Summary
Bile acids are cholesterol-derived signaling molecules that regulate mammalian metabolism through sterol-sensing nuclear receptor transcription factors. In C. elegans, bile acid-like steroids called dafachronic acids (DAs) control developmental timing and longevity by activating the nuclear receptor DAF-12. However, little is known about the biosynthesis of these molecules. Here we show that the DAF-36/Rieske oxygenase works at the first committed step, converting cholesterol to 7-dehydrocholesterol. Its elucidation as a cholesterol 7-desaturase provides crucial biochemical evidence that such oxygenases are key steroidogenic enzymes. By controlling DA production, DAF-36 regulates DAF-12 activities for reproductive development and longevity, and may illuminate related pathways in metazoans.
Keywords: bile acid, nuclear receptor, endocrine signaling, hormone, development, aging
Introduction
Cholesterol homeostasis is critical to animal health and disease, and levels are tightly controlled by the balance of dietary intake, de novo synthesis, and catabolism. Cholesterol is metabolized to oxysterols, bile acids and steroids, which serve as signaling molecules for sterol sensing nuclear hormone receptors, transcription factors that respond to cognate ligands to regulate gene expression. These receptors, including the liver X receptor (LXR), farnesoid X receptor (FXR), and vitamin D receptor (VDR), regulate complex networks governing cholesterol, lipid, and glucose homeostasis, as well as aspects of development and reproduction (Wollam & Antebi 2011).
Sterol homeostasis is less well understood in model invertebrates such as C. elegans, yet its study through metabolic genetics can illuminate novel pathways of regulation and organismal physiology. C. elegans cannot synthesize cholesterol de novo, but requires dietary cholesterol for development, molting, and reproduction (Chitwood 1999). Biochemical studies reveal that cholesterol is first converted to 7-dehydrocholesterol and lathosterol, in steps that are intriguingly opposite of mammalian cholesterol biosynthesis, but the functions of these metabolites and the enzymes making them have remained elusive.
Important clues to their roles have come from the identification of bile acid-like steroids, called the dafachronic acids, which are thought to be derived from these precursors (Motola et al. 2006). At least two endogenous ligands, Δ4- and Δ7-dafachronic acid, act through the nuclear receptor DAF-12 (a homolog of vertebrate FXR, LXR and VDR) to regulate developmental timing, entry into a long-lived larval stage called the dauer diapause, as well as adult longevity in response to signals from the gonad (Antebi et al. 2000; Gerisch et al. 2007; Bethke et al. 2009). More generally, components of the endocrine network controlling dauer formation, such as insulin/IGF signaling, have emerged as important regulators of life span in mammals, though it is unknown whether bile acids also modulate mammalian longevity (Kenyon 2010). The DAF-12-related nuclear receptors and DA signaling pathways are conserved in evolutionarily distant parasitic nematodes, and importantly, regulate exit from the infective stage (Ogawa et al. 2009; Wang et al. 2009). Thus, dissecting the pathways leading to DA production may not only illuminate the biology of aging, but also help identify targets for anti-helminthic therapeutics.
Relatively little is known about the pathways that govern DA synthesis, regulation, and ligand specificity. A cytochrome P450, DAF-9, functions at the last step in the DA biosynthetic pathway, oxidizing the cholesterol side chain of 3-keto steroids (lathosterone and 4-cholesten-3-one) to give Δ7-DA and Δ4-DA, respectively (Motola et al. 2006), a chemistry analogous to mammalian CYP27A1 in mammalian bile acid synthesis (Russell 2003). Null mutations of daf-9 abolish DA production entirely, and animals constitutively enter the dauer diapause with complete penetrance (Gerisch et al. 2001; Jia et al. 2002). HSD-1, a 3-hydroxysteroid-dehydrogenase homolog with weaker phenotypes than daf-9, is proposed to make the 3-keto steroid 4-cholesten-3-one in the synthesis of Δ4-DA, but there is as of yet no biochemical evidence for this activity (Patel et al. 2008; Dumas et al. 2010). DAF-36 is a Rieske-like oxygenase implicated in DA biosynthetic pathways, the loss of which also results in less severe phenotypes than daf-9, suggesting it acts in early biosynthetic branches that converge on DAF-9/CYP450 (Rottiers et al. 2006). The identification of C. elegans DAF-36 and Drosophila neverland provided the first evidence that such enzymes function in animal steroidogenic pathways (Yoshiyama et al. 2006), but their general role in metazoa and their biochemical activity remain uncharacterized. Here we show that DAF-36 works in the very first steps of nematode cholesterol metabolism, converting cholesterol to 7-dehydrocholesterol. As such, daf-36 could be a node of regulation for the partitioning of cholesterol to steroidogenic and bile acid pathways. By controlling the activity of DAF-12/FXR, it thereby influences developmental timing and longevity, and may also prove to be a promising therapeutic target against pathogenic nematodes.
