Abstract
Conjugation of cholesterol moiety to active compounds for either cancer treatment or diagnosis is an attractive approach. Cholesterol derivatives are widely studied as cancer diagnostic agents and as anticancer derivatives either in vitro or in vivo using animal models. In largely growing studies, anticancer agents have been chemically conjugated to cholesterol molecules, to enhance their pharmacokinetic behavior, cellular uptake, target specificity, and safety. To efficiently deliver anticancer agents to the target cells and tissues, many different cholesterol–anticancer conjugates were synthesized and characterized, and their anticancer efficiencies were tested in vitro and in vivo.
Keywords: Cholesterol, Anticancer, Drug delivery systems
1. Introduction
According to the cancer incidence and survival report 2007 by the Saudi cancer registry (scr.org.sa), 12,309 cancer cases were diagnosed in Saudi Arabia in 2007 (Saudi Arabia’s population at that time was approximately 17 million). Breast cancer ranked first in incidence (13.8%), followed by colorectal (9.9%), non-Hodgkin lymphoma (NHL) (7.7%), thyroid (6.4%), leukemia (6.2%), liver (4.8%), and lung cancer (4.5%). The top five cancers in females were breast, thyroid, colorectal, NHL, and leukemia; in males, the top five cancers were colorectal, NHL, leukemia, lung, and liver. Moreover, cancer is the second most common cause of death in the US, exceeded only by heart disease, accounting for nearly 1 of every 4 deaths (American Cancer Society, 2012).
Cholesterol is a neutral lipid that plays an essential role in the maintenance of the integrity of biologic membranes and serves as a precursor in the synthesis of many endocrine mediators. It is also synthesized in mammalian cells via the mevalonate pathway. Recent clinical has demonstrated a possible linkage of cholesterol to prostatic cancer and benign prostatic hyperplasia. Accumulation of cholesterol within the lipid raft component of the cellular plasma membrane may stimulate signaling pathways that promote prostate tumor growth and progression. In addition, cholesterol-lowering drugs, such as statins, have exhibited some promising results for these prostatic diseases (Yat-Ching, 2011). Herein, we shed light upon the anticancer activity of various cholesterol conjugates that have been published in the literatures.
2. The biological properties of cholesterol
Distribution of cholesterol in human organs and tissues varies (Fig. 1) (Alanazi et al., 2003). Cholesterol serves many biological functions. Depending on the cell types, cholesterol content of cell membranes varies (Yeagle, 1988). The membranes of most cells have an intermediate cholesterol/phospholipid ratio and possess both protective and metabolite-transport functions. Cell membranes with a high cholesterol ratio, such as those in the epidermis layer of the skin, have high stability and relatively low permeability, reflecting their major functionality as a protective barrier (Yeagle, 1988; Proksch, 1990). Membranes of the intracellular organelles, such as mitochondria, have a low cholesterol ratio and, thus, are fluidic and permeable (Yeagle, 1988). The total amount of cholesterol bound to lipoproteins in blood circulation is normally about 150–200 mg per 100 ml of serum. The body obtains cholesterol from two routes, either from dietary sources or from de novo biosynthesis.
Figure 1.
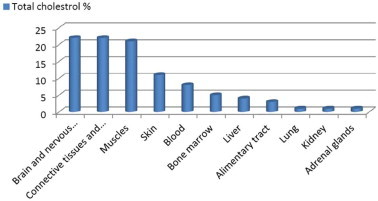
Distribution of cholesterol in human organs and tissues.
Various lipoproteins act as primary carriers in cholesterol transport through blood circulation. Dietary cholesterol is transported from the intestine to the liver in large lipoprotein particles. The liver secretes very low density lipoproteins (VLDL), containing cholesterol and is partially converted into low density lipoprotein (LDL) through the action of lipoprotein lipase. LDL carries cholesterol from the liver to body tissues while high density lipoprotein (HDL) transports cholesterol from various tissues back to the liver (Alanazi et al., 2003).
Cholesterol is the sole precursor to all steroid hormones. These steroids include glucocorticoids responsible for blood sugar regulation, mineralcorticoids that regulate mineral balance and blood pressure and sex hormones responsible for many functions. Cholesterol is the precursor to a hormone called pregnenolone, which has not only its own functions but also be the precursor to all other steroid hormones. Pregnenolone is converted into progesterone, a sex hormone, which in turn is converted into cortisol, which regulates inflammation and blood sugar, aldosterone, which regulates mineral balance and blood pressure, or testosterone, a type of sex hormone referred to as an androgen, which regulates libido, muscle mass, and plays other roles. In females, and to a lesser degree in males, testosterone is further modified, undergoing conversion to estradiol, a different type of sex hormone called an estrogen (Hume and Boyd, 1978). Many neurotransmitter receptors are created with cholesterol, and maintained with the aid of cholesterol (Fantini and Barrantes, 2009; Baier and Barrantes, 2007; Barrantes, 2010). Our nerve cells require cholesterol to function and maintain fluidity (Barres and Smith, 2001). The central nervous system (CNS) comprises the highest concentration of cholesterol in the body, over any other organ (Dietschy and Turley, 2004). For the in vivo synthesis of Vitamin D from the Sun, cholesterol is needed (Bouillon et al., 1995). Bile salts are amphipathic derivatives of cholesterol, and are needed to emulsify dietary fats so they can be digested properly (Denniston et al., 2007).
3. Serum cholesterol and cancer risk
For males with low serum cholesterol levels it has been noted that about 30% increased risk of cancer is expected. For females, some studies examined suggest no more than a 5–10% increased risk associated with having low serum cholesterol (Kritchevsky, 1992). The cancers most consistently associated with low serum cholesterol levels are those of the colon and the lung in males, the cervix and the breast (but only for females under 50 years of age) in females, and leukemia in both sexes. In contrast, high cholesterol levels have been linked with an increase in brain cancer. While immunologic, genetic, and dietary explanations have been offered to explain the association, it is difficult to support the idea that low serum cholesterol causes cancer in any direct manner. In China, counties with the lowest average plasma cholesterol levels have the lowest cancer rates (Peto et al., 1989). While this observation is open to a number of interpretations, it does not support the idea that low serum cholesterol is a tumor initiator. On the other hand, late stage of cancer usually is accompanied by a low level of cholesterol due to the high growth rate of the cancer cells.
4. Rationale for using cholesterol-based conjugates for cancer
Lipoproteins are macromolecules that transport lipids through the blood to various cell types, where they undergo receptor-mediated uptake. Based on densities there are 5 classes of lipoprotein including chylomicrons, very low density lipoproteins (VLDL), intermediate density lipoproteins (IDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL). Chylomicrons are the largest lipoproteins and they are synthesized in the intestinal tract. Their main function is the transport of dietary triglycerides and cholesterol. Very low density lipoproteins (VLDL) are synthesized in the liver and function to export triglycerides to peripheral tissues. LDL is the major vehicle to transport cholesteryl esters to peripheral cells.
LDL contains one major apolipoprotein (i.e. apo B-100), which allows LDL to bind to the LDL receptors on the peripheral cell surfaces and to be internalized by these cells through a receptor mediated endocytosis. High-density lipoproteins (HDL) are the smallest lipoprotein due to high protein/lipid ratio. HDL works as a lipid scavenger, transporting cholesterol from various tissues back to the liver (Fars, 2003).
