Abstract
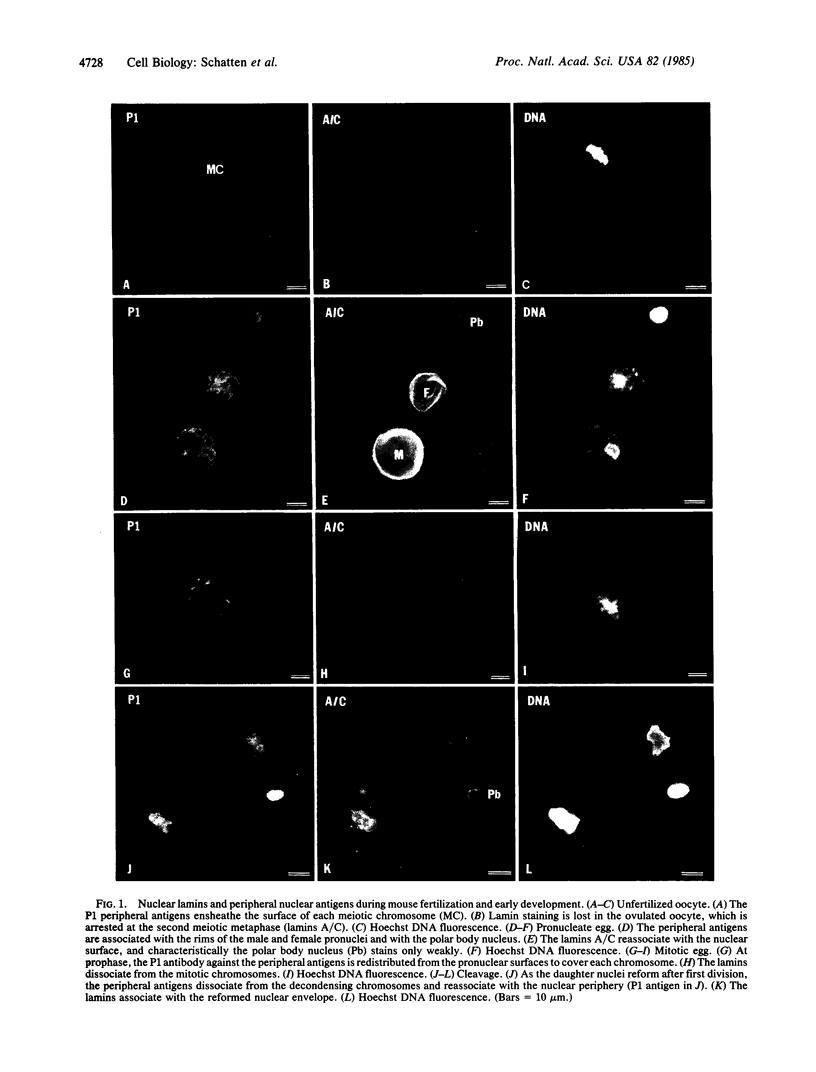
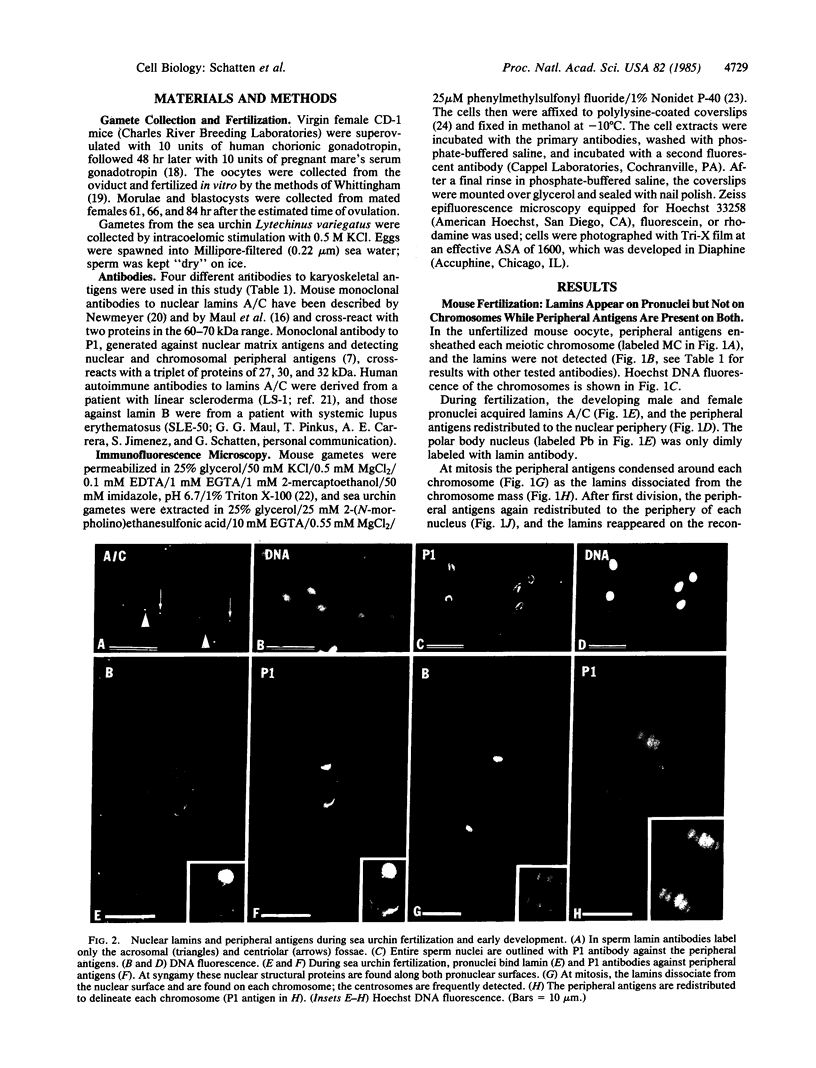
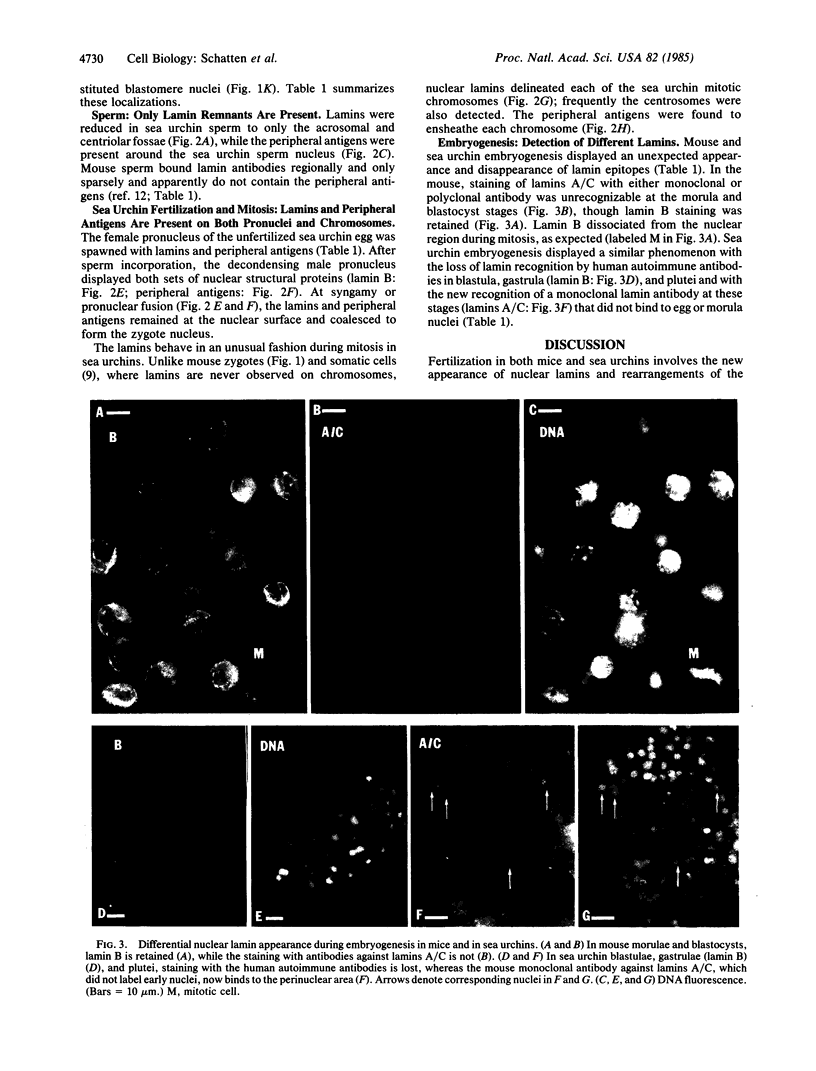
Nuclear structural changes during fertilization and embryogenesis in mice and in sea urchins have been followed by using antibodies against the nuclear lamins A/C and B and against antigens at the periphery of nuclei and chromosomes. Lamins are found on all pronuclei and nuclei during mouse fertilization, but with a diminished intensity on the second polar body nucleus. On sperm in both systems, lamins are reduced and detected only at the acrosomal and centriolar fossae. In sea urchin eggs, lamins are found on both pronuclei. Unlike in other dividing cells, the mitotic chromosomes of sea urchin eggs and embryos retain an association with lamins. The peripheral antibodies delineate each chromosome and nucleus except the mature mouse sperm nucleus. A dramatic change from the expected lamin distribution occurs during early development. In mouse morulae or blastocysts, lamins A/C are no longer recognized, although lamin B remains. In sea urchins both lamins A/C and lamin B, as detected with polyclonal antibodies, are lost after the blastula stage, although a different lamin A/C epitope emerges as recognized by a monoclonal antibody. These results demonstrate that pronucleus formation in both systems involves a new association or exposure of lamins, that the polar body nucleus is largely restricted from the cytoplasmic pool of lamins, and that mitotic chromosomes in the rapidly proliferating sea urchin egg retain associated lamins. They also suggest that changes in the expression or exposure of different lamins are a common feature of embryogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E., Hoppe P. C., Whitten W. K., Lee G. S. In vitro fertilization and early embryogenesis: a cytological analysis. J Ultrastruct Res. 1975 Feb;50(2):231–252. doi: 10.1016/s0022-5320(75)80054-2. [DOI] [PubMed] [Google Scholar]
- Chaly N., Bladon T., Setterfield G., Little J. E., Kaplan J. G., Brown D. L. Changes in distribution of nuclear matrix antigens during the mitotic cell cycle. J Cell Biol. 1984 Aug;99(2):661–671. doi: 10.1083/jcb.99.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. W. On the occurrence of a fibrous lamina on the inner aspect of the nuclear envelope in certain cells of vertebrates. Am J Anat. 1966 Jul;119(1):129–145. doi: 10.1002/aja.1001190108. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Scheer U., Krohne G., Jarasch E. D. The nuclear envelope and the architecture of the nuclear periphery. J Cell Biol. 1981 Dec;91(3 Pt 2):39s–50s. doi: 10.1083/jcb.91.3.39s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. P., Giloh H., Kuo C. H., Saumweber H., Sedat J. Nuclear structure: determination of the fate of the nuclear envelope in Drosophila during mitosis using monoclonal antibodies. J Cell Sci. 1983 Nov;64:331–349. doi: 10.1242/jcs.64.1.331. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blobel G. Nuclear lamina and the structural organization of the nuclear envelope. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):967–978. doi: 10.1101/sqb.1982.046.01.090. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blum A., Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol. 1978 Nov;79(2 Pt 1):546–566. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Gibson W., Shaper J. H. Characterization of the major polypeptides of the rat liver nuclear envelope. J Biol Chem. 1983 Feb 25;258(4):2710–2719. [PubMed] [Google Scholar]