Results
daf-36 mutants exhibit phenotypes typical of mild DA deficiency, forming dauer larvae constitutively at 27°C (Daf-c), and exhibiting gonadal cell migration (Mig) defects at lower temperatures (Rottiers et al. 2006). To determine where in the hormone biosynthetic pathways DAF-36 might act, we performed sterol feeding experiments to rescue these phenotypes. We reasoned that mutants blocked in hormone production should be rescued by compounds lying downstream or parallel to the block, but not by those lying upstream.
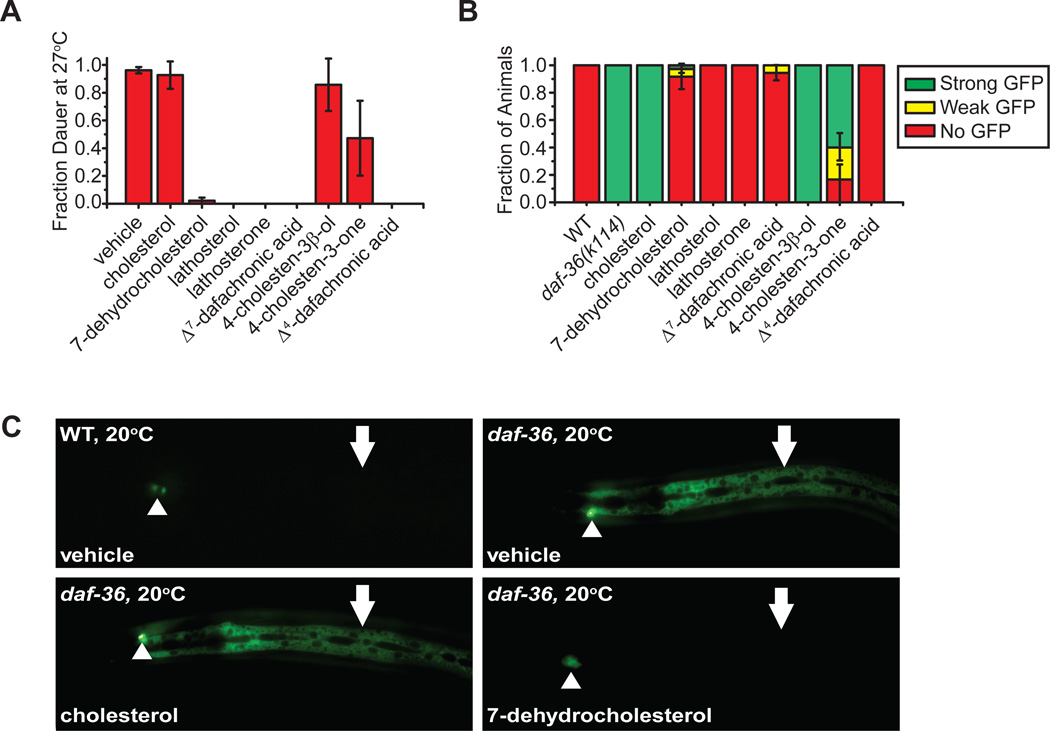
Previously we showed that daf-36 Daf-c phenotypes were rescued by various sterols (Rottiers et al. 2006), which we verified here. 7-Dehydrocholesterol, lathosterol, lathosterone, Δ7-DA, as well as 4-cholesten-3-one and Δ4-DA, rescued Daf-c phenotypes at 27°C, but not cholesterol (Figure 1A, Table S1). This suggests that cholesterol lies upstream of the daf-36 step, while other compounds lie downstream or in parallel. The conversion of cholesterol to 7-dehydrocholesterol is an important initial step in nematode cholesterol metabolism, suggesting that DAF-36 itself could act early at this step. By contrast, the daf-9(k182) hypomorphic allele was fully rescued by the DAs only, consistent with DAF-9/CYP450 working at the last step (Table S1). Additionally, 7-dehydrocholesterol, but not cholesterol, could also rescue daf-36 gonadal Mig phenotypes (Table S1). It is noteworthy that daf-36 animals grown on pure cholesterol (≥99%) had more severe Mig phenotypes (69%) than those grown on the less pure compound (≥92.5% cholesterol, 0% Mig), presumably due to contaminating sterols.
Figure 1. Rescue experiments suggest DAF-36 produces 7-dehydrocholesterol.
(A) daf-36(k114) Daf-c phenotypes at 27°C are rescued with 7-dehydrocholesterol, the dafachronic acids (DAs) and all proposed precursors of the DAs, but not cholesterol. Partial rescue is seen with 4-cholesten-3-one. Experiments were carried out with a compound concentration of 33 µM and ethanol as the vehicle (N≥3, mean ± SD). (B) daf-9::gfp hypodermal expression is upregulated in daf-36(k114) mutants, suggesting loss of negative feedback on daf-9 expression. 7-dehydrocholesterol and downstream DA precursors fully rescue this upregulation. The fraction of animals with strong (green), weak (yellow), or no (red) hypodermal GFP expression is shown (N≥3, mean ± SD). (C) N2 wild-type (WT) and daf-36(k114) animals expressing daf-9::gfp. Arrowheads indicate the XXX R/L neuroendocrine cells in which daf-9 expression is relatively unchanged, and arrows indicate hypodermal expression.
