Abstract
Preeclampsia is a pregnancy-specific condition characterized by an imbalance of circulating angiogenic factors and new-onset hypertension. Although current treatment options are limited, recent studies suggest pravastatin may improve angiogenic profile and reduce blood pressure in preeclampsia. We hypothesized pravastatin would restore angiogenic balance and reduce mean arterial pressure (MAP) in rats with reduced utero-placental perfusion pressure (RUPP)-induced hypertension. Pravastatin was administered i.p. (1 mg/kg/day) in RUPP (RUPP+P) and normal pregnant rats (NP+P) from day 14-19 of pregnancy. On day 19, MAP was measured via catheter, conceptus data was recorded and tissues collected. MAP was increased (p<0.05) in RUPP compared to NP dams and pravastatin ameliorated this difference. Pravastatin attenuated decreased fetal weight and plasma VEGF and the RUPP-induced increased sFlt-1 when compared to NP dams. Pravastatin treatment did not improve angiogenic potential in RUPP serum and decreased (P<0.05) endothelial tube formation in NP rats. RUPP rats presented with indices of oxidative stress such as increased placental catalase activity and plasma TBARS along with decreased plasma total antioxidant capacity compared to NP controls and pravastatin attenuated these effects. MAP, fetal weight, plasma VEGF, and plasma sFlt-1 were unchanged in NP+P compared to NP controls. The present data indicate that treatment with pravastatin attenuates oxidative stress and lowers MAP in placental ischemia-induced hypertension, but may have negative effects on circulating angiogenic potential during pregnancy. Further studies are needed to determine if there are long-term deleterious effects on maternal or fetal health following pravastatin treatment during pregnancy-induced hypertension or preeclampsia.
Keywords: Preeclampsia, pravastatin, angiogenic factors, oxidative stress
INTRODUCTION
Preeclampsia is a pregnancy-specific condition characterized by new-onset hypertension, proteinuria, and intrauterine growth restriction and is a leading cause of maternal and fetal morbidity and mortality worldwide (1;2). Treatment options are currently lacking and delivery of the placenta is the only effective treatment of preeclampsia, making it the leading cause of preterm delivery. Despite the significance of preeclampsia as a women’s health concern, the mechanisms underlying the pathogenesis of this condition are not well understood. Therefore, treatment of this condition remains a challenge.
In preeclampsia, impaired remodeling of the maternal uterine vasculature, leading to placental ischemia and inappropriate hypoxia, is believed to result in an angiogenic imbalance with increased soluble fms-like tyrosine kinase-1 (sFlt-1) and decreased vascular endothelial growth factor (VEGF) (3-5). This angiogenic imbalance, in concert with dysregulation of a variety of other inflammatory mediators including the complement system, is thought to contribute to widespread maternal inflammation and oxidative stress ultimately resulting in endothelial dysfunction and high blood pressure (6;7). These characteristics of preeclampsia are also exhibited in animal models of preeclampsia (8-12). An area of intense investigation is identification of potential therapeutic strategies that may be directed toward the amelioration of maternal inflammation, oxidative stress, and endothelial dysfunction, which in turn will ameliorate hypertension and permit gestation to continue to term. To this end, recent interest has turned to the potential role of 3-hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, or “statins”, to promote vascular endothelial function and mitigate manifestations of preeclampsia. Recent studies have revealed this class of drugs may have a number of pleiotropic effects that are independent of the modification of cholesterol metabolism, including antioxidant, anti-inflammatory, and anti-apoptotic effects both in vivo and in vitro (13;14).
Although several recent studies have revealed the potential for HMG-CoA reductase inhibitors (i.e. statins) to attenuate characteristics of preeclampsia present in several animal and cell culture models used to study pregnancy-induced hypertension (11;15-17), none of these experiments has employed a model of preeclampsia in which angiogenic imbalance and hypertension arise spontaneously following placental ischemia such as the RUPP rat or baboon with uterine ischemia via partial uterine artery ligation (4;5). These studies have generally reported positive outcomes such as lowering blood pressure, increases of pro-angiogenic factors and the promotion of vasculature function (11;15-17). Furthermore, these studies have not reported any deleterious effects in the fetus. However, statin use remains contraindicated during pregnancy due to unknown, but potentially teratogenic, effects on the fetus. Considering the substantial controversy regarding the putative role of statin use in pregnancy, there is need for further study on this matter. Therefore, the purpose of this study was to test the hypothesis that pravastatin administration would restore angiogenic balance, ameliorate oxidative stress, and attenuate high blood pressure in rats with reduced utero-placental perfusion pressure (RUPP)-induced hypertension without any deleterious effects on fetal outcome.
METHODS AND APPARATUS
Animals
Studies were performed in timed-pregnant Sprague-Dawley rats purchased from Charles River (Portage, MI). Animals were housed in a temperature-controlled room (23°C) with a 12:12 light:dark cycle. The experimental procedures in this study were performed strictly in accordance with National Institutes of Health guidelines for use and care of animals and the protocols used in this study were approved by the Institutional Animal Care and Use Committee at the Universities of Minnesota and Oregon. On day 14 of gestation, rat dams were randomly assigned to either RUPP (n=14), RUPP+pravastatin (RUPP+P; n=7), normal pregnant (NP; n =12), or NP+pravastatin (NP+P; n=7) control groups.
Reduced Utero-placental Perfusion Pressure (RUPP) Procedure and Determination of Mean Arterial Pressure (MAP)
The RUPP procedure is a well-established model for studying the link between placental ischemia and hypertension in the pregnant rat and has been described in detail previously (5;18). NP rats underwent a sham surgery, which included the midline incision and suture, on the same day of pregnancy. On days 14-19 of pregnancy, pravastatin was administered at a dose, 1mg/kg/day (IP q.d.), that has previously been shown not to have adverse effects on lipid metabolism in Spraque Dawley rats (19). Animals were instrumented on day 18 of gestation and arterial pressure was determined via an indwelling carotid arterial catheter on day 19 of gestation as described previously (5;18).
