Abstract
Green tea is rich in polyphenol flavonoids including catechins. Epigallocatechin 3-gallate (EGCG) is the most abundant and potent green tea catechin. EGCG has been extensively studied for its beneficial health effects as a nutriceutical agent. Based upon its chemical structure, EGCG is often classified as an antioxidant. However, treatment of cells with EGCG results in production of hydrogen peroxide and hydroxyl radicals in the presence of Fe (III). Thus, EGCG functions as a pro-oxidant in some cellular contexts. Recent investigations have revealed many other direct actions of EGCG that are independent from anti-oxidative mechanisms. In this review, we discuss these novel molecular mechanisms of action for EGCG. In particular, EGCG directly interacts with proteins and phospholipids in the plasma membrane and regulates signal transduction pathways, transcription factors, DNA methylation, mitochondrial function, and autophagy to exert many of its beneficial biological actions.
Abbreviations: EGCG, epigallocatechin 3-gallate; ECG, epicatechin gallate; EGC, epigallocatechin; EC, epicatechin
Keywords: Polyphenol, EGCG, Anti-oxidant, Pro-oxidant
Graphical abstract
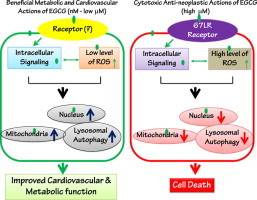
Molecular mechanisms for beneficial health effects of EGCG. Low concentrations of EGCG have beneficial effects on cardiovascular and metabolic functions in normal physiology and pathophysiology. Low concentrations of EGCG directly and indirectly stimulate cellular events including intracellular signaling, nuclear and mitochondrial functions, and lysosomal autophagy. By contrast, high concentrations of EGCG may cause severe stress that damages cellular integrity and disrupts nuclear and mitochondrial function and lysosomal autophagy. These actions can be applied to cancer therapy by inducing cell death. Green oval ( ) indicates EGCG that directly binds or is transported into the indicated organelles.
) indicates EGCG that directly binds or is transported into the indicated organelles.
Highlights
-
•
Many biological actions of EGCG are mediated by specific mechanisms other than its well-known anti-oxidant properties.
-
•
EGCG is a pro-oxidant per se in some biological contexts.
-
•
EGCG directly interacts with cell surface membrane proteins and specific known receptors.
-
•
Treatment of cells with EGCG regulates specific intracellular signaling pathways and transcription.
-
•
Specific biological actions of EGCG are regulated in a concentration-dependent manner.
Introduction
Polyphenols may have therapeutic health effects for a variety of chronic pathological conditions including cancer, neurodegenerative diseases, diabetes, and cardiovascular diseases [16], [51], [106]. Many polyphenols are derived from natural food products. Thus, they are often considered safer and more easily integrated into lifestyle changes than conventional pharmaceutical drugs. Recently, specific molecular targets for various polyphenols have been discovered. Therefore, scientific interest in polyphenols as therapeutic agents is rapidly increasing. However, many studies often report biological effects of food polyphenols are observed without elucidating the underlying molecular, cellular, and physiological mechanisms. Obstacles to defining mechanistic studies in this field may include non-specific effects due to polyphenols with pleiotropic activities, and complex mechanisms of action. Despite difficulties in defining specific mechanisms, recent work has shed light on more detailed molecular mechanisms underlying bioactive actions of polyphenols. Many polyphenols share beneficial effects against a broad range of pathologies, including cancer, inflammation, diabetes, and cardiovascular diseases. However, individual polyphenols have distinct specific molecular targets in various tissues with different efficacies and bioavailabilities.