To independently measure daf-36 physiologic activity, we examined its influence on daf-9/CYP450 regulation. daf-9/CYP450 transcription is tightly regulated in the hypodermis, a syncitial epidermal tissue surrounding the worm (Gerisch & Antebi 2004; Mak & Ruvkun 2004). daf-9 null mutant animals lacking DA are Daf-c with complete penetrance, and hypodermal daf-9::gfp expression is undetectable. By contrast, mutations thought to partially block DA production, such as daf-9 hypomorphs or daf-36 null mutants, often provoke visible upregulation of hypodermal daf-9::gfp and dauer bypass. Such upregulation is hypothesized to reflect homeostatic feedback by hormone. To test directly whether daf-9::gfp levels are regulated by hormonal feedback, we fed the DAs and their proposed precursors to daf-36 mutants. Mutants cultured on vehicle resulted in upregulation of hypodermal daf-9::gfp compared to WT controls (Figure 1B–C). Supplementation with the DAs or 7-dehydrocholesterol, but not cholesterol, dramatically reversed daf-9::gfp upregulation. These results demonstrate that DA serves as a negative feedback signal on the daf-9 promoter, and support the hypothesis that daf-36 defects arise from failure to convert cholesterol to 7-dehydrocholesterol.
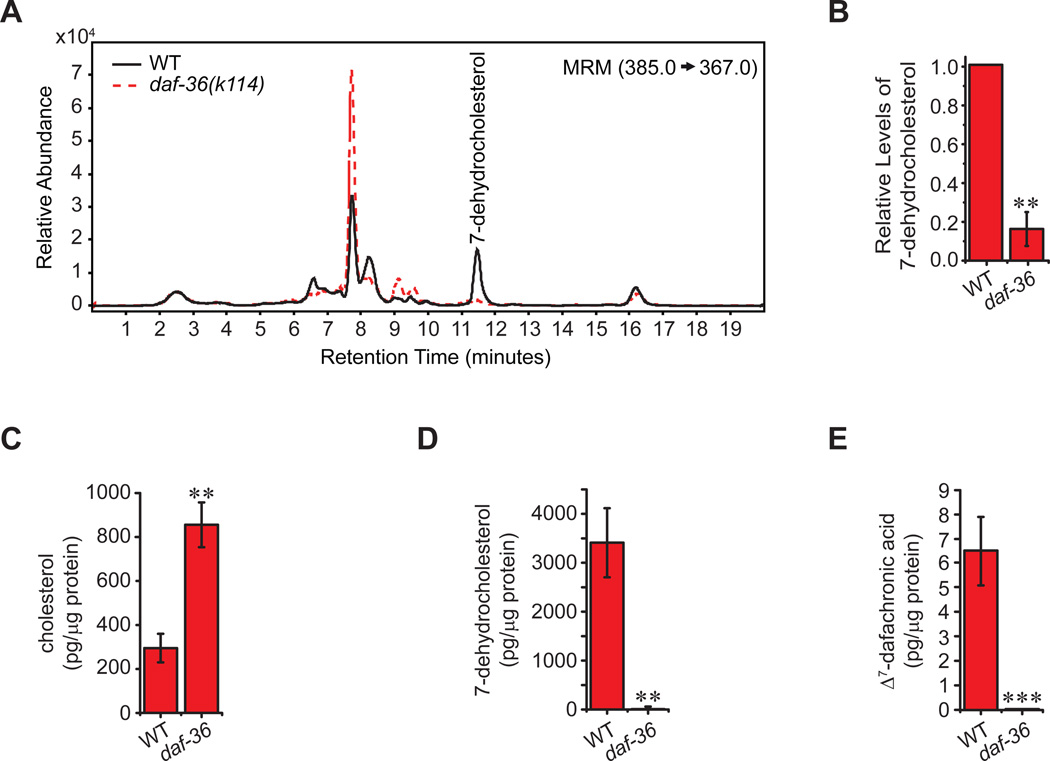
To further test this hypothesis, we examined sterol profiles from mutants. We grew animals in bulk liquid culture, extracted lipids, and analyzed sterols by LC/MS/MS. Strikingly, daf-36 mutants lacked 7-dehydrocholesterol, revealing a 6.5-fold decrease relative to WT (Figure 2A–B). Animals grown on solid media and analyzed by GC/MS/MS gave a similar profile. Mutant extracts were deficient in the putative product, 7-dehydrocholesterol, and accumulated the putative precursor, cholesterol, by 3-fold (Figure 2C–D). The DAF-12 ligand, Δ7-DA, was also undetectable in daf-36 mutants, while Δ4-DA was below the detection limit in both WT and mutants (Figure 2E, data not shown). Thus, DAF-36 supports production of Δ7-DA, and specifically acts in the metabolism of cholesterol to 7-dehydrocholesterol.