Conceptus Measurements
After measurement of MAP, dams were anesthetized with isoflurane and a midline ventral incision was made to isolate the abdominal aorta for plasma and serum collection as reported previously (5;18). Pups and placentas that appeared viable were excised and weighed and placental efficiency (fetal weight/placental weight) was calculated in each dam. Fetal weight was determined as an average weight (g) per litter. Maternal heart and kidney weight was also recorded for each animal. Other tissues were excised, rinsed in saline and snap frozen for later analysis.
Plasma and Tissue Assays
Plasma was collected in BD Vacutainer EDTA-containing tubes. Free plasma VEGF, sFlt-1 and TNF-α concentrations were determined using commercially available enzyme-linked immunosorbant assay (ELISA) kits (Quantikine®, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions as reported previously (5;8). Trolox-equivalent antioxidant capacity of the plasma was determined with a total antioxidant assay (Cayman Chemical, Ann Arbor, MI) and a thiobarbituric acid reactive substances (TBARS) assay (Cayman Chemical, Ann Arbor, MI) was performed to assess oxidative stress with an end measurement of plasma malondialdehyde (MDA) according to the manufacturer’s instructions as described previously (20;21). Bilirubin assay (BioAssays; Haward, CA) was performed according to manufacturer’s instructions. Total soluble protein extracts from placentas were evaluated for catalase activity as per the manufacturer’s (Cayman Chemical, Ann Arbor, MI) specifications.
Western Blot
Western blots were performed as previously described (5;22). Briefly, 50 μg of protein was separated by electrophoresis on 4-20% sodium dodecyl sulfate (SDS) polyacrylamide gels, transferred to nitrocellulose membranes and Ponceau stained to confirm even transfer across each gel. After one hour in casein blocking solution, membranes were incubated in blocking solution containing a commercially available antibody (from Abcam, Cambridge, MA unless noted otherwise) for HSP 27 (ab12351, 1:5000), HSP70 (ab6535, 1:5000), HO-1 (Assay Designs, Ann Arbor, MI, OSA-110, 1:250) overnight at 4°C. β-actin (ab20272, 1:5000; MA5-15739, 1:10000, Thermo Pierce) was incubated 1 hr at room temperature. Membranes were washed and incubated 1hr with the appropriate horseradish peroxidase conjugated secondary antibodies (Cell Signaling, 1:10000-1:20000) or fluorophore conjugated antibodies (Licor 926-68171,1:15000) and incubated in chemiluminescent substrate (West-Femto, Pierce, Rockford, IL) when required. The immunoreactive bands were digitized using an Alpha-Innotech digital imaging system or a LiCor Odyssey infrared imaging system. All digitized images were quantified using Un-Scan-It gel 6.1 software (Silk Scientific, Orem, UT).
Endothelial tube formation assay
Angiogenic potential was assessed in the serum of pregnant rats in vitro using the method of Banek et al (23). Two separate experiments (Group 1 and Group 2) were performed with this assay and both were performed in duplicate. Group 1: Tube formation was measured in cells treated with serum from each rat in the four treatment groups: NP, RUPP, NP+P, and RUPP+P. Group 2: Tube formation was measured in cells treated with serum from NP or RUPP rats with and without the addition of 20 μM pravastatin added directly to the serum during the angiogenesis experiment.
In vitro Experiments
BeWo and JEG cells (ATCC, Manassass, VA) were chosen to study the effects of pravastatin on fusigenic (i.e. syncytium forming) extravillous type trophoblast cells (BeWo) and non-fusigenic (JEG) early pregnancy trophoblast cells (24). Cells plated at 1 ×106 cells/mL in 6 well plates and treated with Ham’s F12 medium with L-glutamine (Cellgro Mediatech -10-080-CV) with 10% fetal bovine serum, 1% penicillin/streptomycin. After 48 hours cells were placed into an incubator chamber (Biospherix; Lacona, NY) fitted with oxygen and carbon dioxide sensors. Chambers were maintained at either: atmospheric control (20% O2, 5% CO2, 75% N2), physiologic normoxic (8% O2, 5% CO2, 87% N2), or hypoxic (1.5% O2, 5% CO2, 93.5 % N2) and cells were treated with 0μM, 10μM, or 20μM of pravastatin then harvested at 12 hours. At the end of the treatment period, cells were placed on ice and rinsed three times with cold phosphate buffered saline (PBS). Laemmli sample buffer without bromophenol blue and β-mercaptoethanol was added and cell extracts collected following scraping. Conditioned media was centrifuged at 1000 × g for 5 minutes and the supernatant was snap frozen and stored at -80° C for analysis.
Statistical Analysis and Calculations
All data are presented as mean ± SEM and statistical significance was accepted when p < 0.05. TNF-α data were square root transformed prior to statistical analysis; raw data is presented. Comparisons between groups were made with one-way or two-way ANOVA as indicated. Statistical calculations were made with GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA USA).
RESULTS
Mean Arterial Pressure
Figure 1 shows that RUPP resulted in increased arterial pressure compared to NP controls. RUPP rats treated with pravastatin had decreased blood pressure compared to untreated RUPP rats, but had blood pressure that was higher than the NP controls (p<0.05). While the blood pressure of the NP rats treated with pravastatin was six mmHg higher than the NP rats, it was not significantly different than any of the treatment groups.
Figure 1. Mean arterial pressure (MAP).