Cholesterol conjugation approach is widely used in delivery of the anticancer agents to tumor tissues selectively. This approach includes physical or biological targeting strategy [5]. Physical targeting is based on the increase of the lipophilicity of the anticancer compounds that helps the intracerebral delivery of anticancer agents to brain tumor [6]. Cholesterol based boron anticancer conjugate (cholesteryl 1,12-dicarba-closo-dodecaborane 1-carboxylate, BCH) showed a rapid distribution from the site of intracerebral injection in tumor-bearing rat (Alanazi et al., 2004). However, biological targeting can be based on two facts. The first one is the difference in the substrate uptake among different human organs. Basically, cholesterol accumulates in the ovarian tissue and is used to synthesize sex hormones. Therefore, cholesteryl drug conjugates could also be used for drug targeting to the ovary. Ades et al. (2001) showed that injection of labeled cholesteryl oleate in patients bearing ovarian tumors resulted in a eightfold uptake by the malignant tumors compared to the contralateral normal ones [93]. The second fact is the difference in the substrate uptake between cancer cells and normal cells. In other words, due to the high growth rate of cancerous cells, they require more nutrients and various receptors, thus, are over expressed, such as folate receptors [12], transferrin receptors [14], growth factor receptors [15], and low-density lipoprotein receptors [18]. Drug delivery systems linked to ligands that target these receptors have been investigated [14], [32].
The high requirement for LDL by malignant cells and thus the overexpression of LDL receptors can be utilized for developing a novel targeted drug delivery system. Because the amount of new membrane synthesized in rapidly growing cancer cells is considerable, they consume large amounts of cholesterol. The LDL receptors (LDL is the major component of the cholesterol-transport pathway) are much more active on many types of cancer cells than on the corresponding normal cells (Rudling et al., 1990, Maletinska et al., 2000). Thus, many types of tumor cells display a higher level of receptor mediated LDL uptakes compared to corresponding normal tissues. The increase in LDL receptor activity in cancer cells is suggested to be due to high cholesterol demand for cell growth and/or a mechanism directly linked to cell transformation (Favre, 1992). LDL has therefore been proposed as a potential carrier for chemotherapeutic agents (Vitols, 1991). There are three main strategies to utilize LDL as a drug delivery system. First, LDL has been utilized as a targeting vector for cancer therapy through loading drugs into LDL particles and subsequent administration of the preloaded LDL. This strategy (preloading drug into LDL) suffers from several limitations, such as being tedious, the possibility of losing LDL targeting capability (due to modification of apo B100 in the presence of such harsh conditions), and risk of cross contamination (i.e. it is a biohazard blood product). A second strategy is developing LDL resembling delivery systems, which follow a similar metabolic pathway to that of natural LDL. This strategy involved using a drug delivery system such as protein free nanoemulsion (Shawer et al., 2002; Fars, 2003) and Apolipoprotein B built-in phospholipid cholesterol liposomes (Klimov et al., 1983). The third strategy, which is a combination of the two previous pioneer strategies, is based on targeting the LDL particle in vivo and allowing the anticancer agent to be transferred to the natural LDL inside of the body. Basically, LDL will function as a secondary carrier of anticancer molecules in vivo and deliver these molecules selectively toward cancerous cells via elevated LDL receptor. This approach required anticancer molecules to have affinity to the LDL particle endogenously and to have certain special physicochemical properties (Fars, 2003). The fourth strategy is the developing LDL-conjugated anti-cancer drug as a new drug delivery system for cancer therapy. This approach is based on the fact that, LDL is the endogenous carrier of cholesterol. The majority of cholesterol is obtained through the LDL receptor-mediated endocytosis mostly in the form of cholesterol ester. Fig. 2 shows that LDL particle is a lipid core, of triglycerides (20%) and cholesteryl esters (80%), that is covered by a monolayer of phospholipid. Basically, only lipophilic drug species partition into the core of the LDL system. Since cholesterol (in its ester form) is the native component of LDL, conjugation of an antitumor moiety with cholesterol facilitates the loading of these compounds into LDL. Synthesizing antitumor conjugates that mimic native cholesteryl esters in its chemical and physical characteristics may result in effectively transferring these compounds into LDL (Fars, 2003). The proposed scenario of targeting these compounds to cancer cells is presented in Fig. 3. Following the administration of cholesterol–antitumor conjugates these compounds will partition into LDL particles in the physiological environment and, thus, utilize the elevated LDL receptor expression on tumor cells and enter the tumor cells via receptor mediated endocytosis. Degradation of LDL particles by endosomal enzymes will result in the liberation of antitumor molecules which act as tumoricides.
Figure 2.
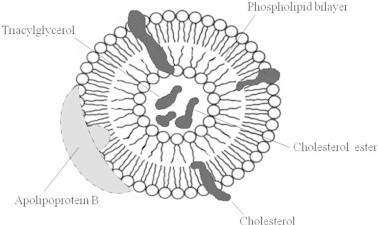
LDL particle.
Figure 3.
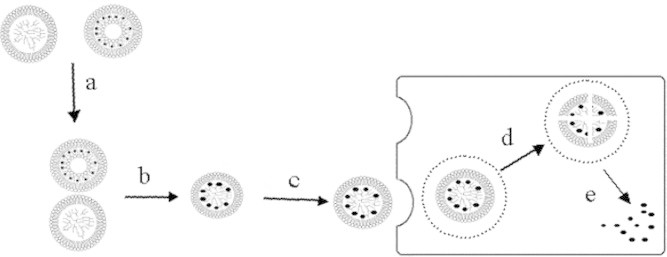
LDL pathway for anticancer-targeted delivery; administration of cholesterol conjugate (a); diffusion of cholesterol conjugate into LDL (b); LDL-cholesterol conjugate binds with LDL-receptor (c); receptor-mediated endocytosis of LDL-particles (d); Releasing of cholesterol conjugate in cytoplasm.
5. Cholesterol-based conjugates for cancer disease diagnosis
The diagnoses of cancer diseases in its early stage play a major role in decreasing the risk of associated complications. Cholesterol has been conjugated with imaging molecules for the detection of manifestations associated with adrenal glands and liver disease. Several cholesteryl conjugates (Fig. 4) are reported for human adrenal imaging which were gamma emitting agent 131I-19-iodocholesterol (NM-145, 1) (Liebermann et al., 1971), 6β-131I iodomethyl-19-norcholest-5-(10)-en-3β-ol (NP-59, 2) (Gross et al., 1994), 37Se-selenomethyl-norcholesterol (Scintadren, 3) (Hawkins et al., 1980) and cholesteryl-p-[18F]fluorobenzoate ([18F] CFB, 4) (Johnson and Welch, 1999) that has used for distinguishing between adrenal producing adenoma (APA) and bilateral adrenal hyperplasia (BHA) (Gums and Wilt, 1997). As the liver plays an important role in lipid metabolism, linking an imaging agent to cholesterol in cholesteryl iopanoate (5) facilitates the radioimaging agent to be taken up by the liver as a potential imaging agent in hepatic adenocarcinoma CT (Longino et al., 1984).
Figure 4.
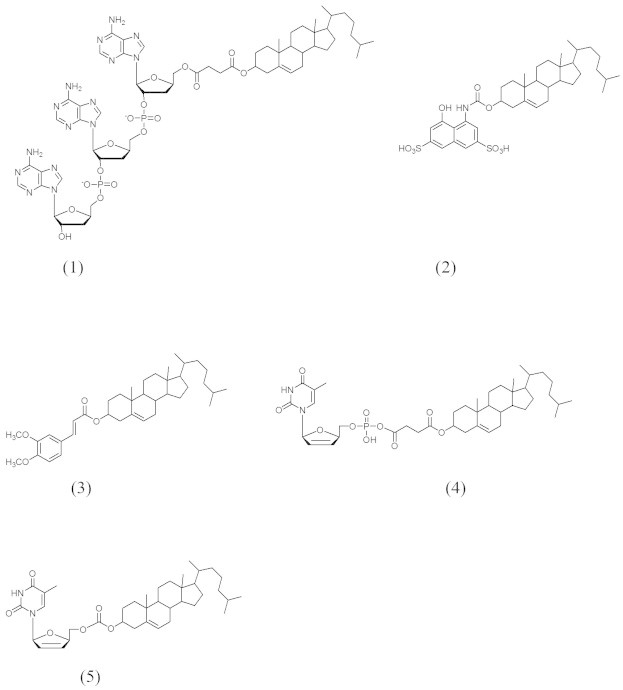
Compounds 1–5.