Dietary sources of polyphenols have received a great deal of attention. In particular the polyphenolic components in different tea preparations have been examined in detail. Tea is the second most frequently consumed beverage after water. Tea polyphenols have received considerable public attention due to the positive association between tea consumption and beneficial health effects [56]. Epidemiological studies show correlations between tea consumption and decreased risk of cardiometabolic disorders and mortality in a dose-dependent fashion [56]. Green tea extract contains a number of catechins, including epigallocatechin-gallate (EGCG), epigallocatechin (EGC), epicatechin-gallate (ECG), and epicatechin (EC). The profiles of catechins in human plasma and urine after tea consumption have been analyzed [29], [61], [112]. Although the absorption, excretion, and modification of catechins may affect the bioavailabilities and bioactive potencies, structural characteristics seems to play important roles in differential bioactivities. It has been proposed that the galloyl moiety of tea catechins may play the critical roles in specific activities of tea catechins, especially in lipid lowering effect [39], [40]. Furthermore, the two catechins with galloyl moiety (EGCG and ECG) have the most potent biological activities as listed in Table 1. The most abundant green tea polyphenol, epigallocatechin 3-gallate (EGCG) may be responsible for many of the beneficial effects of green tea in clinical and animal studies as well as in cell culture studies [7], [14], [47], [48], [49], [87], [101]. One potential mechanism for beneficial health effects of EGCG may be attributable to its anti-oxidative function [9]. However, more recent findings suggest many additional mechanisms of action for EGCG including interactions with plasma membrane proteins, activation of second messengers and signal transduction pathways, modulation of metabolic enzymes, and autophagy [47], [49], [55], [110], [111], [124]. Furthermore, the biological actions of EGCG are concentration-dependent. Bioavailability studies after tea consumption demonstrate levels of EGCG in human plasma in the low μM range [60]. Circulating levels of EGCG reach about 10 μM in animal studies after oral intake of pure EGCG [57]. In this review, the concentration-dependent biological actions of EGCG (low defined as ≤10 µM and high defined as >10 μM) and recent progress in elucidation of specific molecular mechanisms of action are discussed (Table 2).
Table 1.
Relative biological potency of green tea polyphenols.
| Biological actions | EGCG | ECG | EGC | EC | Ref. |
|---|---|---|---|---|---|
| Inhibition of histamine release | Strong | Moderate | Moderate | No effect | [70] |
| Inhibition of leukotriene B4 release | Strong | Moderate | Moderate | No effect | [70] |
| Angiogenesis | Strong | Strong | Weak | Weak | [53] |
| RyR1 activation | EGCG> | ECG> | EGC> | EC | [27] |
| Cytotoxicity in oral cavity | EGCG> | ECG> | EGC> | EC | [5] |
| FAS inhibition | EGCG< | ECG | [105] | ||
| SIRT1 activation | 1.75 fold | 1.85 fold | [19] | ||
| Na/H exchanger inhibition | EGCG< | ECG< | EGC< | EC | [92] |
Table 2.
Effects of EGCG treatment on various cellular responses.
| Cellular function | Cell types | Con. EGCG (μM) | Mechanism | Reference |
|---|---|---|---|---|
| Glucose uptake | 3T3-L1 | <5 | Anti-oxidant | [118] |
| Adipocyte differentiation | 3T3-L1 | 5–10 | Up-regulate Adipogenic Genes | [98] |
| Inhibition of gluconeogenesis | Hepatocyte | <1 | CaMKKβ/AMPK pro-oxidant | [15] |
| Autophagy | Endothelial cells | 10 | Ca2++/CaMKKβ | [47] |
| Autophagy/Anti-tumor (cell death) | Cancer | >50 | Pro-oxidant | [63], [99], [124] |
| Transformation | Cancer | 20 | AP-1 inhibition | [21] |
| Protection | HaCaT | >20 | Pro-oxidant | [22] |
| Anti-proliferation | Cancer | >20 | Pro-oxidant | [36], [64] |
Pro-oxidant or anti-oxidant?