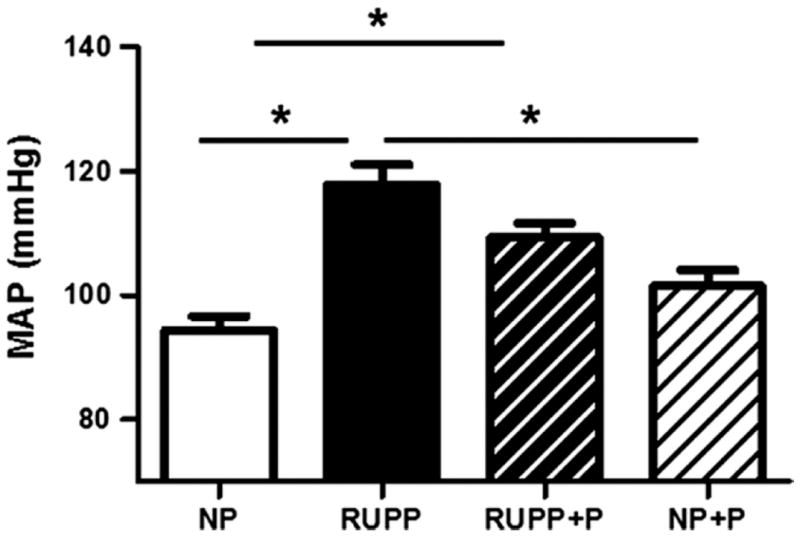
Pravastatin decreased blood pressure compared to untreated reduced utero-placental perfusion pressure (RUPP) rats, but was higher than normal pregnant (NP) controls. Data are expressed as mean ± SEM, * indicates p<0.05.
Conceptus and Maternal Morphometrics
Fetal weight was decreased in RUPP compared to NP dams (Figure 2A). Fetuses from RUPP rats treated with pravastatin were not smaller than the NP rats or the NP rats treated with pravastatin. While there was a treatment effect by one-way ANOVA for placental weight (data not shown), there were no significant differences among specific treatment groups when data were further analyzed with a Newman-Keuhls post-hoc test. Fetal resorptions were increased due to the RUPP procedure and this was not altered by pravastatin (Figure 2B). Placental efficiency was decreased by RUPP and this was attenuated by treatment with pravastatin (Figure 2C). Heart weight (Figure 2D) was increased (p<0.05) in the RUPP rats compared to the NP rats and this was attenuated in the RUPP+P group. Pravastatin had no adverse effect on heart weight in the NP rats. Kidney weight was not different between any of the groups (data not shown).
Figure 2. Fetal weight, fetal resorptions, placental efficiency and heart weight.
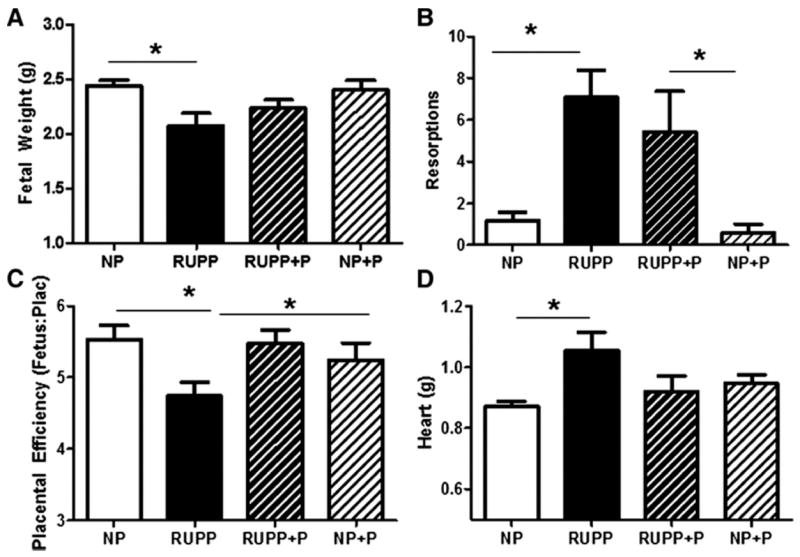
Fetal weight (Panel A) was decreased in reduced utero-placental perfusion pressure (RUPP) compared to normal pregnant (NP). Pravastatin administration resulted in fetuses that were not smaller than fetuses from NP dams or from NP dams treated with pravastatin. There were an increased number of fetal resorptions in the RUPP compared to the NP rats and pravastatin had no effect in either group (Panel B). Placental efficiency was decreased by RUPP and this was ameliorated by pravastatin (Panel C). Heart weight was increased by RUPP and this was ameliorated by pravastatin (Panel D). Data are expressed as mean ± SEM, * indicates p<0.05.
Circulating Factors
Figure 3A illustrates that plasma sFlt-1 was increased (p<0.05) in the RUPP compared to NP rats and this was attenuated by treatment with pravastatin. Circulating levels of free VEGF were decreased (p<0.05) in the RUPP compared to the NP rats and this was also attenuated by treatment with pravastatin (Figure 3B). Figure 3C shows that the sFlt-1:VEGF ratio, which was increased in RUPP compared to NP rats, was ameliorated by pravastatin treatment. There was no difference between NP and NP+P controls with respect to either sFlt-1 or VEGF. Figure 3D illustrates that TNF-α was not significantly increased in the RUPP compared to NP rats, but was increased in the RUPP+P rats compared to the NP controls. Serum total bilirubin was not different between the NP, RUPP, RUPP+P and NP+P (1.40 ± 0.27 vs. 1.91 ± 0.25 vs. 1.10 ± 0.21 vs.1.52 ± 0.21 mg/dL).
Figure 3. Circulating Cytokines and Anti-angiogenic Factors.