5.1. Cholesterol analogs as anticancer
Many steroids and their triterpene precursors (Fig. 5) (Mihai et al., 2011) such as betulinic, oleanolic, and ursolic acids and stigmasterol have been reported as antitumor agents (Awad and Fink, 2000; Ma et al., 2005). Moreover, many previous studies have shown that some phytocomponents related to cholesterol presented induced apoptosis (Itoh et al., 2008). However, most of the steroids presented in the literature as cytotoxic compounds are polyhydroxylated or contain a α,β-unsaturated ketone function (Amagata et al., 1999; Wang et al., 2006; Samadi et al., 2010; Shang et al., 2011). Besides, these terpenoids caused cell death when they are presented as sulfate (Zhang et al., 2007) or quaternary ammonium salts (Brassart et al., 2007).
Figure 5.
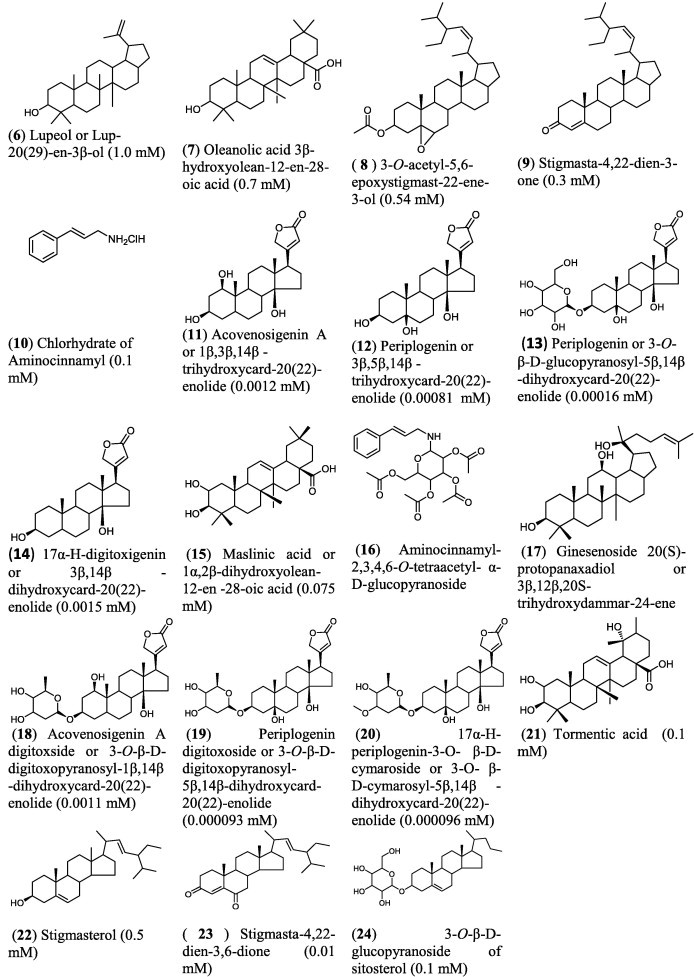
Some hemisynthetic and isolated natural compounds (6–23) along with their IC50 cytotoxic activity on human fibrosarcoma cell HT1080.
6. Prostate cancer
It has long been established that prostatic growth is stimulated by 5α-dihydrotestosterone (DHT), rather than by the classic testicular hormone testosterone (T) (Bruchovsky and Wilson, 1968). Metabolically DHT is made from T by the action of the enzyme steroid 5α-reductase (5AR). A deficiency of 5AR in males results in an incomplete differentiation of external genitalia at birth (Abul-Hajj, 1972). On the other hand, abnormally high 5AR activity in humans results in excessively high DHT levels in peripheral tissues, which is implicated in the pathogenesis of prostate cancer, benign prostatic hyperplasia (BPH), acne and male pattern baldness (Andersson et al., 1989; Cabeza et al., 2004). Two distinct isozymes of 5AR are differentially expressed in human tissues and referred to as type 1 5AR (5AR1) and type 2 5AR (5AR2) (Andersson and Russell, 1990; Thigpen et al., 1993; Russell and Wilson, 1994). Although 5AR1 is predominantly expressed in the skin and the liver, 5AR2 is mainly expressed in prostate, seminal vesicles, liver and epididymis (Hartmann et al., 2000).
Various steroidal (Ramírez et al., 2002; Flores et al., 2003) and non-steroidal (Audia et al., 1993; Jones et al., 1993; Kurup et al., 2000) inhibitors have been synthesized and tested against steroid 5α-reductase enzyme (5AR). Of these, finasteride (24) (PROSCAR®, Fig. 6), a type II-selective 5α-reductase inhibitor, was the first 5AR inhibitor approved in the USA for the treatment of BPH and prostate cancer. It has also been demonstrated that dutasteride (25) (Fig. 6) acts as a type 1 5AR (5AR1) and type 2 5AR (5AR2) inhibitor (Lazier et al., 2004) and turosteride (26) (Fig. 6) is selective for the type II isoform of 5α-reductase. However, since finasteride is slow acting and produces side effects affecting sexual function (Sudduth and Koronkowski, 1993), this has caused us and others to seek new classes of steroidal and non-steroidal inhibitors.
Figure 6.
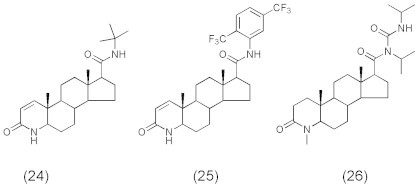
Steroid 5α-reductase enzyme inhibitors (24–26).
A series of C21 steroids (Fig. 7) showed less activity than finasteride (IC50: 1.2 nM) as inhibitors against 5α-reductase (Kim and Ma, 2009; Sujeong et al., 2012). Of these steroids 4-azasteroid-20-oximes (28, 30) showed good inhibitory activity (IC50: 26, 10 and 11 nM) and were more active than corresponding 4-azasteroid 20-ones (27, 29) (IC50: 2300, 6100 nM). On the other hand, introduction of a double bond at 1-position of these 4-azasteroides results in 4-azasteroid-20-oxime (32) of good inhibitory activity (IC50: 11 nM) as well as its analog 4-azasteroid-20-one (31) (IC50: 14 nM).
Figure 7.
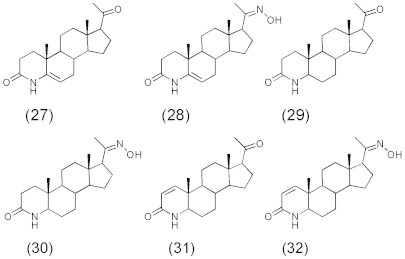
C21 steroids (27–32); 5α-Reductase inhibitors.
For some non azasteroid derivatives (Fig. 8), pregnenolone (33) showed high inhibitory activity of 5α-reductase enzyme (IC50: 6.5 nM) whereas its derivatives 3β-Acetoxypregnenolone (34) and 20-oxime derivative (35), 3β-hydroxy-5α,6α-epoxypregnane and 20-oxime (36 and 37), 3β-hydroxy pregn-4-ene-20-oxime (38) exhibited no inhibition for 5α-reductase enzyme (IC50 > 10000 nM). This leads to the indication that reduction or acetylation of carbonyl group at C-3 position will abolish the activity. Also, the introduction of a hydrophilic epoxy group will greatly diminish the activity. Moreover, compound 39, the 20-oxime analog of progesterone, was less active (IC50: 14 nM) than the corresponding carbonyl compound (33). 1,4-Pregnadiene-20-oxime (40) exhibited inhibitory activity similar to that of compound (41) (IC50: 760 nM) and was more active than 6α,7α-epoxy-1,4-pregnadiene-20-one (42) (IC50: >10000 nM) having an epoxy ring at the 6,7 position.
Figure 8.