Oxidant properties of polyphenols may be both anti-oxidant and/or pro-oxidant based upon the structure of the particular polyphenol and the cellular redox context that may include increased levels of oxidant scavenging proteins or decreased levels of oxidized proteins and lipids [11], [76], [122]. Thus, some studies claim EGCG is an anti-oxidant [7], [44], [83], [118]. For example, mitochondrial function is improved by anti-oxidative action of EGCG [72]. Moreover, EGCG ameliorates lipid infusion-mediated insulin resistance, which is associated with increased expression of anti-oxidant enzymes including superoxide dismutase (SOD) and glutathione peroxidase by EGCG in vivo [66]. However, these reports are associative and do not directly demonstrate that a decrease in oxidative stress by EGCG per se is the direct mechanism preventing lipid-induced insulin resistance. On the other hand, other reports suggest that green tea extract and EGCG exert pro-oxidant actions [23], [36], [64], [97], [121]. EGCG auto-oxidizes, and produces hydrogen peroxide in cell culture media with and without cells. Addition of SOD and catalase abolishes some cellular actions of EGCG by inhibiting the auto-oxidation and dimerization of EGCG [64]. EGCG works in two ways to promote cytotoxicity in anti-tumor activity; one is directly by producing hydrogen peroxide with its pyrogallol moiety, and the other is reducing Fe (III) to Fe (II) which triggers the Fenton reaction to create more potent reactive oxygen species (ROS) such as hydroxyl radicals [77], [78]. The combination of hydroxyl radicals and hydrogen peroxide contributes to the cytotoxic effects of EGCG at high µM concentrations (>50 μM). Another study has shown that N-acetyl cysteine (NAC) is able to protect from hydrogen peroxide-induced cytotoxicity but not EGCG-induced cell death. Thus, the cytotoxic effect of EGCG in tumor cells is not mimicked by hydrogen peroxide alone [117]. This suggests that EGCG has tumor-specific mechanisms for mitochondrial damage other than production of hydrogen peroxide. However, these results are based upon cell culture studies using >50 μM EGCG (50 μM EGCG generates about 1 μM hydrogen peroxide in Jurkat cells) [77]. In fact, more modest nM and biologically realistic concentrations (1–2 μM up to 10 μM) of EGCG produce lower levels of intracellular reactive oxygen species that stimulate multiple signal transduction pathways to promote cellular protective mechanisms [15], [22]. This suggests that EGCG-mediated production of reactive oxygen species contributes to the beneficial biological actions of EGCG. It is possible that different amount of anti-oxidants, including glutathione, thioredoxin, catalase and SOD in various tissues and serum components in animal models may underlie discrepancies in the activities of EGCG under in vitro and in vivo conditions. Moreover, expression levels of modifying enzymes and their stability may also be factors determining bioavailability of EGCG. EGCG is converted to dimer and multimer, and modified to glucuronated and/or methylated forms. Since many cellular actions of EGCG are acute and occur within minutes, it is likely that EGCG has direct cellular actions that are independent of its metabolites. However, the various metabolites of EGCG may also have some bioactivity, particularly with respect to chronic actions of green tea extracts. Thus, distinct actions and roles of these derivatives may also contribute to pleiotropic biological effects of EGCG [107]. Although it has not been proven that oxidation of EGCG occurs in vivo, all of the forms, including intact, oxidized or modified EGCG are potentially bioactive substances. Therefore, the combination of direct and indirect effects of EGCG may create synergistic or additive mechanisms for various actions of green tea.