- Krohne G., Debus E., Osborn M., Weber K., Franke W. W. A monoclonal antibody against nuclear lamina proteins reveals cell type-specificity in Xenopus laevis. Exp Cell Res. 1984 Jan;150(1):47–59. doi: 10.1016/0014-4827(84)90700-6. [DOI] [PubMed] [Google Scholar]
- Longo F. J., Anderson E. The fine structure of pronuclear development and fusion in the sea urchin, Arbacia punctulata. J Cell Biol. 1968 Nov;39(2):339–368. doi: 10.1083/jcb.39.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G., Avdalović N. Nuclear envelope proteins from Spisula solidissima germinal vesicles. Exp Cell Res. 1980 Nov;130(1):229–240. doi: 10.1016/0014-4827(80)90059-2. [DOI] [PubMed] [Google Scholar]
- Maul G. G., Baglia F. A., Newmeyer D. D., Ohlsson-Wilhelm B. M. The major 67 000 molecular weight protein of the clam oocyte nuclear envelope is lamin-like. J Cell Sci. 1984 Apr;67:69–85. doi: 10.1242/jcs.67.1.69. [DOI] [PubMed] [Google Scholar]
- Mazia D., Schatten G., Sale W. Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J Cell Biol. 1975 Jul;66(1):198–200. doi: 10.1083/jcb.66.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon F. D., Tuffanelli D. L., Fukuyama K., Kirschner M. W. Autoimmune response directed against conserved determinants of nuclear envelope proteins in a patient with linear scleroderma. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4374–4378. doi: 10.1073/pnas.80.14.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon F. D., Tuffanelli D. L., Kobayashi S., Kirschner M. W. The redistribution of a conserved nuclear envelope protein during the cell cycle suggests a pathway for chromosome condensation. Cell. 1984 Jan;36(1):83–92. doi: 10.1016/0092-8674(84)90076-x. [DOI] [PubMed] [Google Scholar]
