Abstract
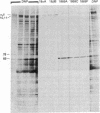
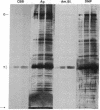
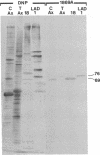
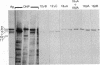
Monoclonal antibodies that recognize individual polypeptides of the outer arm dyneins of Chlamydomonas flagella were obtained and used to study the structural relationships between the various polypeptides. Immunoblot analysis showed that the gamma heavy chain of 12S dynein and the alpha and beta heavy chains and Mr 69,000 intermediate chain of 18S dynein each contain immunoreactive sites not found in the other dynein chains or in any other axonemal protein. We also used these antibodies to investigate possible structural similarities between dynein polypeptides from Chlamydomonas and phylogenetically distant species. No crossreactivity was observed with antibodies against either the alpha, beta, or gamma heavy chains, demonstrating that each Chlamydomonas heavy chain has structural features distinct from those present in dyneins from the other species tested. However, one antibody against the Mr 69,000 polypeptide recognized an intermediate chain (Mr 76,000) of latent-activity dynein-1 from the sea urchin Tripneustes gratilla. This result provides further evidence that 18S dynein and latent-activity dynein-1 are related. In the course of the above studies, we modified existing procedures to achieve efficient transfer of high molecular weight proteins from NaDodSO4/polyacrylamide gels to nitrocellulose sheets, and to detect small quantities of protein on nitrocellulose. Our modified procedure for staining total protein on nitrocellulose is rapid, inexpensive, and as sensitive as silver-staining of polyacrylamide gels. These methods should be useful to investigators working with small amounts of protein or requiring resolution of closely migrating polypeptides after transfer to nitrocellulose.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. W., Fronk E., Gibbons I. R. Polypeptide subunits of dynein 1 from sea urchin sperm flagella. J Supramol Struct. 1979;11(3):311–317. doi: 10.1002/jss.400110305. [DOI] [PubMed] [Google Scholar]
- Bloom G. S., Schoenfeld T. A., Vallee R. B. Widespread distribution of the major polypeptide component of MAP 1 (microtubule-associated protein 1) in the nervous system. J Cell Biol. 1984 Jan;98(1):320–330. doi: 10.1083/jcb.98.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Gardner J. M., Fambrough D. M. Fibronectin expression during myogenesis. J Cell Biol. 1983 Feb;96(2):474–485. doi: 10.1083/jcb.96.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P. Fodrin is the general spectrin-like protein found in most cells whereas spectrin and the TW protein have a restricted distribution. Cell. 1983 Sep;34(2):503–512. doi: 10.1016/0092-8674(83)90383-5. [DOI] [PubMed] [Google Scholar]
- Goodenough U. W., Heuser J. E. Substructure of the outer dynein arm. J Cell Biol. 1982 Dec;95(3):798–815. doi: 10.1083/jcb.95.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U., Heuser J. Structural comparison of purified dynein proteins with in situ dynein arms. J Mol Biol. 1984 Dec 25;180(4):1083–1118. doi: 10.1016/0022-2836(84)90272-9. [DOI] [PubMed] [Google Scholar]
- Harrison R. A. Glycolytic enzymes in mammalian spermatozoa. Activities and stabilities of hexokinase and phosphofructokinase in various fractions from sperm homogenates. Biochem J. 1971 Oct;124(4):741–750. doi: 10.1042/bj1240741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A., Porter M. E., Shimizu T. Mechanism of force production for microtubule-dependent movements. J Cell Biol. 1984 Jul;99(1 Pt 2):132s–136s. doi: 10.1083/jcb.99.1.132s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart D. P., Kaiser D. A., Pollard T. D. Monoclonal antibodies demonstrate limited structural homology between myosin isozymes from Acanthamoeba. J Cell Biol. 1984 Sep;99(3):1002–1014. doi: 10.1083/jcb.99.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler J. M., Meisler N. T., Viceps-Madore D., Cidlowski J. A., Thanassi J. W. A general immunochemical method for detecting proteins on blots. Anal Biochem. 1984 Feb;137(1):210–216. doi: 10.1016/0003-2697(84)90372-5. [DOI] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Ogata K., Arakawa M., Kasahara T., Shioiri-Nakano K., Hiraoka K. Detection of toxoplasma membrane antigens transferred from SDS-polyacrylamide gel to nitrocellulose with monoclonal antibody and avidin-biotin, peroxidase anti-peroxidase and immunoperoxidase methods. J Immunol Methods. 1983 Dec 16;65(1-2):75–82. doi: 10.1016/0022-1759(83)90305-8. [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Fay R. B., Witman G. B. Purification and polypeptide composition of dynein ATPases from Chlamydomonas flagella. Cell Motil. 1982;2(6):525–547. doi: 10.1002/cm.970020604. [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Haley B. E., Witman G. B. The photoaffinity probe 8-azidoadenosine 5'-triphosphate selectively labels the heavy chain of Chlamydomonas 12 S dynein. J Biol Chem. 1984 Jul 10;259(13):8499–8504. [PubMed] [Google Scholar]
- Pfister K. K., Witman G. B. Subfractionation of Chlamydomonas 18 S dynein into two unique subunits containing ATPase activity. J Biol Chem. 1984 Oct 10;259(19):12072–12080. [PubMed] [Google Scholar]
- Piperno G., Luck D. J. Axonemal adenosine triphosphatases from flagella of Chlamydomonas reinhardtii. Purification of two dyneins. J Biol Chem. 1979 Apr 25;254(8):3084–3090. [PubMed] [Google Scholar]
- Tang W. J., Bell C. W., Sale W. S., Gibbons I. R. Structure of the dynein-1 outer arm in sea urchin sperm flagella. I. Analysis by separation of subunits. J Biol Chem. 1982 Jan 10;257(1):508–515. [PubMed] [Google Scholar]
- Tash J. S., Means A. R. Regulation of protein phosphorylation and motility of sperm by cyclic adenosine monophosphate and calcium. Biol Reprod. 1982 May;26(4):745–763. doi: 10.1095/biolreprod26.4.745. [DOI] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtkowiak Z., Briggs R. C., Hnilica L. S. A sensitive method for staining proteins transferred to nitrocellulose sheets. Anal Biochem. 1983 Mar;129(2):486–489. doi: 10.1016/0003-2697(83)90581-x. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yuen K. C., Johnson T. K., Denell R. E., Consigli R. A. A silver-staining technique for detecting minute quantities of proteins on nitrocellulose paper: retention of antigenicity of stained proteins. Anal Biochem. 1982 Nov 1;126(2):398–402. doi: 10.1016/0003-2697(82)90534-6. [DOI] [PubMed] [Google Scholar]