Figure 2. daf-36 mutant animals are deficient in 7-dehydrocholesterol.
(A) LC/MS/MS analysis of lipid extracts from L3 stage animals reveals that 7-dehydrocholesterol levels are significantly reduced in daf-36(k114) mutant animals relative to N2 wild-type (WT) animals, shown quantitatively in (B) (N=4, mean ± SEM, **P<0.005, determined by paired t-test). (C) Analysis of daf-36 lipid extracts by GC/MS/MS shows that cholesterol levels are significantly elevated, whereas animals are deficient in (D) 7-dehydrocholesterol and (E) Δ7-DA (N=5, mean ± SEM, **P<0.005, determined by unpaired t-test, ***below limit of detection).
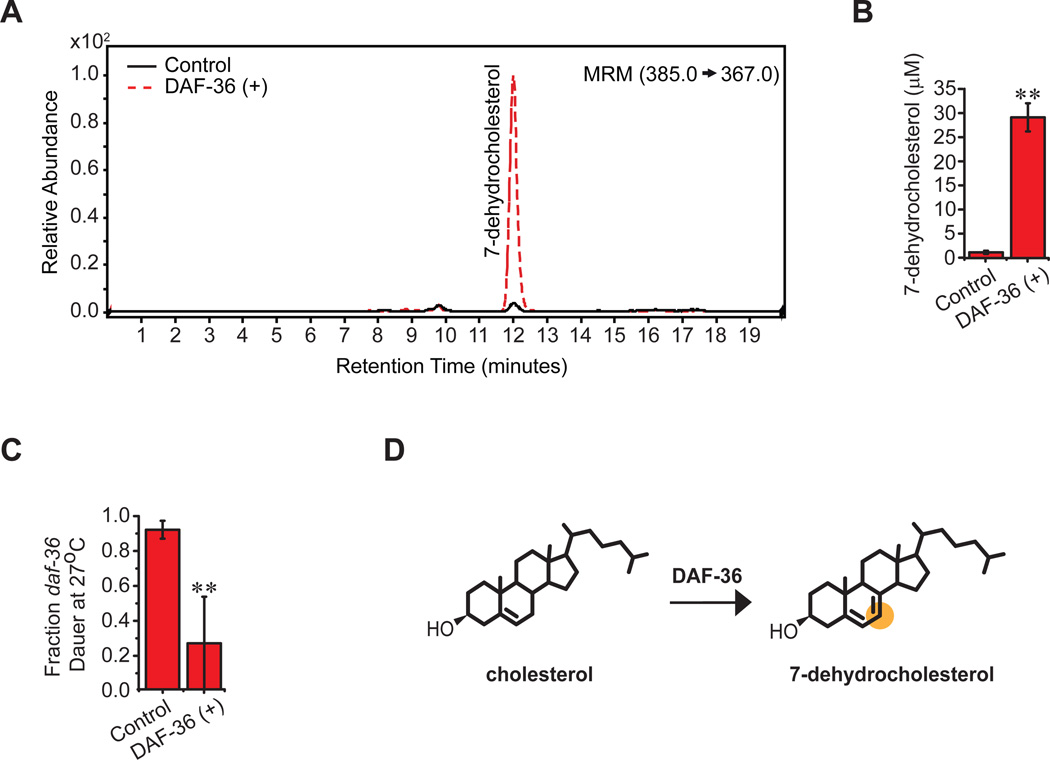
To analyze DAF-36 biochemical activities, we expressed daf-36 cDNA in cultured Sf9 cells and isolated microsomes. We found that the presence of DAF-36 resulted in a significant increase in 7-dehydrocholesterol relative to controls (Figure 3A–B), and extracts from these microsomes rescued the Daf-c phenotypes of daf-36 mutants (Figure 3C). We conclude that DAF-36 converts cholesterol to 7-dehydrocholesterol, and serves as a cholesterol 7-desaturase (Figure 3D).
Figure 3. DAF-36 produces 7-dehydrocholesterol.
(A) LC/MS/MS scans of lipid extracts from microsomes expressing either human CYP450 oxidoreductase (hOR) (Control) or DAF-36+hOR (DAF-36(+)), showing that production of 7-dehydrocholesterol is detected in the presence of DAF-36, whereas it is absent in control microsomes, shown quantitatively in (B) (N=3, mean ± SEM, **P<0.001, determined by unpaired t-test). (C) Supplementation of daf-36(k114) with lipid extracts from microsomes expressing human CYP450 oxidoreductase (hOR) (Control) or DAF-36+hOR (DAF-36 (+)) shows DAF-36 and 7-dehydrocholesterol dependent rescue of dauer formation at 27°C (N=2, mean ± range, **P<0.001, determined by Fisher’s exact test of grouped data). (D) DAF-36 acts as a cholesterol 7-desaturase, introducing a double bond at the 7 position, forming 7-dehydrocholesterol, shown highlighted in orange.