Cell surface receptor
One important mechanism frequently overlooked in considering the biological effects of polyphenols is their potential interaction with receptors capable of initiating cell signaling. Interestingly, when EGCG is incubated with cells, 75% of radioactively labeled EGCG is found in the cytosolic compartment while some radioactivity is found in the membrane fraction [34]. This suggests that EGCG directly binds to membrane components, including proteins and lipids. In fact, EGCG inhibits PDGFR-BB-stimulated signal transduction pathway in vascular smooth muscle cells by direct binding to PDGFR-BB thereby causing inhibition of PDGF-stimulated restenosis [1], [95], [114]. Moreover, EGCG regulates activities of cell surface growth factor receptors, especially receptor tyrosine kinases (RTK), including epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptor (VEGFR), insulin-like growth factor receptor (IGFR), and the insulin receptor (InsR) (Table 3). [1], [53], [67], [93], [96], [102], [113]. Most RTKs are involved in cell proliferation, survival and angiogenesis. In particular, InsR is important for regulation of metabolism and cell survival. It is notable that EGCG inhibits activities of EGFR, VEGFR, and IGFR. By contrast, EGCG mimics or augments InsR signaling [113]. One mechanism proposed for activation of InsR is that EGCG inhibits tyrosine phosphatases through a redox mechanism that results in increased and sustained tyrosine phosphorylation of InsR [113] by increasing the level of hydrogen peroxide. Thus, inhibitory actions of EGCG on proliferation may be specific to abnormally proliferating or cancer cells, but not to normal cells. In fact, by using surface plasmon resonance technique, Tachibana et al. discovered that EGCG, but not other tea catechins, directly binds to the laminin receptor (67LR) (Kd~nanomolar) [81]. The binding site in 67LR is located within the peptide LR161-170 and the two basic amino acids K(166) and H(169) are critical for the binding of EGCG [28]. The expression of 67LR is elevated in cancer cells but not in normal cells [71]. This suggests that EGCG may specifically target cancer cells in some contexts. More recently, Kumazoe et al. suggested a mechanism for EGCG-induced apoptosis of cancer cells through 67LR that may provide a potential therapeutic strategy using inhibitors of phosphodiesterase 5 (PDE5) (Fig. 1) [116]. Another role of EGCG-stimulated 67LR is to mediate anti-inflammatory actions. Treatment with EGCG reduces expression of toll–like receptor 4 (TLR4) and increases expression of tollip, a negative regulator of TLRs, through a 67LR-dependent mechanism [33]. Because TLR2 and TLR4 are involved in innate immunity in response to bacterial infection, this activity of EGCG inhibits lipopolysaccharide-stimulated pro-inflammatory responses in macrophages [33]. Identifying the detailed mechanisms for 67LR-mediated reduction of TLR4 and increased tollip expression will provide more detailed mechanisms for anti-inflammatory actions of EGCG. Growing evidence suggests that 67LR plays important roles in biological actions of EGCG (Fig. 2). Despite the importance of 67LR in the activities of EGCG, detailed downstream signaling pathways for 67LR are yet to be elucidated.
Table 3.
Effects of EGCG on receptor tyrosine kinases.
| Biological actions | Signaling molecule | Conc. of EGCG (μM) | Inhibition/Activation | Reference |
|---|---|---|---|---|
| Atherosclerosis | PDGF-BB | 5–100 | Inhibition | [1] |
| Angiogenesis | VEGFR | 1.56–100 | Inhibition | [53] |
| Cancer | EGFR | >10 | Inhibition | [67] |
| Angiogenesis | VEGFR | 0.5–10 | Inhibition | [93] |
| Cancer | EGFR | 30–50 | Inhibition | [96] |
| Gluconeogenesis | InsR | 5–50 | Activation | [113] |
Fig. 1.
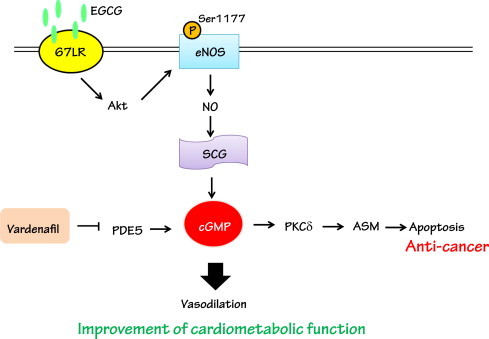
EGCG-activated eNOS pathways that improve cardiovascular function and anti-cancer effect in the presence of PDE5 inhibitor. EGCG elevates cGMP levels through a specific receptor 67LR that stimulates the Akt/eNOS pathway. This pathway leads to vasodilation which contributes to improvement of cardiovascular function. In cancer cells, inhibition of PDE5 by treatment with Vardenafil leads to sustained elevations of cGMP levels. This elevated cGMP activates PKCδ/acidic sphingomyelinase (ASM) that contributes to apoptosis.
