DK55615 (HHF). The authors would like to thank Dr. Laura Pollard and Stephen McGee at the Greenwood Genetic Center for their help with the NGS analysis.
ABBREVIATIONS
- CTP
cytosine triphosphate
- EDTA
ethylenediaminetetraacetic acid
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Freeze HH, Eklund EA, Ng BG, Patterson MC. Neurology of inherited glycosylation disorders. Lancet Neurol. 2012;11:453–466. doi: 10.1016/S1474-4422(12)70040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eklund EA, Freeze HH. The Congenital Disorders of Glycosylation: A Multifaceted Group of Syndromes. NeuroRx. 2006;3:254–263. doi: 10.1016/j.nurx.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapusta L, Zucker N, Frenckel G, Medalion B, Gal TB, Birk E, et al. From discrete dilated cardiomyopathy to successful cardiac transplantation in congenital disorders of glycosylation due to dolichol kinase deficiency (DK1-CDG) Heart Fail Rev. 2013;18:187–196. doi: 10.1007/s10741-012-9302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kranz C, Jungeblut C, Denecke J, Erlekotte A, Sohlback C, Debus V, et al. A defect in dolichol phosphate biosynthesis causes a new inherited disorder with death in early infancy. Am. J. Hum. Genet. 2007;80:433–440. doi: 10.1086/512130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantagrel V, Lefeber DJ. From glycosylation disorders to dolichol biosynthesis defects: a new class of metabolic diseases. J. Inherit. Metab. Dis. 2011;34:859–867. doi: 10.1007/s10545-011-9301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helander A, Stödberg T, Jaeken J, Matthijs G, Eriksson M, Eggertsen G. Dolichol kinase deficiency (DOLK-CDG) with a purely neurological presentation caused by a novel mutation. Mol. Genet. Metab. 2013 doi: 10.1016/j.ymgme.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Jones MA, Bhide S, Chin E, Ng BG, Rhodenizer D, Zhang VW, et al. Targeted polymerase chain reaction-based enrichment and next generation sequencing for diagnostic testing of congenital disorders of glycosylation. Genet. Med. 2011;13:921–932. doi: 10.1097/GIM.0b013e318226fbf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumbilla C, Waechter CJ. Dolichol kinase, phosphatase, and esterase activity in calf brain. Meth. Enzymol. 1985;111:471–482. doi: 10.1016/s0076-6879(85)11032-3. [DOI] [PubMed] [Google Scholar]
- 9.Al-Gazali L, Hertecant J, Algawi K, El Teraifi H, Dattani M. A new autosomal recessive syndrome of ocular colobomas, ichthyosis, brain malformations and endocrine abnormalities in an inbred Emirati family. Am. J. Med. Genet. Part A. 2008;146:813–819. doi: 10.1002/ajmg.a.32114. [DOI] [PubMed] [Google Scholar]
- 10.Lefeber DJ, de Brouwer APM, Morava E, Riemersma M, Schuurs-Hoeijmakers JHM, Absmanner B, et al. Autosomal Recessive Dilated Cardiomyopathy due to DOLK Mutations Results from Abnormal Dystroglycan O-Mannosylation. PLoS Genetics. 2011;7:e1002427. doi: 10.1371/journal.pgen.1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefeber DJ, Schönberger J, Morava E, Guillard M, Huyben KM, Verrijp K, et al. Deficiency of Dol-P-Man Synthase Subunit DPM3 Bridges the Congenital Disorders of Glycosylation with the Dystroglycanopathies. Am. J. Hum. Genet. 2009;85:76–86. doi: 10.1016/j.ajhg.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang AC, Ng BG, Moore SA, Rush J, Waechter CJ, Raymond KM, et al. Congenital disorder of glycosylation due to DPM1 mutations presenting with dystroglycanopathy-type congenital muscular dystrophy. Mol. Genet. Metab. 2013 doi: 10.1016/j.ymgme.2013.06.016. http://dx.doi.org/10.1016/j.ymgme.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barone R, Aiello C, Race V, Morave E, Foulquier F, Riemersma M, et al. DPM2-CDG: A Muscular Dystrophy-Dystroglycanopathy Syndrome with Severe Epilepsy. Ann. Neurol. 2012;72:550–558. doi: 10.1002/ana.23632. [DOI] [PubMed] [Google Scholar]
- 14.Margeta M, Connolly AM, Winder TL, Pestronk A, Moore SA. Cardiac pathology exceeds skeletal muscle pathology in two cases of limb-girdle muscular dystrophy type 2I. Muscle Nerve. 2009;40:883–889. doi: 10.1002/mus.21432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar P, Henikoff S, Ng PC PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 16.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]