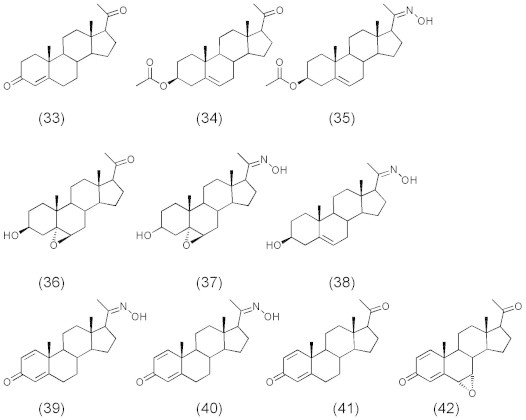
Compounds 33–42.
7. Cholesterol derivatives as DNA topoisomerase and cancer cell growth inhibitor
The inhibitory activities of cholesterol derivatives (Fig. 9) such as cholesterol, sodium cholesteryl sulfate, cholesteryl-5a, 6a-epoxide, cholesteryl chloride, cholesteryl bromide, and cholesteryl hemisuccinate (43–48,) against DNA polymerase (pol), DNA topoisomerase (topo), and human cancer cell growth were reported (Ishimaru et al., 2008). In the human body, cholesterol derivatives cholesteryl sulfate (44) and cholesteryl-5a, 6aepoxide (45) were produced by cholesterol sulfotransferase (Chida et al., 1995) and autoxidation (Ishimaru et al., 2008), respectively. Compound 44 is a second messenger of the g isoform of protein kinase C mediating squamous differentiation, and inhibits the tumor promotional phase in mouse skin carcinogenesis (Chida et al., 1995). Compound 44 is present at approximately 300 μg/100 ml (i.e., 6.1 l M) in human body (Strott and Higashi, 2003), and this concentration is almost the same inhibitory dose in pol/topo activities and cancer cell growth. Since synthesized derivatives from cholesterol in the body may have possible work in anti-cancer activity, a diet containing cholesterol prevents cancer disease. Compounds 44 and 48 revealed themselves to be potent inhibitors of animal pols, and the IC50 values for pols were 0.84–11.6 and 2.9–148 μM, respectively. Compounds 44, 45 and 48 inhibited the activity of human topo II, with IC50 values of 5.0, 12.5 and 120 μM, respectively. Compounds 44, 45 and 48 also suppressed the human cancer cell (promyelocytic leukemia cell line, HL-60) growth, and LD50 values were 8.8, 20.2 and 72.3 μM, respectively, suggesting that cell growth inhibition had the same tendency as the inhibition of topos rather than pols. Compounds 44 and 48 arrested the cells in S and G2/M phases, compound 45 arrested the cells in the G2/M phase, and these compounds also increased the sub-G1phase in the cell cycle. These results suggested that the effect of cell cycle arrest might be effective on both pol and topo activities.
Figure 9.
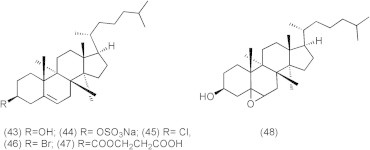
Cholesterol derivatives.
8. Cholesterol-induced antitumor synergism
Betulin (BE) which is a precursor of betulinic acid (BetA) is isolated from different plants (Cichewicz and Kouzi, 2004). It was found that BE induces apoptosis utilizing a similar mechanism as BetA and is prevented by cyclosporin A (CsA). In comparison with BetA, BE showed a more rapid death, but achieves similar amounts of cell death at a considerably higher concentration. At the same time, it was observed that cholesterol sensitized cells to BE-induced apoptosis, while there was no effect of cholesterol when combined with BetA. Despite the significantly enhanced cytotoxicity, the mode of cell death was not changed as CsA completely abrogated cell death. These findings indicate that BE has potent anti-tumor activity especially in combination with cholesterol (Mullauer et al., 2009).
Furthermore, cholesterol sequestration by nystatin, a polyene antifungal drug, significantly enhances endostatin uptake by endothelial cells through the switching of endostatin internalization predominantly to the clathrin-mediated pathway. Nystatin-enhanced internalization of endostatin also increases its inhibitory effects on endothelial cell tube formation and migration. More importantly this enhancement of the uptake and therapeutic efficacy of endostatin with cholesterol-sequestering agents selectively enhances endostatin uptake and biodistribution in tumor blood vessels and tumor tissues but not in normal tissues of tumor-bearing mice, ultimately resulting in elevated antiangiogenic and antitumor efficacies of endostatin in vivo (Chen et al., 2011).
9. Cholesterol-based intracerebral delivery of chemotherapeutics
The lack of effective treatment options results in poor prognosis of brain tumor. It is crucial to deliver a sufficient amount of therapeutic agents to the brain tumor site. However, delivery of therapeutic agents to the brain is technically challenging due to the presence of blood brain barrier (BBB) (Zee-Cheng and Cheng, 1989). Many attempts of special drug delivery have been made to overcome this obstacle. One approach is to intracerebrally administer the agents within brain parenchyma through local delivery to tumor tissue (Lu et al., 1997). The advantage of this approach is it results in high drug concentrations at the tumor site with limited exposure to normal tissues and organs. Another approach developed a cholesterol-based anticancer agent containing carborane as the anticancer unit for boron neutron capture therapy. The new compound (Fig. 10), cholesteryl 1,12-dicarba-closo-dodecaborane 1-carboxylate (BCH) (49) mimics the native cholesteryl ester in structure and was found to be effectively taken up by brain glioma cells in vitro (Peacock et al., 2004). In addition, BCH was formulated in liposomes and was administrated intracerebrally to tumor-bearing rats. The ratio of BCH between tumor tissue and normal tissue at 2, 6, 8, and 14 h were 5.46, 1.56, 1.12, and 1.01, respectively (Alanazi et al., 2004).
Figure 10.
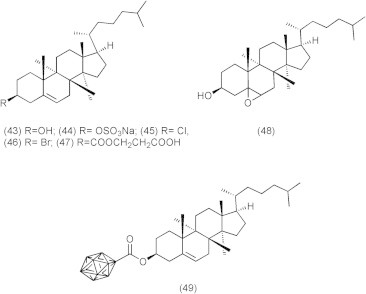
Cholesteryl 1,12-dicarba-closododecarborane 1-carboxylate (BCH).
In a new approach, doxorubicin and cholesterol derivatives (50) (Fig. 11) were bound by pH-sensitive hydrazone bonds to N -(2-hydroxypropyl)methacrylamide (HPMA) copolymers. At pH 5.0, the hydrazone bond showed a hydrolysis rate strongly dependent on the microenvironment around the bond. This property led to the site-specific release of the doxorubicin and hydrophobic domains because of the decrease in pH in the endosomes or lysosomes of tumor cells. Doxorubicin would be released in the first stage, whereas the polymer macromolecule would be disintegrated very slowly into short fragments that are small enough to be eliminated by glomerular filtration (Chytil et al., 2012).
Figure 11.
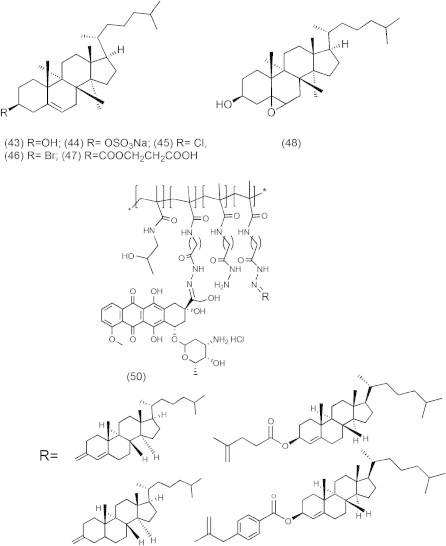
Schematic structure of cholesterol/Dox conjugates.