Fig. 2.
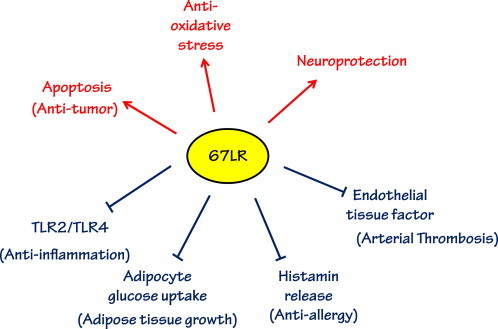
The roles of 67LR as a specific receptor for EGCG. EGCG has multiple biological actions mediated through 67LR by enhancing (red) and inhibiting (blue) specific pathways.
Intracellular signaling pathways
In cell culture, the majority of [3H]–EGCG is found in the cytosolic fraction [34]. This suggests that biological actions of EGCG may occur through EGCG metabolites or interaction with intracellular molecules. As mentioned above, EGCG produces low level reactive oxygen species, including hydrogen peroxide that may act as a second messenger for downstream signaling pathways [23], [36], [64], [97], [121]. This action may be mediated by direct chemical reactions of EGCG with compounds at the cell surface. However, additional unknown receptor-mediated signaling pathways cannot be excluded. EGCG also increases other intracellular second messengers including Ca2+, cAMP, and cGMP.
Calcium
EGCG elevates cytosolic Ca2+ levels in excitable and non-excitable cells [13], [47], [50]. Elevation of Ca2+ is achieved after stimulation with nanomolar concentrations of EGCG and ECG, but not by EGC or EC. This increases the sensitivity of the ryanodine receptor (RyR1) in response to extracellular Ca2+ inward current or electrical stimulation without changing basal cytosolic Ca2+ [27]. This effect is independent of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) activity at concentrations of EGCG up to 2 μM. However, 10 μM EGCG elevates cytosolic Ca2+ without Ca2+ challenge or electrical stimulation. This is inhibited by depletion of endoplasmic reticulum Ca2+ stores by treatment with cyclopiazonic acid in bovine aortic endothelial cells [47]. This suggests that concentrations of EGCG>10 μM elevate cytosolic Ca2+ by inhibition of SERCA [43], [103]. This elevated cytosolic Ca2+ affects activities of various Ca2+ requiring enzymes including calmodulin (CaM)-dependent protein kinase II and CaMKKβ [47], [50]. CaMKKβ is an upstream regulator for AMP-dependent kinase (AMPK), an energy sensing enzyme that plays crucial roles in energy metabolism and cardiovascular functions [79], [94], [108]. EGCG-stimulated elevation of cytosolic calcium contributes to NO production by binding to calmodulin in the heart and vascular endothelium [32], [35], [73]. These actions of EGCG are closely related to beneficial cardiovascular actions of EGCG.
Cyclic-nucleotides (cAMP/cGMP)
EGCG treatment increases cAMP in endothelial cells and platelets that participate in phosphorylation of eNOS and vasodilator-stimulated phosphoprotein (VASP) [68], [82]. However, this seems to be a cell type-specific response because elevation of intracellular cAMP by EGCG is not observed in other cell types. Although the detailed molecular mechanism for elevation of cAMP is not known, elevation of cAMP may be achieved through activation of adenylate cyclase, but not through inhibition of phosphodiesterase 4 (PDE4) [82]. This elevated cAMP stimulates protein kinase A that contributes to various biological events [24], [68]. By contrast, EGCG stimulates production of NO through a 67LR-mediated mechanism leading to apoptosis in cancer cells and acute myeloid leukemia cells [54], [55], [119]. This effect is potentiated by the PDE5 inhibitor Vardenafil in cancer cells but not in normal cells. Thus, prolonged elevation of cGMP by EGCG and Vardenafil leads to potentiated cell death. Interestingly, cancer cells express abnormally high levels of 67LR and PDE5. This may aid in cancer-specific targeted treatment. This novel mechanism provides a combinatorial therapeutic strategy to treat cancer patients with EGCG and PDE5 inhibitors [55]. EGCG also stimulates vasorelaxation by increasing both cAMP and cGMP in rat aorta [3]. Thus, EGCG may stimulate production of cyclic nucleotides that may be one of the important mechanisms underlying beneficial biological actions of EGCG in metabolic and cardiovascular physiology and in anti-neoplastic actions [41], [49], [68] (Fig. 1).