- Paweletz N. Membranes in the mitotic apparatus. Cell Biol Int Rep. 1981 Apr;5(4):323–336. doi: 10.1016/0309-1651(81)90001-1. [DOI] [PubMed] [Google Scholar]
- Schatten G., Simerly C., Schatten H. Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubule-mediated motility during mammalian fertilization. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4152–4156. doi: 10.1073/pnas.82.12.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stick R., Krohne G. Immunological localization of the major architectural protein associated with the nuclear envelope of the Xenopus laevis oocyte. Exp Cell Res. 1982 Apr;138(2):319–313. doi: 10.1016/0014-4827(82)90181-1. [DOI] [PubMed] [Google Scholar]
- Stick R., Schwarz H. Disappearance and reformation of the nuclear lamina structure during specific stages of meiosis in oocytes. Cell. 1983 Jul;33(3):949–958. doi: 10.1016/0092-8674(83)90038-7. [DOI] [PubMed] [Google Scholar]
- Stick R., Schwarz H. The disappearance of the nuclear lamina during spermatogenesis: an electron microscopic and immunofluorescence study. Cell Differ. 1982 Jun;11(4):235–243. doi: 10.1016/0045-6039(82)90071-9. [DOI] [PubMed] [Google Scholar]
- Whittingham D. G. Fertilization of mouse eggs in vitro. Nature. 1968 Nov 9;220(5167):592–593. doi: 10.1038/220592a0. [DOI] [PubMed] [Google Scholar]
- Zirkin B. R., Soucek D. A., Chang T. S. Sperm nuclear packing and regulation during spermatogenesis and fertilization. Johns Hopkins Med J. 1982 Sep;151(3):101–112. [PubMed] [Google Scholar]