10. Cholesterol-based targeted delivery of anticancer compounds
One of the successful chemotherapeutic approaches is the boron neutron capture therapy (BNCT) that is currently used to treat brain tumors such as glioblastoma multiforme (GBM) and anaplastic astrocytomas (AA) (Barth, 2003; Zamenhof et al., 2004; Barth et al., 2005). BNCT delivers boron-10 to the tumor tissue followed by external radiation of low energy (e.g., ∼0.025 keV) thermal neutron that results in fission reactions which produce high linear energy transfer (LET) α-particles and recoiling 7Li nuclei (10B + 1n → [11∗B] → 4He (α) + 7Li + 2.39 MeV), which are highly lethal to surrounding cells. It is crucial for efficient BNCT to selectively deliver a sufficient amount of 10B (∼20 μg/g tumor) to tumor cells (Vicente, 2006; Wu et al., 2006). Delivery of 10B containing agents via liposomes conjugated to the anti-EGFR monoclonal antibody (MAb) cetuximab (C225) and L8A4, which is directly against EGFRvIII is an attractive delivery approach for BNCT, because of their high payload capacity (Barth et al., 2002; Wu et al., 2006). These liposomes consist of cholesterol anchored folate (folate-PEG-Chol) (51) in EGFR-folate receptor targeted liposomes (Fig. 12) (Lee and Low, 1994; Guo et al., 2000) or consist of cholesterol anchored MAb (MAb-PEG-Chol) (53) in the preparation of immunoliposomes (55) (Figs. 13 and 14) (Pan et al., 2007).
Figure 12.
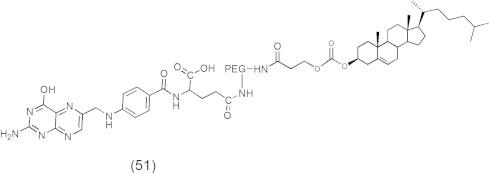
Chemical structure of folate-PEG-Cholesterol.
Figure 13.
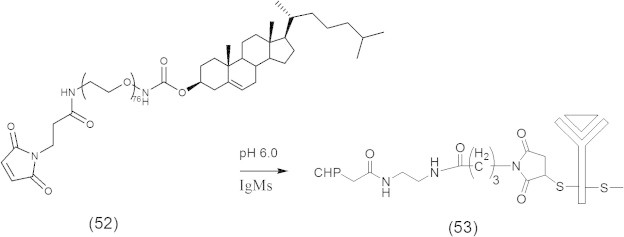
Synthetic scheme for Mal-PEG-Chol.
Figure 14.
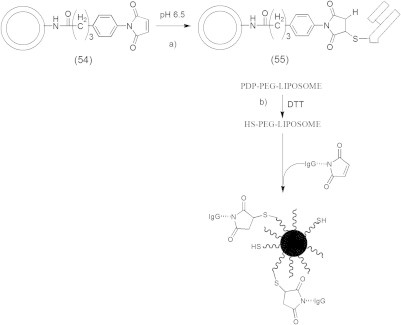
Covalent conjugation of Fab’ fragments to MPB-PE liposomes.
11. Cholesterol-sugar conjugates as anticancer agents against peritoneal dissemination of tumor cells
Peritoneal dissemination of cancer cells is a major form of recurrence in patients with advanced stages of gastric, colorectal, ovarian, and pancreatic cancers after curative resection of tumors (Vogel and Kalthoff, 2001; Sugarbaker, 2005; Koppe et al., 2006; de Bree et al., 2006; Yan et al., 2006). In this phenomenon, tumor cells exfoliate from the tumors to the abdominal cavity, adhere to the surface of the peritoneum to invade the basement membrane, and, in particular, adhere to the greater omentum and the mesenterium (Asao et al., 1994; Asao et al., 1995; Okamura et al., 2000). In this process cell surface glycoconjugates have an essential role. Chemically synthesized sugars have been developed as anti-adhesion molecules against the peritoneal dissemination of cancer cells (Asao et al., 1994; Asao et al., 1995; Okamura et al., 2000). Basically, chemically synthesized sugar-cholestanols with mono-, di-, and tri-saccharides attached to cholestanol (56) showed a strong inhibiting activity against the proliferation of colorectal and gastric cancer cells by inducing apoptotic cell death. Also, these sugar-cholestanols were suggested to have clinical potential as a novel anticancer agent based on their strong inhibiting activity against peritoneal dissemination in a mouse model. In contrast, cholestanol without sugar moieties was totally ineffective. Furthermore, when cancer cells were exposed to GlcNAcRβ-cholestanol (R = (−) or β1–3Gal; Fig. 15) (56), the compound was rapidly taken up via the lipid rafts/microdomains on the cell surface. The uptake of sugar-cholestanol in mitochondria increased gradually and was followed by the release of cytochrome c from mitochondria and the activation of apoptotic signals through the mitochondrial pathway and the caspase cascade, leading to apoptotic cell death, characterized by DNA ladder formation and nuclear fragmentation (Shinji et al., 2008).
Figure 15.
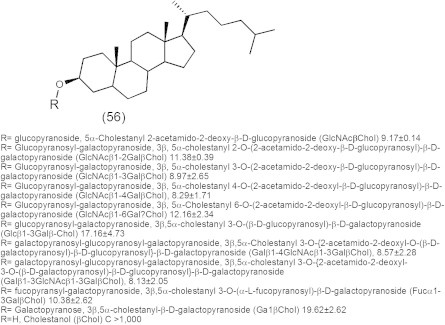
Chemical structure of sugar-cholestanols with mono-, di-, and tri-saccharides attached to cholestanol and their IC50 in μM against colorectal cells.
In recent work, chemically synthesized sugar-cholestanols (57) (Fig. 16) were demonstrated to possess potential multi-target anticancer activity against human esophageal cell lines because they induced apoptotic cell death of esophageal cancer cells through modulating the Bcl-2 family, caspase cascade, PARP and VEGF-A (Ahmad et al., 2007).
Figure 16.
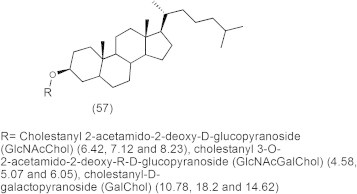
Cholestanol-sugar structures and their 50% of cell proliferation inhibition (CPI50, μM) of TE-2, TE-13 and CHEK-1 cells respectively.
In summary, this review focused not only on the cholesterol blood level during some types of cancer diseases but also on the great benefit of using cholesterol derivatives in diagnosis and in the perioperative and postoperative chemotherapy for the treatment of various types of cancer diseases.
12. Future prospective
Conjugation of the cholesterol moiety to active medicinal compounds for either cancer disease diagnosis or treatment is an attractive approach for targeted drug delivery. There are many additional areas in which this targeting tactic could be applied to improve the chemotherapeutic efficacy and to reduce the toxicity to normal cells. For example, brain drug delivery presents a challenge due to the presence of blood brain barrier (BBB). Several anticancer agents were victorised by cholesterol for brain delivery and the experimental results appeared promising.
It is well known that LDL receptors are present on the BBB capillary endothelial cells and, therefore, they could be utilized for transporting cholesteryl-based or other compounds to the brain. Furthermore, cholesterol accumulates in the ovarian tissue and is used to synthesize sex hormones. Therefore, cholesteryl drug conjugates could also be used for drugs targeted to the ovary.
Since most of the cholesteryl drug conjugates are very hydrophobic, suitable pharmaceutical formulations are also required to enhance the solubility of these conjugates in blood circulation and to improve their interactions with lipoproteins and other functional proteins. Risks from the reaching of cholesteryl conjugates to non-target tissues and cells need to be investigated to fully assess the risk/benefit ratio. Since the liver most likely takes up significant amount of these cholesteryl conjugates, the associated pharmacological and toxic effects should be specifically examined. Literature data have indicated that local excess of cholesterol, the metabolic product of cholesteryl conjugates, can be cytotoxic due to the build-up of free cholesterol hydrolysate in the plasma membrane.