Other signaling pathways
AMPK is an energy sensing molecule that is also activated by EGCG in hepatocytes, adipocytes, cancer cells, and endothelial cells. AMPK contributes to inhibition of gluconeogenesis, stimulation of lipolysis, apoptosis, and reduction of endothelin-1 expression, respectively (Fig. 3) [15], [38], [74], [85], [90]. This activity may contribute to improvement of insulin sensitivity and vasodilation [12], [37], [41]. In fact, EGCG plays an important role in lipid metabolism by regulating lipolytic and lypogenic enzymes [12], [62]. EGCG inhibits fatty acid synthase (FAS), PPAR gamma, and C/EBP. Fatty acid binding protein 4 (FABP4) and FAS are reduced by upregulation of nuclear beta-catenin [58]. Knock-down of beta-catenin attenuates inhibition of intracellular lipid accumulation [58]. Upstream kinases for AMPK include CaMKKβ and liver kinase B1 (LKB1) that are activated by EGCG [10], [15], [75]. The previously mentioned Ca2+ signaling contributes to stimulation of CaMKKβ in response to EGCG treatment of endothelial cells [47]. The activation of AMPK is dependent on reactive oxygen species because catalase and N-acetyl cysteine suppress this action of EGCG [15], [38], [74]. This is an example of beneficial pro-oxidant actions of EGCG. In addition to Ca2+ and cyclic nucleotides, a number of studies demonstrate that other intracellular signaling pathways are regulated by EGCG. It is difficult to determine unified EGCG-stimulated signal transduction pathways. This seems to be highly dependent on cell type and EGCG concentrations. For example, EGCG stimulates Src-family kinases, including Fyn in endothelial cells [49], while EGCG inhibits concanavalin A-stimulated Src in mesenchymal stromal cells [123]. The concentrations of EGCG in both studies are in a similar range (10–50 μM). Ca2+ signaling and Src activation is not observed in NIH-3T3 fibroblast cells (unpublished observation). Furthermore, EGCG-stimulated Fyn/PI 3-kinase/Akt/eNOS pathway contributes to vasorelaxation and protection from ischemia reperfusion injury of cardiac tissues. However, this does not seem to be mediated through a 67LR-dependent mechanism [49], [87]. Puzzlingly, a similar pathway in cancer cells is mediated by 67LR and leads to apoptosis [55]. It is conceivable that opposing actions of EGCG may have differential effects depending on whether they are integrated into normal physiology or intervene in dysregulated pathophysiology.
Fig. 3.
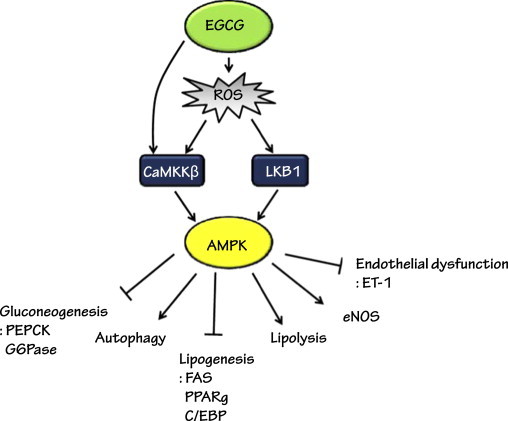
Metabolic and vascular functions of EGCG. A schematic diagram showing the mechanisms for the activation of AMPK by EGCG. AMPK is a key molecule that regulates enzymes involved with energy metabolism and endothelial functions.