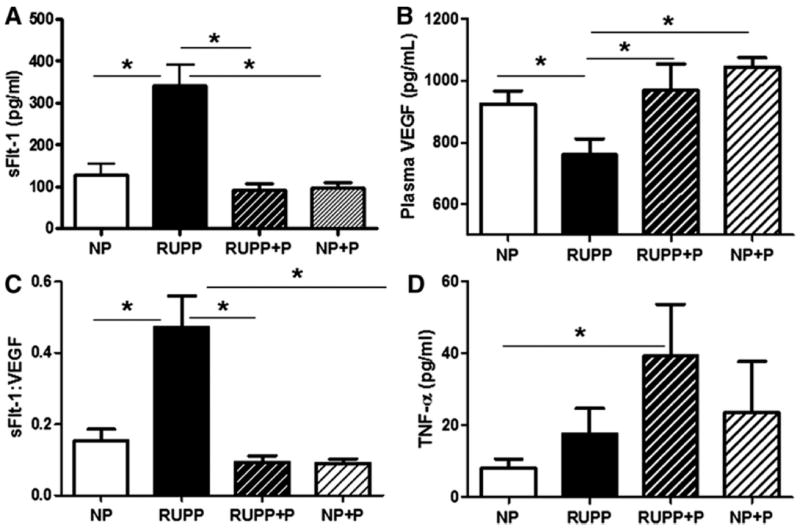
Circulating sFlt-1 (Panel A) was increased and VEGF (Panel B) was decreased in reduced utero-placental perfusion pressure (RUPP) compared to normal pregnant (NP) controls. Pravastatin administration restored the sFlt-1:VEGF ratio to NP levels (Panel C). Panel D shows that TNF-α was increased in the RUPP+P group compared to NP rats. Data are expressed as mean ± SEM, * indicates p<0.05. TNF-α was square root transformed prior to analysis.
Total Antioxidant Capacity and Catalase Activity
Figure 4 illustrates that TBARS (panel A) were increased (p < 0.05) in the plasma of RUPP compared to NP rats and this increase was attenuated (p < 0.05) by treatment with pravastatin. Total antioxidant capacity, shown in Figure 4B, was decreased (p < 0.05) in plasma of RUPP compared to NP controls and pravastatin attenuated (p < 0.05) this difference in the treated RUPP rats. There was no difference between NP and NP+P controls. Figure 5 shows that placental catalase activity was increased (p < 0.05) in RUPP compared to NP and this was attenuated by treatment with pravastatin. Pravastatin had no effect on catalase activity in the NP placentas.
Figure 4. Plasma TBARS and trolox antioxidant capacity.
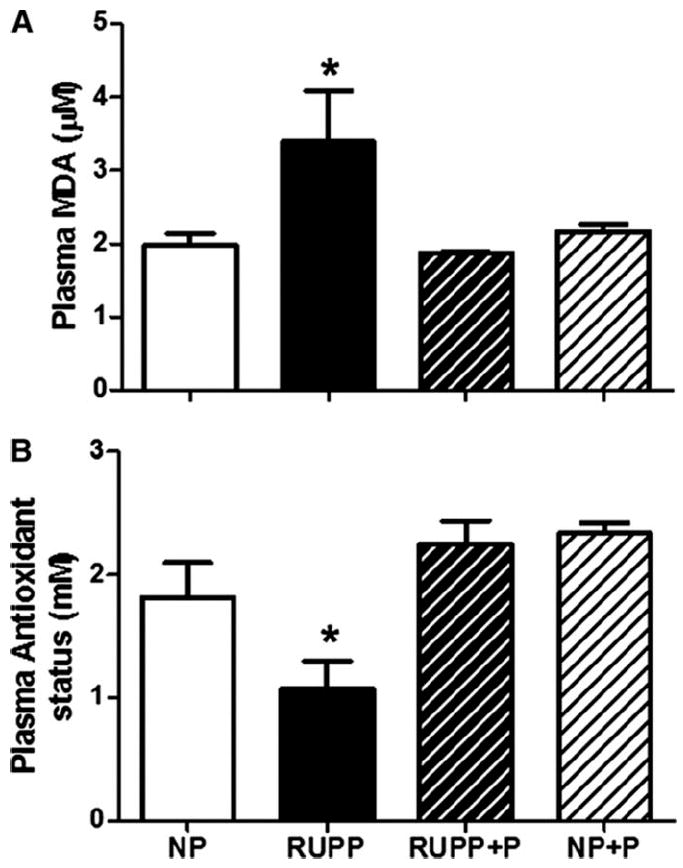
Plasma levels of TBARS (Panel A) were increased and antioxidant capacity (Panel B) was decreased in reduced utero-placental perfusion pressure (RUPP) compared to normal pregnant (NP) rats. Pravastatin restored both TBARS and antioxidant capacity to NP levels. Data are expressed as mean ± SEM, * indicates p<0.05.
Figure 5. Placental catalase activity.
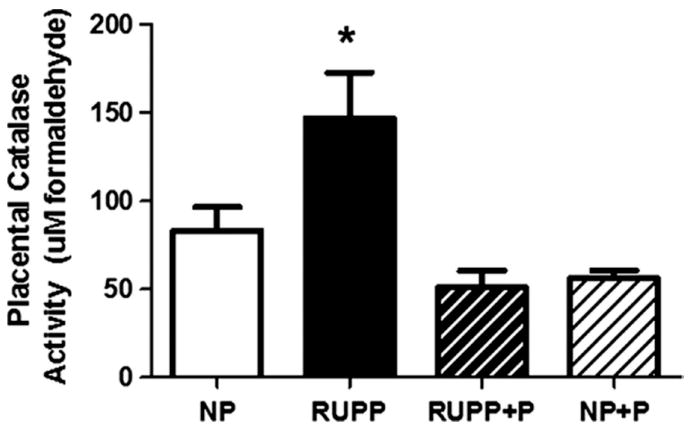
Catalase activity was increased in reduced utero-placental perfusion pressure (RUPP) compared to normal pregnant (NP) rats. Treatment with pravastatin lowered catalase activity in the RUPP dams. Data are expressed as mean ± SEM, * indicates p<0.05.