The information related to biodistribution and pharmacokinetics of various cholesteryl drug conjugates is also essential for their practical use. When active drug molecules are conjugated to cholesterol, their properties, such as hydrophilicity/lipophilicity and molecular weight, are largely altered and consequently their biodistribution, pharmacokinetics as well as efficacy are significantly affected. Therefore, the delivery mechanism of these new cholesteryl conjugates at cellular and molecular levels involving their interactions with proteins, cells, receptors and membranes becomes important and requires extensive investigation. Nevertheless, utilization of cholesteryl drug conjugates for targeted delivery provides a novel approach which has the potential to enhance their therapeutic efficacy and benefit the medical treatment of various human diseases.
Acknowledgements
This research project is fully supported by National Plan for Science and Technology “NPST” program by King Saud University”, Project Number 10-NAN1286-02.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abul-Hajj Y.J. Stereospecificity of hydrogen transfer from NADPH by steroid Δ4-5α-and Δ4-5β-reductase. Steroids. 1972;20:215–222. doi: 10.1016/0039-128x(72)90081-5. [DOI] [PubMed] [Google Scholar]
- Ahmad F., Leri S.F., Takashi N., Takahito Y., Mami K., Hiroyuki S., Toyo N., Nurhayat U., Hiroyuki K., Takayuki A., Hiroyuki K., Shin Y. Chemically synthesized sugar-cholestanols possess a preferential anticancer activity involving promising therapeutic potential against human esophageal cancer. Cancer Sci. 2007;98:1358–1367. doi: 10.1111/j.1349-7006.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanazi F., Halpern D.S., Lu D.R. Development of cholesterol-based conjugates for targeted drug delivery. S.T.P. Pharma Sci. 2003;13:27–35. [Google Scholar]
- Alanazi F., Halpern D.S., Lu D.R. Intracerebral diffusion of new cholesterol-based anticancer conjugate in tumor-bearing rat model. J. Appl. Res. 2004;4:127–134. [Google Scholar]
- Amagata T., Doi M., Tohgo M., Minoura K., Numata A. Dankasterone, a new class of cytotoxic steroid produced by a Gymnascella species from a marine sponge. Chem. Commun. 1999;14:1321–1322. [Google Scholar]
- American Cancer Society . American Cancer Society; Atlanta: 2012. Cancer facts and figures: 2012. [Google Scholar]
- Andersson S., Russell D.W. Structural and biochemical properties of cloned expressed human and rat steroid 5α-reductase. Proc. Natl. Acad. Sci. USA. 1990;87:3544–3640. doi: 10.1073/pnas.87.10.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S.R., Bishop R.W., Russell D.W. Expression cloning and regulation of steroid 5α–reductase, an enzyme essential for male sexual differentiation. J. Biol. Chem. 1989;264:16249–16255. [PMC free article] [PubMed] [Google Scholar]
- Asao T., Yazawa S., Kudo S., Takenoshita S., Nagamachi Y. A novel ex vivo method for assaying adhesion of cancer cells to the peritoneum. Cancer Lett. 1994;78:57–62. doi: 10.1016/0304-3835(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Asao T., Nagamachi Y., Morinaga N., Shitara Y., Takenoshita S., Yazawa S. Fucosyltransferases of the peritoneum contributed to the adhesion of cancer cells to the mesothelium. Cancer. 1995;75:1539–1544. doi: 10.1002/1097-0142(19950315)75:6+<1539::aid-cncr2820751526>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Audia J.E., Lawhorn D.E., Deeter J.B. Synthesis of the individual enantiomers of the benzoquinolinone human type 1 steroid 5α-reductase inhibitors LY191704 and LY266111. Tetrahedron Lett. 1993;34:7001–7004. [Google Scholar]
- Awad A.B., Fink C.S. Phytosterols as anticancer dietary components: evidence and mechanism of action. J. Nutr. 2000;130:2127–2130. doi: 10.1093/jn/130.9.2127. [DOI] [PubMed] [Google Scholar]
- Baier C.J., Barrantes F.J. Sphingolipids are necessary for nicotinic acetylcholine receptor export in the early secretory pathway. J. Neurochem. 2007;101:1072–1084. doi: 10.1111/j.1471-4159.2007.04561.x. [DOI] [PubMed] [Google Scholar]
- Barrantes F.J. Cholesterol effects on nicotinic acetylcholine receptor: cellular aspects. Subcell Biochem. 2010;51:467–487. doi: 10.1007/978-90-481-8622-8_17. [DOI] [PubMed] [Google Scholar]
- Barres B.A., Smith S.J. Cholesterol—making or breaking the synapse. Science. 2001;294:1296–1297. doi: 10.1126/science.1066724. [DOI] [PubMed] [Google Scholar]
- Barth R.F. A critical assessment of boron neutron capture therapy: an overview. J. Neurooncol. 2003;62:1–210. doi: 10.1007/BF02699929. [DOI] [PubMed] [Google Scholar]
- Barth R.F., Yang W., Adams D.M., Rotaru J.H., Shukla S., Sekido M., Tjarks W., Fenstermaker R.A., Ciesielski M., Nawrocky M.M., Coderre J.A. Molecular targeting of the epidermal growth factor receptor for neutron capture therapy of gliomas. Cancer. 2002;62:3159–3166. [PubMed] [Google Scholar]
- Barth R.F., Coderre J.A., Vicente M.G., Blue T.E. Boron neutron capture therapy of cancer: current status and future prospects. Clin. Canc. Res. 2005;11:3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- Bouillon R., Okamura W.H., Norman A.W. Structure–function relationships in the vitamin D endocrine system. Endocr. Rev. 1995;16:200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- Brassart B., Gomez D., De Cian A., Paterski R., Montagnac A., Qui K.-H., Temime-Smaali N., Trentesaux C., Mergny J.-L., Gueritte F. A new steroid derivative stabilizes G-quadruplexes and induces telomere uncapping in human tumor cells. Mol. Pharmacol. 2007;72:631–640. doi: 10.1124/mol.107.036574. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J.D. The conversion of testosterone to 5-androstan-17-ol-3-one by rat prostate in vivo and in vitro. J. Biol. Chem. 1968;242:2012–2021. [PubMed] [Google Scholar]
- Cabeza M., Flores E., Heuze I., Sanchez M., Bratoeff E., Ramirez E., Francolugo V.A. Novel 17-substituted pregnadiene derivatives as 5α-reductase inhibitors and their binding affinity for the androgen receptor. Chem. Pharm. Bull. 2004;52:535–539. doi: 10.1248/cpb.52.535. [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang S., Lu X., Zhang H., Fu Y., Luo Y. Cholesterol sequestration by nystatin enhances the uptake and activity of endostatin in endothelium via regulating distinct endocytic pathways. Blood. 2011;117:6392–6403. doi: 10.1182/blood-2010-12-322867. [DOI] [PubMed] [Google Scholar]
- Chida K., Murakami A., Tagawa T., Ikuta T., Kuroki T. Cholesterol sulfate, a second messenger for the g isoform of protein kinase C, inhibits promotional phase in mouse skin carcinogenesis. Cancer Res. 1995;55:4865–4869. [PubMed] [Google Scholar]
- Chytil P., Etrych T., Kostka L., Ulbrich K. Hydrolytically degradable polymer micelles for anticancer drug delivery to solid tumors. Macromol. Chem. Phys. 2012;213:858–867. [Google Scholar]
- Cichewicz R.H., Kouzi S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004;24:90–114. doi: 10.1002/med.10053. [DOI] [PubMed] [Google Scholar]
- de Bree E., Theodoropoulos P.A., Rosing H., Michalakis J., Romanos J., Beijnen J.H., Tsiftsis D.D. Treatment of ovarian cancer using intraperitoneal chemotherapy with taxanes: from laboratory bench to bedside. Cancer Treat. Rev. 2006;32:471–482. doi: 10.1016/j.ctrv.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Denniston, K., Topping, J., Caret, R., 2007. General Organic and Biochemistry: 6th Edition. McGraw Hill, New York, pp. 602–603.