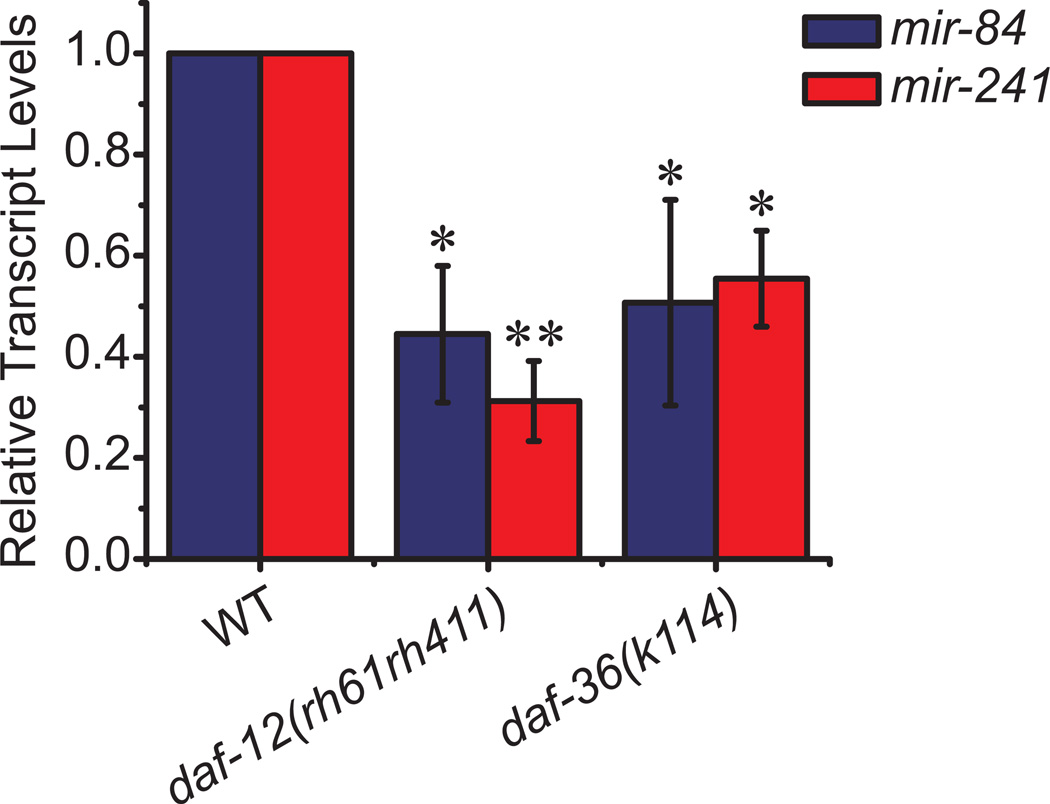
If daf-36 mutants diminish Δ7-DA production, expression of DAF-12 target genes should also be reduced. To test this, we examined two direct DAF-12 target genes, the microRNAs mir-84 and mir-241, by qPCR (Bethke et al. 2009). Both transcripts were significantly reduced by about 50% in daf-36 mutants, illustrating perturbation of the DAF-12 transcriptional response (Figure 4).
Figure 4. Loss of daf-36 impacts DAF-12 activity.
daf-36(k114) mutant animals have significantly lower transcript levels of two known targets of DAF-12, mir-84 and mir-241, relative to N2 wild-type (WT) animals, determined by quantitative RT-PCR of L3 stage worms. This reduction is similar to that seen in daf-12(rh61rh411) null mutants (N=3, mean ± SD, *P<0.05, **P<0.005, determined by unpaired t-test).
Discussion
In this work we demonstrate that the DAF-36/Rieske oxygenase functions as a cholesterol 7-desaturase involved in the production of the bile acid-like steroid, Δ7-DA. By controlling DA production, DAF-36 regulates DAF-12/FXR transcriptional activity and hence developmental timing and longevity. This study provides crucial biochemical evidence that such enzymes play an important role in the first steps of cholesterol metabolism towards the production of bioactive steroids.