Western Blots for HO-1 and Heat shock proteins 27 and 70
No differences were found between any of the treatment groups in regards to placental expression of heme oxygenase-1 (HO-1 or heat shock protein 32), heat shock protein 27, or heat shock protein 70 (data not shown). In addition, hepatic expression of HO-1 relative to β-actin was not altered due to pravastatin treatment between the NP, RUPP, RUPP+P and NP+P (2.45 ± 1.01 vs. 3.12 ± 1.27 vs. 2.83 ± 1.06 vs. 3.23 ± 0.90) groups.
Angiogenic Potential
Endothelial cell tubule formation was assessed as a measure of the angiogenic potential of serum from animals in each treatment group. Figure 6A shows that angiogenic potential as determined by the tube formation assay was decreased (p<0.05) in the RUPP compared to the NP rats. Despite the observed increase in VEGF and decrease in sFlt-1 in pravastatin treated animals (Figure 3A-C), there was no improvement in endothelial tube formation in the treated RUPP+P rats. Moreover, we observed a decrease (p<0.05) in endothelial tube formation in the NP rats that were treated with pravastatin.
Figure 6. Effects of pravastatin on endothelial cell (EC) tube formation.
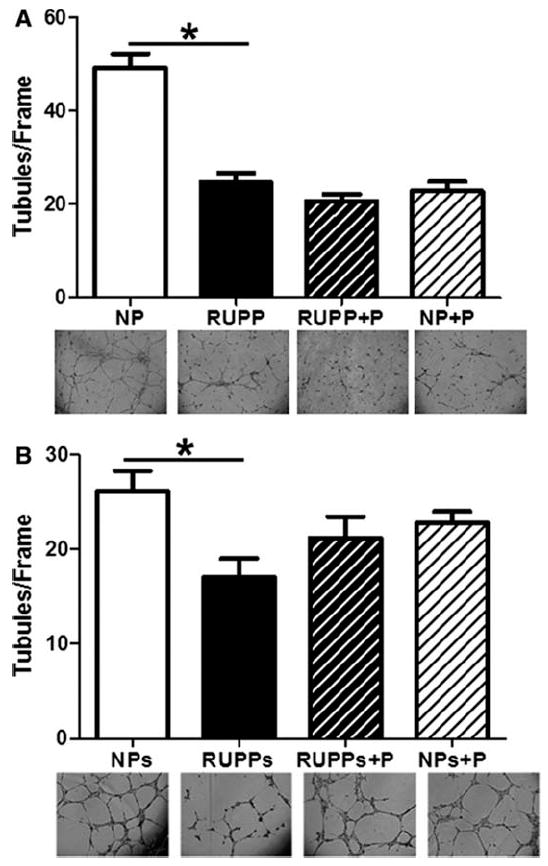
Panel A shows that EC tube formation was decreased in reduced utero-placental perfusion pressure (RUPP) rats compared to normal pregnant (NP) controls. Pravastatin treatment had no effect in RUPP rats and decreased EC tube formation in NP rats compared to NP controls. Panel B shows that when 20 μM pravastatin was added directly to NP and RUPP serum post-mortem to determine if there were direct effects on EC tube formation EC tube formation was decreased in reduced utero-placental perfusion pressure (RUPPs) compared to normal pregnant (NPs) controls. Pravastatin did not directly affect EC tube formation (RUPPs+P, NPs+P). Data are expressed as mean ± SEM, * indicates p<0.05.
In order to determine if pravastatin directly affected endothelial cell tube formation, we added 20 μM pravastatin to serum collected from NP and RUPP rats. Figure 6B shows that the RUPP serum decreased (P<0.05) tube formation, but addition of pravastatin to either RUPP or NP serum did not result in a statistically significant change in tubule formation.
Cell Culture Studies
To determine if pravastatin had differential effects on trophoblast cells compared to endothelial cells, we treated BeWo and JEG cells with varying oxygen concentrations and pravastatin doses. BeWo cells secreted increased (P<0.05) concentrations of VEGF in hypoxic (1.5% O2) conditions with and without pravastatin, thus the main statistical effect was due to O2 concentration (Figure 7A). In contrast, figure 7B shows JEG cells decreased (P<0.05) VEGF secretion with pravastatin treatment and showed no effect of O2 concentrations.
Figure 7. Effects of oxygen concentration and pravastatin on vascular endothelial growth factor (VEGF) secretion by BeWo and JEG trophoblast cells.

BeWo cells secreted increased (panel A) concentrations of VEGF in hypoxic (1.5% O2) conditions with and without pravastatin (Prav), thus the main statistical effect was due to O2 concentration. In contrast, JEG cells decreased (panel B) VEGF secretion with pravastatin treatment and showed no effect of O2 concentrations. Data are expressed as mean ± SEM, * indicates p<0.05.
DISCUSSION
This study reports a combination of potentially positive and negative effects of pravastatin use during pregnancies with placental ischemia-related hypertension. Animal studies are required to investigate potential treatments for complications of pregnancy, but animal models often have limitations and ours is no different. Nevertheless, we feel our rat model of placental ischemia-induced hypertension, which mimics many of the features of preeclampsia, is robust and contributes important findings to the body of literature in this area.
Foremost, we report that pravastatin administration ameliorates RUPP-induced hypertension. Further, we found that pravastatin increased circulating free VEGF concentrations and normalized sFlt-1:VEGF ratio, but did not restore angiogenic potential of the serum of these animals as determined by an endothelial tube formation assay. While pravastatin treatment also improved markers of oxidative stress in the RUPP rats and resulted in no obvious adverse effects on fetal outcome, there was an increase in circulating TNF-α in the RUPP+P rats compared to the NP controls. Thus, the present study shows that pravastatin treatment during pregnancy may have effects that are specific to the concurrent presence of placental ischemia.