- Dietschy J.M., Turley S.D. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid. Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Fantini J., Barrantes F.J. Sphingolipid/cholesterol regulation of neurotransmitter receptor conformation and function. Biochim. Biophys. Acta. 2009;1788:2345–2361. doi: 10.1016/j.bbamem.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Fars K. A., 2003. Application of lipoprotein as drug delivery system for anticancer drug and DNA-based vaccine. Thesis pp. 11-14.
- Favre G. Targeting of tumor cells by low-density lipoproteins: principle and use of ellipticin derivatives. C. R. Seances Soc. Biol. Fil. 1992;186:73–87. [PubMed] [Google Scholar]
- Flores E., Bratoeff E., Cabeza M., Ramirez E., Quiroz A., Heuze I. Steroid 5-reductase inhibitors. Mini Rev. Med. Chem. 2003;3:225–237. doi: 10.2174/1389557033488196. [DOI] [PubMed] [Google Scholar]
- Gross M.D., Shapiro B., Francis I.R., Glazer G.M., Bree R.L., Arcomano M.A., Schteingart D.E., McLeod M.K., Sanfield J.A., Thompson N.W. Scintigraphy evaluation of clinically silent adrenal masses. J. Nucl. Med. 1994;35:1145–1152. [PubMed] [Google Scholar]
- Gums J.G., Wilt V.M. Disorders of the adrenal gland. In: Dipiro J.T., editor. Pharmacotherapy, A pathophysiologic approach. Appleton and Lange; Connecticut: 1997. pp. 1547–1583. [Google Scholar]
- Guo W.J., Lee T., Sudimack J., Lee R.J. Receptor-specific delivery of liposomes via folate-PEG-Chol. J. Liposome Res. 2000;10:179–195. [Google Scholar]
- Hartmann R.W., Hector M., Haidar S., Ehmer P.B., Reichert W., Jose J. Synthesis and evaluation of novel steroidal oxime inhibitors of P450 17 (17α-hydroxylase/C17,20-lyase) and 5α-reductase types 1 and 2. J. Med. Chem. 2000;43:4266–4277. doi: 10.1021/jm001008m. [DOI] [PubMed] [Google Scholar]
- Hawkins L.A., Brritton K.E., Shairo B. Selenium-75 selenomethyl cholesterol: a new agent for quantitative functional scintigraphy of the adrenals: physical aspects. Br. J. Radiol. 1980;53:883–889. doi: 10.1259/0007-1285-53-633-883. [DOI] [PubMed] [Google Scholar]
- Hume R., Boyd G.S. Cholesterol metabolism and steroid-hormone production. Biochem. Soc. Trans. 1978;6:893–898. doi: 10.1042/bst0060893. [DOI] [PubMed] [Google Scholar]
- Ishimaru C., Yonezawa Y., Kuriyama I., Nishida M., Yoshida H., Mizushina Y. Inhibitory effects of cholesterol derivatives on DNA polymerase and topoisomerase activities, and human cancer cell growth. Lipids. 2008;43:373–382. doi: 10.1007/s11745-007-3149-y. [DOI] [PubMed] [Google Scholar]
- Itoh H., Ito H., Hibasami H. Blazein of a new steroid isolated from Agaricus blazei Murrill (himematsutake) induces cell death and morphological change indicative of apoptotic chromatin condensation in human lung cancer LU99 and stomach cancer KATO III cells. Oncol. Rep. 2008;20:1359–1361. [PubMed] [Google Scholar]
- Johnson S.D., Welch M.J. Synthesis, biological and baboon PET imaging of the potential adrenal imaging agent cholesteryl-p-[18F]fluorobenzoate. Nucl. Med. Biol. 1999;26:131–138. doi: 10.1016/s0969-8051(98)00081-x. [DOI] [PubMed] [Google Scholar]
- Jones C.D., Audia J.E., Lawhorn D.E., McQuaid L.A., Neubauer B.L., Pike A.J., Pennington P.A., Stamm N., Toomey R.E., Hirsch K.S. Nonsteroidal inhibitors of human type I steroid 5α-reductase. J. Med. Chem. 1993;36:421–423. doi: 10.1021/jm00055a014. [DOI] [PubMed] [Google Scholar]
- Kim S., Ma E. Synthesis of pregnane derivatives, their cytotoxicity on LNCap and PC-3 cells, and screening on 5α-reductase inhibitory activity. Molecules. 2009;14:4655–4668. doi: 10.3390/molecules14114655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimov A.N., Korovkin B.F., Kuznetsov A.S., Popov I.N. Apolipoprotein B of plasma lipoproteins incorporated in liposomes: immunological properties and organ distribution when administered to rabbits. Bull. Eksp. Biol. Med. 1983;96:47–50. [PubMed] [Google Scholar]
- Koppe M.J., Boerman O.C., Oyen W.J., Bleichrodt R.P. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann. Surg. 2006;243:212–222. doi: 10.1097/01.sla.0000197702.46394.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritchevsky S.B. Serum cholesterol and cancer risk: an epidemiologic perspective. Annu. Rev. Nutr. 1992;12:391–416. doi: 10.1146/annurev.nu.12.070192.002135. [DOI] [PubMed] [Google Scholar]
- Kurup A., Garg R., Hansch C. Comparative QSAR analysis of 5α-reductase inhibitors. Chem. Rev. 2000;100:909–924. doi: 10.1021/cr990028x. [DOI] [PubMed] [Google Scholar]
- Lazier C.B., Thomas L.N., Douglas R.C., Vessey J.P., Rittmaster R.S. Dutasteride, the dual 5α-reductase inhibitor, inhibits androgen action and promotes cell death in the LNCaP prostate cancer cell line. Prostate. 2004;58:130–144. doi: 10.1002/pros.10340. [DOI] [PubMed] [Google Scholar]
- Lee R.J., Low P.S. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J. Biol. Chem. 1994;269:3198–3204. [PubMed] [Google Scholar]
- Liebermann L.M., Beierwaltes W.H., Conn J.W., Ansari A.N., Nishiyama H. Diagnosis of adrenal disease by visualization of human adrenal glands with 131I-19-iodocholesterol. N. Engl. J. Med. 1971;285:1387–1393. doi: 10.1056/NEJM197112162852501. [DOI] [PubMed] [Google Scholar]
- Longino M.A., Glazer G.M., Weichert J.P., Groziak M.P., Schwendner S.W., Counsell R.E. Esters of iopanoic acid as liver-specific CT contrast agents: biodistribution and CT evaluation. J. Comput. Assist. Tomogr. 1984;8:1099–1104. doi: 10.1097/00004728-198412000-00010. [DOI] [PubMed] [Google Scholar]
- Lu D.R., Mehta S.C., Chen W. Selective boron drug delivery to brain tumors for boron neutron capture therapy. Adv. Drug Deliv. Rev. 1997;26:231–247. doi: 10.1016/s0169-409x(97)00037-9. [DOI] [PubMed] [Google Scholar]
- Ma C.-M., Cai S.-Q., Cui J.-R., Wang R.-Q., Tu P.-F., Hattori M., Daneshtalab M. The cytotoxic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2005;40:582–589. doi: 10.1016/j.ejmech.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Maletinska L., Blakely E.A., Bjornstad K.A., Deen D.F., Knoff L.J., Forte T.M. Human glioblastoma cell lines: levels of low-density lipoprotein receptor and low-density lipoprotein receptor-related protein. Cancer Res. 2000;60:2300–2303. [PubMed] [Google Scholar]