Nuclear function
Cellular responses in intracellular signal transduction pathways generally occur acutely. However, chronic cellular responses often involve nuclear functions that regulate gene expression and chromosomal modifications. In this section, we will discuss the effects of EGCG in nuclear function.
Regulation of transcription factors
EGCG modulates gene expression by inhibiting various transcription factors including Sp1, NF-κB, AP-1, STAT1, STAT3 and FOXO1 (Table 4) [2], [9], [21], [46], [59], [90], [91], [109], [120]. NF-κB and AP-1 expression are inhibited by EGCG in rats exposed to ischemia-reperfusion (I/R) injury [4]. EGCG inhibits STAT-1 to mediate protective effects of EGCG in myocardial I/R injury [104], [109]. Multiple studies show that nuclear activities of EGCG inhibit inflammatory responses that are usually accompanied by increased oxidative stress [6], [86]. Thus, one may claim that this anti-inflammatory action is mainly due to direct anti-oxidant activity of EGCG. However, it is not clear whether anti-oxidative effects of EGCG are a major mechanism for anti-inflammatory actions of EGCG. Interestingly, EGCG-stimulated production of ROS that causes activation of NF-κB and NF-E2-related factor 2 (Nrf2) leading to increased expression of HO-1 and glutathione [88], [115]. Scavenging ROS by using various anti-oxidants abolishes EGCG-stimulated induction of HO-1 while pretreatment with EGCG has protective effects against hydrogen peroxide-induced cytotoxicity [115]. It is noteworthy that most in vitro studies use high concentrations of EGCG to elicit inhibitory actions on transcription factors. However, in vivo studies may not achieve the concentrations of EGCG used with in vitro studies. These potential differences in conditions and concentrations of EGCG need to be carefully considered when interpreting results of experiments designed to reveal mechanisms for the physiological actions of EGCG in vivo. Protective mechanisms that EGCG uses to defend against oxidative stress may be secondary to induction of various endogenous anti-oxidant proteins. Inhibition of the transcription factor FOXO1 by EGCG leads to suppression of basal levels of endothelin-1 (ET-1) and differentiation of adipocytes [45], [46]. Both of these mechanisms are linked to activation of Akt that inhibits FOXO1 by direct phosphorylation of FOXO1 and may have cardiometabolic implications [56], [80].
Table 4.
Effects of EGCG on transcription factors.
| Transcription factor | Cell type | Con. EGCG (μM) | Inhibition/Activation | Reference |
|---|---|---|---|---|
| Sp1 | LNCaP | 20 | Inhibition | [91] |
| NF-κB | HSC | 20–100 | Inhibition | [9] |
| RAW264.7 | 100 | Inhibition | [120] | |
| Nrf2 | HAEC | 2.5 | Activation | [88] |
| BAEC | 25–100 | Activation | [115] | |
| AP-1 | HSC | 20–100 | Inhibition | [9] |
| Epidermal cell | 5–20 | Inhibition | [21] | |
| STAT1 | Cardiac myocyte | 100 | Inhibition | [109] |
| STAT3 | A549, HPAEpiC | 10 | Inhibition | [59] |
| FOXO1 | 3T3-L1 | 100 | Inhibition | [45], [46] |
| BAEC | 10 | Inhibition | [90] | |
DNA methylation
EGCG has epigenetic functions in chromosomes [25]. Aberrant methylation on CpG islands cause gene silencing that leads to altered cellular physiology and cell proliferation. EGCG inhibits DNA methyltransferase (DNMT) which reverses methylation-induced gene silencing by directly binding to DNMT with an IC50 of less than 1 μM EGCG [26]. This suggests that EGCG is transported to the nucleus. Although this function of EGCG has been known for a decade, the specific genes affected by this mechanism are not well defined and this area requires further investigation.