Our present findings are in agreement with previous studies reporting that pravastatin treatment has beneficial effects on blood pressure in hypertensive pregnant rodents (11;16). This is an important consideration as elevations in blood pressure are of primary concern in the treatment and management of preeclamptic patients. In addition, we also report that pravastatin treatment increased circulating free VEGF concentrations. Again, this is similar to other recent reports that have shown that pravastatin increases PlGF and VEGF in other models of pregnancy-induced hypertension (11;16) or otherwise complicated pregnancies with angiogenic imbalance (25). We are presently unsure of the mechanism by which pravastatin increases VEGF in the pregnant rat, but it appears to be ischemia-dependent as the same dose did not increase VEGF concentrations in the NP rats. Further, it remains unclear if the alterations in VEGF due to pravastatin treatment presage the reduction in blood pressure or occur due to the alteration in blood pressure and further studies are needed to clarify the mechanisms underlying these observations. Alternatively, previous work has shown that statins may stimulate eNOS in endothelial cells (26) and improve vascular function via increased NO or cGMP signaling.
We also observed a decrease in endothelial tube formation in HUVECs incubated with serum from both the RUPP and NP rats treated with pravastatin when compared to NP rats. Similarly, Frick et al. reported endothelial cells treated directly with 10μM simvastatin formed fewer tubes compared to cells treated with 0.1μM simvastatin (27). In contrast, we performed an additional study using NP and RUPP serum with pravastatin added post-mortem and did not observe any direct effects on tube formation. One possibility for the differences in these observations is that factors in the rat serum interfere with the direct effects of statins on tubule formation that has been reported previously (27). Alternatively, it is possible that TNF-α which we found to be increased by RUPP in the present study, has a direct effect on tubule formation that is independent of pravastatin treatment. Further studies are being planned to evaluate this possibility.
We also evaluated the effects of pravastatin on VEGF secretion from trophoblast cells in vitro at different oxygen concentrations and found differing effects depending on the cell type. In fusigenic BeWo cells we found that hypoxia increased VEGF expression, but that there was no effect of pravastatin on VEGF expression. In contrast, we found in the non-fusigenic JEG cells that pravastatin decreased VEGF secretion and there was no effect of hypoxia. This suggests that the effects of pravastatin may be different in different populations of trophoblast cells and that these effects are also different than effects in HUVECs. Nevertheless, these observations point to the need for further study into the effects of statins on reproductive tissues during pregnancy.
Previous studies in cell culture have reported that statins increase HO-1 expression in endothelial and smooth muscle cells (28;29) and increase VEGF expression (27;30). However, following pravastatin treatment in vivo, we did not find an increase in HO-1 or other heat shock proteins in the placenta, molecules which are known to have a variety of cytoprotective roles (31;32). Further, we did not observe any alterations in hepatic HO-1 expression nor did we find any changes in circulating bilirubin levels either due to RUPP or to pravastatin treatment. These findings regarding HO-1 are in contrast to previous work showing that pravastatin decreased HO-1 in the liver in non-pregnant rats with obstructive cholestasis (33). Thus our findings do not support this as a putative mechanism linking pravastatin to increased VEGF in the RUPP rat. We have not evaluated this possibility in endothelial cells from this model and presently cannot rule this out. Further studies are planned to examine this possibility.
We also noted an increased cardiac weight in the RUPP compared to the NP rats that was attenuated with pravastatin treatment. Increased heart weight is consistent with chronic elevations in blood pressure observed in this model and has been reported previously (34). A previous study by Gutkowska et al., reported that treatment with Etanercept decreased RUPP-induced hypertension and mitigated associated hypertrophy (34). In contrast, our present findings indicate that increased cardiac mass was decreased along with decreased blood pressure, but independent of circulating levels of TNF-α as we found them to be increased in RUPP rats treated with pravastatin. We did not measure other markers of cardiac inflammation as part of the present study, thus further work is needed to determine the significance of this observation.
Another beneficial effect we observed in the RUPP rats treated with pravastatin was restoration of total antioxidant capacity and a decrease in oxidative stress as measured by decreases in TBARS and catalase activity. Increased oxidative stress has long been recognized as a key characteristic of preeclampsia (6;35;36) and the RUPP model has previously been shown by us and others (23;25) to mimic this observation. The present study is the first to report on catalase activity in the RUPP model and we found that, similar to reports from preeclamptic women (6), there was an increase in catalase activity in the RUPP rat. Since catalase activity is increased in response to oxidative stress, the decrease observed in the RUPP+P rats further supports the notion that pravastatin decreases oxidative stress in this model. Further, oxidative stress is known to play a key role in RUPP hypertension (21) and the antioxidant effects of pravastatin may be an important mediator of the blood pressure reduction we presently report.
In addition to the beneficial effects of pravastatin treatment on blood pressure, there was no evidence of fetal distress due to pravastatin treatment in either RUPP or NP rats. We also observed the placental efficiency, which was decreased in the RUPP compared to NP rats, was restored by pravastatin treatment. This observation is in agreement with previous work showing improved placental development and VEGF expression in a transgenic mouse model of preeclampsia in which mice have a complement component C1q deficiency (11). However, our study only examined the effects of one dose of pravastatin. It is possible that other doses may have different effects.
In contrast to previous studies that have reported only positive outcomes associated with pravastatin use in models of preeclampsia and miscarriage, we report for the first time that pravastatin treatment is associated with potentially detrimental effects on maternal physiology. In contrast to previous studies that have reported improved endothelial function in various models of preeclampsia and angiogenic imbalance in pregnancy (16;17;25;37), we observed that, despite increases in VEGF, pravastatin treatment did not improve endothelial tube formation in RUPP hypertensive rats. Moreover, we found that pravastatin treatment decreased the angiogenic potential of the serum from NP rats. Since pravastatin did not alter VEGF expression in the NP rats, it appears that this effect is VEGF independent. In addition, a dose of pravastatin that was higher than the in vivo dose used in our studies did not have the same effect on endothelial tube formation when added to NP and RUPP serum post-mortem. Thus it appears that pravastatin has a pregnancy-specific interaction with maternal cells that is not dependent on placental ischemia and results in an alteration in angiogenic potential of the maternal serum. This may represent an area of concern with respect to use of pravastatin during pregnancy and further studies are warranted to further evaluate this observation.