Several lines of evidence show that DAF-36 is a cholesterol 7-desaturase responsible for the production of 7-dehydrocholesterol, thereby impacting DA synthesis and DAF-12 activity. First, daf-36 mutants exhibit the phenotypic profile associated with DA deficiency, including Daf-c phenotypes, gonadal Mig defects, and abrogated longevity in animals lacking germline stem cells (Rottiers et al. 2006). daf-36 mutants also exhibit reduced expression of the DAF-12 dependent target genes, the let-7 related microRNAs, mir-84 and mir-241, which function in developmental timing (Bethke et al. 2009). Second, sterol feeding experiments reveal that Δ7-DA and the early precursor, 7-dehydrocholesterol, rescue daf-36 mutant phenotypes, whereas cholesterol does not. Such sterol supplementation experiments implicate daf-36 as an important first step in nematode cholesterol catabolism, the conversion of cholesterol to 7-dehydrocholesterol.
Direct evidence for this activity stems from biochemical analysis. Lipid extracts from daf-36 mutants accumulate the proposed precursor, cholesterol, and are dramatically deficient in the predicted product, 7-dehydrocholesterol, as well as the end product of the pathway, Δ7-DA. The fact that daf-36 mutants have undetectable Δ7-DA, yet have weaker phenotypes than daf-9/CYP450 mutants, implies that residual amounts of a known DAF-12 ligand or that alternate ligand(s) are present. Finally, microsomes expressing the DAF-36 protein in a heterologous system produce 7-dehydrocholesterol, establishing DAF-36 as a cholesterol 7-desaturase. It has long been noted that several nematode sterols contain the Δ7 double bond (Chitwood 1999); DAF-36 activity is the likely source of such modifications. In the future it will be interesting to dissect the co-factors and molecular mechanism underlying this activity.
Rieske-like oxygenases are non-heme iron-dependent oxygenases that carry out diverse chemical reactions, including oxygenation and desaturation of ring structures (Bugg & Ramaswamy 2008). Studied most intensively in bacteria, including Mycobacterium tuberculosis, the Rieske oxygenase kshA has been shown to function as a 9α-hydroxylase on 3-ketosteroids and contributes to pathogenesis (Hu et al. 2010). Among eukaryotes, the Rieske-like oxygenases are conserved in plants, worms, insects, sea urchins, amphibians, fish, and birds, but have not been studied in vertebrates. Functional studies in worms and flies are amongst the first to ascribe a role of such enzymes to steroidogenesis. Like DAF-36, Drosophila neverland is implicated in the first steps of ecdysone synthesis, and is involved in the conversion cholesterol to 7-dehydrocholesterol, based on sterol supplementation experiments (Yoshiyama et al. 2006). In C. elegans, 7-dehydrocholesterol not only supports DA production, but can serve as a source of sterols for growth and molting (Chitwood 1999). By inference, DAF-36 may contribute to sterol pools for these other processes. Of particular interest are DAF-36 homologs in pathogenic nematodes, as they may present novel therapeutic targets to combat animal and plant parasitism (Wang et al. 2009).
Although mammals lack a clear DAF-36 homolog, it remains possible that the function has been assumed by another gene product. Rieske domains and non-heme iron binding motifs are found in different proteins but not together, raising the possibility that a DAF-36-like function could be separated. Extracts from the guinea pig gut have the ability to convert cholesterol to 7-dehydrocholesterol (Glover et al. 1952). The first step of mammalian bile acid synthesis entails hydroxylation of the 7-position of cholesterol (Russell 2003). Speculatively, modification of the 7-position, either by hydroxylation or desaturation, may be a conserved feature in the first step of partitioning cholesterol to bile acid production. As such, we hypothesize daf-36 may be a key point of control, and indeed, insulin signaling is known to regulate its expression (Rottiers et al. 2006). Ultimately it will be interesting to understand the biochemical activities and physiologic functions of potential vertebrate homologs, particularly with respect to steroidogenesis and bile acid metabolism, and how such bioactive steroids impact longevity.
Experimental Procedures
C. elegans Growth Conditions
Worms were grown on NGM agar seeded with the E. coli bacteria strain OP50 at 20°C unless noted otherwise. NGM contains 5 µg/mL cholesterol (≥92.5%, Sigma-Aldrich, St. Louis, MO), which is omitted in low cholesterol conditions. Rescue with pure steroids was performed as described (Rottiers et al. 2006).
Microsomal Incubations
Sf9 cells were cultured and harvested 60 hours post-infection and microsomes prepared as described (Motola et al. 2006). Microsomes expressing DAF-36 and human oxidoreductase (hOR) or hOR alone were thawed on ice, and brought to 0.5 mg/mL in either 0.1 M KPO4 buffer alone or including a NADPH regenerating system (50 U/ml isocitrate dehydrogenase, 0.1 M isocitrate and 0.1 M MgCl2). Substrates were added at 100 µM in 0.5 mL total volume, preincubated for 3 minutes at 37°C and reacted with 1 mM NADPH for 16 hours at 37°C. Reactions were processed by extracting twice with 2 mL methyl tert-butyl ether (MTBE), and drying under nitrogen. In some experiments, microsomes were processed without incubation with substrates. 0.5 µg of cholesterol-d7 was added as an internal standard for extractions. For rescues, extracts were resuspended in 50 µL methanol, mixed with 5X concentrated OP50, vacuum dried, resuspended in 100 µL 1X OP50, and plated on 3 cm plates containing 3 mL NGM agar.