- Mihai V.P., Marius L., Louis P.S. Quantitative structure inter-activity relationship (QSInAR). Cytotoxicity study of some hemisynthetic and isolated natural steroids and precursors on human fibrosarcoma cells HT1080. Molecules. 2011;16:6603–6620. doi: 10.3390/molecules16086603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullauer F.B., Kessler J.H., Medema J.P. Betulin Is a potent anti-tumor agent that is enhanced by cholesterol. PLoS One. 2009;4:e5361. doi: 10.1371/journal.pone.0005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura A., Yazawa S., Nishimura T., Tanaka S., Takai I., Kudo S., Asao T., Kuwano H., Matta K.L., Akamatsu S., Kochibe N. A new method for assaying adhesion of cancer cells to the greater omentum and its application for evaluating antiadhesion activities of chemically synthesized oligosaccharides. Clin. Exp. Metastasis. 2000;18:37–43. doi: 10.1023/a:1026526829010. [DOI] [PubMed] [Google Scholar]
- Pan X., Wu G., Yang W., Barth R.F., Tjarks W., Lee R.J. Synthesis of cetuximab-immunoliposomes via a cholesterol-based membrane anchor for targeted delivery of a neutron capture therapy (NCT) agent to glioma cells. Bioconjug. Chem. 2007;18:101–108. doi: 10.1021/bc060174r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock G., Sidwell R., Pan G., Oie S., Lu D.R. In vitro uptake of a new cholesteryl carborane ester compound in human glioma cell lines. J. Pharm. Sci. 2004;93:13–19. doi: 10.1002/jps.10452. [DOI] [PubMed] [Google Scholar]
- Peto R., Borenham J., Chen J., Li J., Campbell T.C., Brun T. Plasma cholesterol, coronary heart disease, and cancer. Br. Med. J. 1989;298:1249. doi: 10.1136/bmj.298.6682.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proksch E. The epidermis as metabolically active tissue: regulation of lipid synthesis by the barrier function. Z. Hautkr. 1990;65:296–300. [PubMed] [Google Scholar]
- Ramírez E., Cabeza M., Heuze I., Gutierrez E., Bratoeff E., Membrillo M., Lira A. Synthesis and pharmacological evaluation of new 16-methyl pregnenolone derivatives. Chem. Pharm. Bull. 2002;50:15–20. doi: 10.1248/cpb.50.15. [DOI] [PubMed] [Google Scholar]
- Rudling M.J., Angelin B., Peterson C.O., Collins V.P. Low density lipoprotein receptor activity in human intracranial tumors and its relation to the cholesterol requirement. Cancer Res. 1990;50:483–487. [PubMed] [Google Scholar]
- Russell D.W., Wilson J.D. Steroid 5 alpha-reductase: two genes/two enzymes. Ann. Rev. Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- Samadi A.K., Tong X., Mukerji R., Zhang H., Timmermann B.N., Cohen M.S. Withaferin A, a cytotoxic steroid from Vassobia breviflora, induces apoptosis in human head and neck squamous cell carcinoma. J. Nat. Prod. 2010;73:1476–1481. doi: 10.1021/np100112p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang X.-Y., Li J.-J., Liu M.-T., Li S., Liu Y., Wang Y.-F., Huang X., Jin Z.-L. Cytotoxic steroids from Monascus purpureus-fermented rice. Steroids. 2011;76:1185–1189. doi: 10.1016/j.steroids.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Shawer M., Greenspan P., Øie S., Lu D.R. VLDL-resembling phospholipid-submicron emulsion for cholesterol-based drug targeting. J. Pharm. Sci. 2002;91:1405–1413. doi: 10.1002/jps.10117. [DOI] [PubMed] [Google Scholar]
- Shinji H., Shin Y., Takayuki A., Ahmad F., Toyo N., Kaori T., Takashi N., Takahito Y., Noriyuki K., Ken U., Abbi R.S., Hiroyuki K. Novel sugar-cholestanols as anticancer agents against peritoneal dissemination of tumor cells. Glycoconj J. 2008;25:531–544. doi: 10.1007/s10719-008-9108-x. [DOI] [PubMed] [Google Scholar]
- Strott C.A., Higashi Y. Cholesterol sulfate in human physiology: what’s it all about? J. Lipid Res. 2003;44:1268–1278. doi: 10.1194/jlr.R300005-JLR200. [DOI] [PubMed] [Google Scholar]
- Sudduth S.L., Koronkowski M.J. Finasteride, the first 5 alpha-reductase inhibitor. Pharmacotherapy. 1993:309–325. [PubMed] [Google Scholar]
- Sugarbaker P.H. Strategies for the prevention and treatment of peritoneal carcinomatosis from gastrointestinal cancer. Cancer Invest. 2005;23:155–172. [PubMed] [Google Scholar]
- Sujeong K., Yong-ung K., Eunsook M. Synthesis and 5α-reductase inhibitory activity of C21 steroids having 1,4-diene or 4,6-diene 20-ones and 4-azasteroid 20-oximes. Molecules. 2012;17:355–368. doi: 10.3390/molecules17010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen A.E., Silver R.I., Guileyardo J.M., Casey M., McConnel J.D., Russell D.W. Tissue distribution and ontogeny of steroid 5alpha-reductase isozyme expression. J. Clin. Invest. 1993;92:903–910. doi: 10.1172/JCI116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente M.G.H. Boron in medicinal chemistry. Anticancer Agents Med. Chem. 2006;6:73–181. doi: 10.2174/187152006776119135. [DOI] [PubMed] [Google Scholar]
- Vitols S. Uptake of low-density lipoprotein by malignant cells—possible therapeutic applications. Cancer Cells. 1991;3:488–495. [PubMed] [Google Scholar]
- Vogel I., Kalthoff H. Disseminated tumour cells. Their detection and significance for prognosis of gastrointestinal and pancreatic carcinomas. Virchows Arch. 2001;439:109–117. doi: 10.1007/s004280100476. [DOI] [PubMed] [Google Scholar]
- Wang Y.-S., Yang J.-H., Luo S.-D., Zhang H.-B., Li L. New cytotoxic steroid from Stachyurus himalaicus var. himalaicus. Molecules. 2006;11:536–542. doi: 10.3390/12030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Barth R.F., Yang W., Lee R.J., Tjarks W., Backer M.V., Backer J.M. Boron containing macromolecules and nanovehicles as delivery agents for neutron capture therapy. Anticancer Agents Med. Chem. 2006;6:167–184. doi: 10.2174/187152006776119153. [DOI] [PubMed] [Google Scholar]
- Yan T.D., Stuart O.A., Yoo D., Sugarbaker P.H. Preoperative intraperitoneal chemotherapy for peritoneal surface malignancy. J. Transl. Med. 2006;4:1–7. doi: 10.1186/1479-5876-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yat-Ching T. The role of cholesterol in prostatic diseases. Urol. Sci. 2011;22:97–102. [Google Scholar]
- Yeagle P.L. Cholesterol and cell membrane. In: Yeagle P.L., editor. Biology of Cholesterol. CRC Press; Boca Raton, Florida: 1988. pp. 121–146. [Google Scholar]
- Zamenhof R.G., Coderre J.A., Rivard M.J., Patel H. Topics in neutron capture therapy. Proceedings of the eleventh world congress on neutron capture therapy. Appl. Radiat. Isot. 2004;61:731–1130. [Google Scholar]
- Zee-Cheng R.K., Cheng C.C. Delivery of anticancer drugs. Methods Find. Exp. Clin. Pharmacol. 1989;11:439–529. [PubMed] [Google Scholar]
- Zhang H.-J., Sun J.-B., Lin H.-W., Wang Z.-L., Tang H., Cheng P., Chen W.-S., Yi Y.-H. A new cytotoxic cholesterol sulfate from marine sponge Halichondria rugosa. Nat. Prod. Res. 2007;21:953–958. doi: 10.1080/14786410701371330. [DOI] [PubMed] [Google Scholar]