PERSPECTIVES
Our findings in the present study are unique in that we show that pravastatin attenuates high blood pressure in a model in which hypertension develops spontaneously after initiation of placental ischemia, a situation thought to be very similar to the manifestation of preeclampsia in women. While previous studies have reported that pravastatin and other statin class drugs have been shown to have pleiotropic effects on oxidative stress and endothelial function, our findings only support potential anti-oxidant effects in pregnancy. Further, pravastatin treatment had no effect on blood pressure in the normal pregnant rats suggesting that the effects were specific to the RUPP condition. The present data suggest that a decrease in oxidative stress may play an important role in these observations and that this may be independent of angiogenic balance. Lastly, these studies reveal the need for caution as well as further study to determine whether the benefits of statin use in hypertensive pregnancies outweigh the potential risks to the mother and fetus.
NOVELTY AND SIGNIFICANCE.
1) What is new?
In this study we report for the first time that pravastatin treatment reduced high blood pressure, attenuated oxidative stress, and restored angiogenic balance in the reduced utero-placental perfusion pressure (RUPP) rat, a robust model of preeclampsia. However, in contrast to previous reports we found that pravastatin treatment did not improve angiogenic potential in rats with placental ischemia-induced hypertension suggesting pravastatin use during pregnancy may have deleterious effects on maternal physiology.
What is relevant?
There are currently no treatments available for women with preeclampsia except for delivery of the baby when necessary. Our findings provide important evidence that pravastatin treatment attenuates many symptoms of placental ischemia-induced hypertension including high blood pressure, angiogenic imbalance, and oxidative stress. Nevertheless, we also report pravastatin treatment may have negative effects on maternal physiology. Therefore, further consideration must be given to whether reported benefits of statin use in hypertensive pregnancies outweigh the potential risks.
Summary
Pravastatin treatment attenuates high blood pressure, angiogenic imbalance and oxidative stress in placental ischemia-induced hypertension but may stimulate circulating antiangiogenic factors.
Acknowledgments
Sources of Funding
This work was supported in part by grants from the American Heart Association 10SDG2600040 and National Institutes of Health (NIH) HL114096 to JSG and NIH HL109843 to JFR.
Footnotes
Disclosures
Ashley Bauer- NONE
Christopher Banek- NONE
Karen Needham- NONE
Haley Gillham-NONE
Susan Capoccia-NONE
Jean Regal- funding from NIH
Jeffrey Gilbert- funding from AHA and NIH
References
- 1.August P, Lindheimer M. Pathophysiology of preeclampsia. In: Laragh J, Brenner BM, editors. Hypertension. New York: Raven Press; 1995. pp. 2407–2426. [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 3.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. Journal of Clinical Investigation. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–984. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert JS, Babcock SA, Granger JP. Hypertension Produced by Reduced Uterine Perfusion in Pregnant Rats Is Associated With Increased Soluble Fms-Like Tyrosine Kinase-1 Expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Walsh SW. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. J Soc Gynecol Investig. 1996;3:179–184. [PubMed] [Google Scholar]
- 7.Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertens. 1991;4:700–708. doi: 10.1093/ajh/4.8.700. [DOI] [PubMed] [Google Scholar]
- 8.Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative Stress Contributes to Soluble Fms-Like Tyrosine Kinase-1 Induced Vascular Dysfunction in Pregnant Rats. Am J Hypertens. 2009;22:564–568. doi: 10.1038/ajh.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedeek MH, Wang YP, Granger JP. Increased oxidative stress in a rat model of preeclampsia. American Journal of Hypertension. 2004;17:142A. [Google Scholar]
- 10.Parrish MR, Wallace K, Tam Tam KB, Herse F, Weimer A, Wenzel K, Wallukat G, Ray LF, Arany M, Cockrell K, Martin JN, Dechend R, LaMarca B. Hypertension in response to AT1-AA: role of reactive oxygen species in pregnancy-induced hypertension. Am J Hypertens. 2011;24:835–840. doi: 10.1038/ajh.2011.62. [DOI] [PubMed] [Google Scholar]
- 11.Singh J, Ahmed A, Girardi G. Role of Complement Component C1q in the Onset of Preeclampsia in Mice. Hypertension. 2011;58:716–724. doi: 10.1161/HYPERTENSIONAHA.111.175919. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant Vascular Endothelial Growth Factor 121 Infusion Lowers Blood Pressure and Improves Renal Function in Rats With Placental Ischemia-Induced Hypertension. Hypertension. 2009;55:380–385. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maron DJ, Fazio S, Linton MF. Current Perspectives on Statins. Circulation. 2000;101:207–213. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- 14.Strazzullo P, Kerry SM, Barbato A, Versiero M, D’Elia L, Cappuccio FP. Do Statins Reduce Blood Pressure?: A Meta-Analysis of Randomized, Controlled Trials. Hypertension. 2007;49:792–798. doi: 10.1161/01.HYP.0000259737.43916.42. [DOI] [PubMed] [Google Scholar]