Nematode Lipid Extracts
Worms were grown on 10 cm NGM agar plates seeded with OP50 bacteria. Gravid adults were bleached and the resulting embryos transferred to liquid culture (S-complete medium supplemented with 100X concentrated OP50). Two to three rounds of growth and lysis were performed. For the final round, worms were grown at 20°C until the L3-L4 stage, harvested, frozen in liquid nitrogen and stored at -80°C. Thawed worms were homogenized and total lipids (plus 1 µg cholesterol-d7 or chenodeoxycholic acid (CDCA)-d4/107 worms) were extracted with 2:1 chloroform:methanol. The resulting organic phase was dried under nitrogen. For GC/MS/MS analysis, growth of worms was carried out on plates only.
LC/MS/MS Analysis
Samples were analyzed by LC/MS/MS using 6410 Triple Quadrupole LC/MS instrument (Agilent Technologies, Santa Clara, CA) equipped with an ESI source and a Zorbax XDB-C18 column (4.6 × 50 mm, 3.5 µm). Briefly, lipid extracts were resuspended in methanol, spiked with cholesterol-d7 as an internal standard, and analyzed in Multiple Reaction Monitoring (MRM) mode. The following transitions were observed: cholesterol-d7 (m/z 411→376) and 7-dehydrocholesterol (m/z 385→369).
GC/MS/MS Analysis
Samples were analyzed by GC/MS/MS on a 7000A Triple Quadrupole GC/MS instrument (Agilent Technologies, Santa Clara, CA) equipped with an ESI source and an HP-5MS column. Briefly, lipid extracts were spiked with cholesterol-d7 or 5β-cholanic acid as internal standards and derivitized with (trimethylsilyl)diazomethane. Compounds were analyzed in MRM mode using the following transitions: cholesterol-d7 (m/z 465.4→360.3), cholesterol (m/z 458.4→353.3), 7-dehydrocholesterol (m/z 350.2→195.0), 5β-cholanic acid (m/z 374.3→264.0) and Δ7-dafachronic acid (m/z 428.3→229).
qRT-PCR
Real-time quantification for microRNAs by RT-PCR was performed with a protocol modified from a previous report (Chen et al. 2005). Briefly, total RNA was purified from L3 stage larvae using TRIzol (Invitrogen, Carlsbad, CA) and the miRNeasy kit (Qiagen, Hilden, Germany). TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA) was used to generate cDNA with microRNA-specific primers. qRT-PCR was performed with Power SYBR Green master mix (Applied Biosystems, Carlsbad, CA) according to the manufacturer’s instructions. Sno-RNA U18 was used as an internal control. The following primers were used: 5’-CAGTGCAGGGTCCGAGGT-3’ (U18-RT); 5’-GGCAGTGATGATCACAAATC-3’(U18-f); 5’-TGGCTCAGCCGGTTTTCTAT-3’ (U18-r); 5’-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCATTT-3’ (mir-241-RT); 5’-CGCTGAGGTAGGTGCGAG-3’ (mir-241-f); 5’-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTACAAT-3’(mir-84-RT); 5’-GCGCGCTGAGGTAGTATGTAAT-3’(mir-84-f); 5’-GTGCAGGGTCCGAGGT-3’ (microRNA reverse primer).
Statistical Analysis
Results are presented as mean ± SD or SEM, as indicated. P values were calculated where appropriate using GraphPad Prism (GraphPad Software Inc., La Jolla, California).
Supplementary Material
Acknowledgements
We thank Antebi lab members for manuscript comments. This work was supported by the NIA (RO1AG027498) and the Max Planck Society (A. Antebi); the Howard Hughes Medical Foundation, NIH (U19DK62434), and the Welch Foundation (D.J. Mangelsdorf); the Natural Sciences and Engineering Research Council of Canada (356873-08), the Canadian Institutes of Health Research (MOP-89361 and 97904), and the Canada Foundation for Innovation (C.L. Cummins).
Footnotes
Author Contributions: JW, LM, DBM, YS, VR, DLM and AA conducted experiments, AA, CLC, and DJM supervised projects, and JW and AA wrote the paper.
Contributor Information
Joshua Wollam, Email: JWollam@age.mpg.de.
Lilia Magomedova, Email: Lilia.Magomedova@utoronto.ca.
Daniel B Magner, Email: DMagner@age.mpg.de.
Yidong Shen, Email: YShen@age.mpg.de.
David J Mangelsdorf, Email: Davo.Mango@UTSouthwestern.edu.
Carolyn L Cummins, Email: Carolyn.Cummins@utoronto.ca.