- 15.Cudmore MJ, Ahmad S, Al-Ani B, Fujisawa T, Coxall G, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW, Ahmed A. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 16.Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, Takakura N, Kimura T, Okabe M. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proceedings of the National Academy of Sciences. 2010;108:1451–1455. doi: 10.1073/pnas.1011293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox KA, Longo M, Tamayo E, Kechichian T, Bytautiene E, Hankins GDV, Saade GR, Costantine MM. Effects of pravastatin on mediators of vascular function in a mouse model of soluble Fms-like tyrosine kinase-1induced preeclampsia. American Journal of Obstetrics and Gynecology. 2011;205:366. doi: 10.1016/j.ajog.2011.06.083. [DOI] [PubMed] [Google Scholar]
- 18.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 19.van TA, Jansen EH, Koomans HA, Joles JA. Hyperlipoproteinemia in Nagase analbuminemic rats: effects of pravastatin on plasma (apo)lipoproteins and lecithin:cholesterol acyltransferase activity. J Lipid Res. 1991;32:1719–1728. [PubMed] [Google Scholar]
- 20.Heltemes A, Gingery A, Soldner ELB, Bozadjieva N, Jahr K, Johnson B, Gilbert JS. Chronic placental ischemia alters amniotic fluid milieu and results in impaired glucose tolerance, insulin resistance and hyperleptinemia in young rats. Experimental Biology and Medicine. 2010;235:892–899. doi: 10.1258/ebm.2010.009357. [DOI] [PubMed] [Google Scholar]
- 21.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of Reactive Oxygen Species in Hypertension Produced by Reduced Uterine Perfusion in Pregnant Rats. Am J Hypertens. 2008;21:1152–1156. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert JS, Banek CT, Bauer AJ, Gingery A, Dreyer HC. Placental and vascular adaptations to exercise training before and during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol. 2012;303:R520–R526. doi: 10.1152/ajpregu.00253.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banek CT, Bauer AJ, Gingery A, Gilbert JS. Timing of ischemic insult alters fetal growth trajectory, maternal angiogenic balance, and markers of renal oxidative stress in the pregnant rat. Am J Physiol Regul Integr Comp Physiol. 2012;303:R658–R664. doi: 10.1152/ajpregu.00250.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vatish M, Tesfa L, Grammatopoulos D, Yamada E, Bastie CC, Pessin JE. Inhibition of Akt Activity and Calcium Channel Function Coordinately Drive Cell-Cell Fusion in the BeWO Choriocarcinoma Placental Cell Line. PLoS ONE. 2012;7:e29353. doi: 10.1371/journal.pone.0029353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed A, Singh J, Khan Y, Seshan SV, Girardi G. A New Mouse Model to Explore Therapies for Preeclampsia. PLoS ONE. 2010;5:e13663. doi: 10.1371/journal.pone.0013663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of Endothelial Nitric Oxide Synthase by HMG CoA Reductase Inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 27.Frick M, Dulak J, Cisowski J, Jozkowicz A, Zwick R, Alber H, Dichtl W, Schwarzacher SP, Pachinger O, Weidinger F. Statins differentially regulate vascular endothelial growth factor synthesis in endothelial and vascular smooth muscle cells. Atherosclerosis. 2003;170:229–236. doi: 10.1016/s0021-9150(03)00299-5. [DOI] [PubMed] [Google Scholar]
- 28.Grosser N, Hemmerle A, Berndt G, Erdmann K, Hinkelmann U, Schurger S, Wijayanti N, Immenschuh S, Schroder H. The antioxidant defense protein heme oxygenase 1 is a novel target for statins in endothelial cells. Free Radic Biol Med. 2004;37:2064–2071. doi: 10.1016/j.freeradbiomed.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW, Ahmed A. Negative Regulation of Soluble Flt-1 and Soluble Endoglin Release by Heme Oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 30.Jozkowicz A, Huk I, Nigisch A, Weigel G, Dietrich W, Motterlini R, Dulak J. Heme oxygenase and angiogenic activity of endothelial cells: stimulation by carbon monoxide and inhibition by tin protoporphyrin-IX. Antioxid Redox Signal. 2003;5:155–162. doi: 10.1089/152308603764816514. [DOI] [PubMed] [Google Scholar]
- 31.Jayakumar J, Suzuki K, Sammut IA, Smolenski RT, Khan M, Latif N, Abunasra H, Murtuza B, Amrani M, Yacoub MH. Heat Shock Protein 70 Gene Transfection Protects Mitochondrial and Ventricular Function Against Ischemia-Reperfusion Injury. Circulation. 2001;104:I–303. doi: 10.1161/hc37t1.094932. [DOI] [PubMed] [Google Scholar]
- 32.Mymrikov EV, Seit-Nebi AS, Gusev NB. Large Potentials of Small Heat Shock Proteins. Physiol Rev. 2011;91:1123–1159. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- 33.Kolouchova G, Brcakova E, Hirsova P, Cermanova J, Fuksa L, Mokry J, Nachtigal P, Lastuvkova H, Micuda S. Modification of hepatic iron metabolism induced by pravastatin during obstructive cholestasis in rats. Life Sci. 2011;89:717–724. doi: 10.1016/j.lfs.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Gutkowska J, Granger JP, LaMarca B, Coderre L, Pelletier A, Gangal M, Jankowski M. Cardiac hypertrophy and cardiac insulin responsive amino-peptidase (IRAP) in the reduced uterine perfusion (RUPP) model of preeclampsia. Hypertension. 2005;46:841. [Google Scholar]
- 35.Wang Y, Walsh SW. Placental mitochondria as a source of oxidative stress in preeclampsia. Placenta. 1998;19:581–586. doi: 10.1016/s0143-4004(98)90018-2. [DOI] [PubMed] [Google Scholar]
- 36.Walsh SW, Vaughan JE, Wang Y, Roberts LJ. Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000;14:1289–1296. doi: 10.1096/fj.14.10.1289. [DOI] [PubMed] [Google Scholar]
- 37.Costantine MM, Tamayo E, Lu F, Bytautiene E, Longo M, Hankins GD, Saade GR. Using pravastatin to improve the vascular reactivity in a mouse model of soluble fms-like tyrosine kinase-1-induced preeclampsia. Obstet Gynecol. 2010;116:114–120. doi: 10.1097/AOG.0b013e3181e10ebd. [DOI] [PubMed] [Google Scholar]