References
- Antebi A, Yeh WH, Tait D, Hedgecock EM, Riddle DL. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev. 2000;14:1512–1527. [PMC free article] [PubMed] [Google Scholar]
- Bethke A, Fielenbach N, Wang Z, Mangelsdorf DJ, Antebi A. Nuclear Hormone Receptor Regulation of MicroRNAs Controls Developmental Progression. Science. 2009;324:95–98. doi: 10.1126/science.1164899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg TD, Ramaswamy S. Non-heme iron-dependent dioxygenases: unravelling catalytic mechanisms for complex enzymatic oxidations. Curr Opin Chem Biol. 2008;12:134–140. doi: 10.1016/j.cbpa.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ, Livak KJ, Guegler KJ. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood DJ. Biochemistry and Function of Nematode Steroids. Crit Rev Biochem Mol Biol. 1999;34:273–284. doi: 10.1080/10409239991209309. [DOI] [PubMed] [Google Scholar]
- Dumas KJ, Guo C, Wang X, Burkhart KB, Adams EJ, Alam H, Hu PJ. Functional divergence of dafachronic acid pathways in the control of C. elegans development and lifespan. Dev Biol. 2010;340:605–612. doi: 10.1016/j.ydbio.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch B, Antebi A. Hormonal signals produced by DAF-9/cytochrome P450 regulate C. elegans dauer diapause in response to environmental cues. Development. 2004;131:1765–1776. doi: 10.1242/dev.01068. [DOI] [PubMed] [Google Scholar]
- Gerisch B, Rottiers V, Li D, Motola DL, Cummins CL, Lehrach H, Mangelsdorf DJ, Antebi A. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc Natl Acad Sci U S A. 2007;104:5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A. A Hormonal Signaling Pathway Influencing C. elegans Metabolism, Reproductive Development, and Life Span. Dev Cell. 2001;1:841–851. doi: 10.1016/s1534-5807(01)00085-5. [DOI] [PubMed] [Google Scholar]
- Glover M, Glover J, Morton RA. Provitamin D3 in Tissues and the Conversion of Cholesterol to 7-Dehydrocholesterol in vivo. Biochem J. 1952;51:1–9. doi: 10.1042/bj0510001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, van der Geize R, Besra GS, Gurcha SS, Liu A, Rohde M, Singh M, Coates A. 3-Ketosteroid 9α-hydroxylase is an essential factor in the pathogenesis of Mycobacterium tuberculosis. Mol Microbiol. 2010;75:107–121. doi: 10.1111/j.1365-2958.2009.06957.x. [DOI] [PubMed] [Google Scholar]
- Jia K, Albert PS, Riddle DL. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development. 2002;129:221–231. doi: 10.1242/dev.129.1.221. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Mak HY, Ruvkun G. Intercellular signaling of reproductive development by the C. elegans DAF-9 cytochrome P450. Development. 2004;131:1777–1786. doi: 10.1242/dev.01069. [DOI] [PubMed] [Google Scholar]
- Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, Mangelsdorf DJ. Identification of Ligands for DAF-12 that Govern Dauer Formation and Reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Streit A, Antebi A, Sommer RJ. A Conserved Endocrine Mechanism Controls the Formation of Dauer and Infective Larvae in Nematodes. Curr Biol. 2009;19:67–71. doi: 10.1016/j.cub.2008.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DS, Fang LL, Svy DK, Ruvkun G, Li W. Genetic identification of HSD-1, a conserved steroidogenic enzyme that directs larval development in Caenorhabditis elegans. Development. 2008;135:2239–2249. doi: 10.1242/dev.016972. [DOI] [PubMed] [Google Scholar]
- Rottiers V, Motola DL, Gerisch B, Cummins CL, Nishiwaki K, Mangelsdorf DJ, Antebi A. Hormonal Control of C. elegans Dauer Formation and Life Span by a Rieske-like Oxygenase. Dev Cell. 2006;10:473–482. doi: 10.1016/j.devcel.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhou XE, Motola DL, Gao X, Suino-Powell K, Conneely A, Ogata C, Sharma KK, Auchus RJ, Lok JB, Hawdon JM, Kliewer SA, Xu HE, Mangelsdorf DJ. Inaugural Article: Identification of the nuclear receptor DAF-12 as a therapeutic target in parasitic nematodes. Proc Natl Acad Sci U S A. 2009;106:9138–9143. doi: 10.1073/pnas.0904064106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollam J, Antebi A. Sterol Regulation of Metabolism, Homeostasis, and Development. Annu Rev Biochem. 2011;80:885–916. doi: 10.1146/annurev-biochem-081308-165917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama T, Namiki T, Mita K, Kataoka H, Niwa R. Neverland is an evolutionally conserved Rieske-domain protein that is essential for ecdysone synthesis and insect growth. Development. 2006;133:2565–2574. doi: 10.1242/dev.02428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.