Mitochondrial function
Mitochondria are organelles that play important roles in energy production. EGCG intake reduces obesity and expression of leptin and stearyl-coA desaturase in white adipose cells while increasing fat oxidation [52]. This suggests that EGCG actions enhance mitochondrial fat utilization and reduce adipogenesis in fat tissue. EGCG stimulates mitochondrial biogenesis and promotes oxidative phosphorylation through a cAMP/PKA- and sirtuin-dependent mechanism [111]. With respect to anti-tumor activity, EGCG promotes apoptosis through mitochondrial damage, membrane depolarization, and cytochrome c release [89]. This apoptotic action of EGCG is inhibited by NAC or catalase suggesting that excess hydrogen peroxide may contribute to mitochondrial damage-induced cell death. By contrast, the metabolic function of EGCG with regard to mitochondrial function occurs with much lower concentrations of EGCG. In some animal models, EGCG enhances mitochondrial function that reduces oxidative stress in alcoholic fatty liver or diet-induced obesity [42], [52]. In addition, pretreatment with EGCG (30 mg/kg) protects against mitochondrial damage in isoproterenol-induced cardiac toxicity in albino Wistar rats [20]. A study using rat cerebellar granule neurons shows >90% of [3H]–EGCG is found in the mitochondrial fraction. Moreover, pre-incubation with EGCG protects against mitochondrial damage-caused cell death without changes in SOD, glutathione peroxidase, Nrf2, or Bcl2 expression and oxidative stress. However, toxin-, serum withdrawal-, and proteasome inhibitor-induced cell death are not prevented by EGCG [100]. This effect of EGCG may be considered a direct anti-oxidant property of EGCG. However, it is not completely clear if these effects are due to direct anti-oxidant effects of EGCG alone or additional secondary molecular interactions including changes in mitochondrial transcription activity. These issues require further clarification with specific experiments designed to test well-defined hypotheses.
Autophagy
Autophagy is a lysosomal catabolic process that degrades accumulated and unnecessary intracellular materials [17], [18], [69]. Autophagy requires a number of molecules that interact in a highly organized manner to help determine cell survival or death. Most polyphenols, including EGCG, resveratrol, quercetin, and curcumin induce autophagy. This may contribute to anti-aging effects of polyphenols [84]. Anti-aging actions of polyphenols mimic effects of calorie restriction [84]. Some studies show that high concentrations of EGCG (100 μM) inhibit autophagy leading to apoptosis in macrophage cell lines (Raw 264.7 cells) and cancer cells [31], [124]. By contrast, low concentrations of EGCG (10 μM) induces autophagy that facilitates degradation of endotoxin-induced aggregation of high mobility group B-1 (HMGB1) leading to anti-inflammatory actions [65]. We recently reported that EGCG (10 μM) stimulates autophagy and autophagic flux in endothelial cells that helps degradation of lipid droplets through a Ca2+/CaMKKβ/AMPK dependent mechanism [47]. This may be an additional mechanism for protective effects of EGCG related to inflammation, lipotoxicity, and cell death. Thus, the regulation of autophagy by EGCG is dependent on concentration, stress conditions, and cell types. Further studies elucidating more detailed mechanisms for EGCG or other polyphenols to regulate cell survival, death, and metabolism will shed light on potential novel roles for EGCG in promoting health and preventing chronic diseases characterized by inflammation and oxidative stress.
Summary and perspectives
In this review, we emphasize biological actions of EGCG that do not directly involve anti-oxidant properties (graphic summary). EGCG directly interacts with plasma membrane proteins and phospholipids which stimulates intracellular signaling pathways. In addition, EGCG is transported to intracellular compartments, cytosol, mitochondria, lysosome, and nucleus where it mediates additional biological actions. These various effects are dependent on cell type, stress conditions, and concentrations of EGCG. Two major points should be emphasized: one is that the EGCG effects observed in vitro may not be the same as in vivo. The major reason for this is that EGCG is readily modified after absorption through the gut which modulates bioavailability. Moreover, the concentrations of EGCG often used in in vitro experiments cannot be reached in vivo in either plasma or tissues in whole animals. Despite these caveats, we can extend some in vitro results to in vivo physiology. With moderate and low concentrations of EGCG, low level oxidative stress may be a beneficial cue for the body to initiate induction of protective anti-oxidant systems and boost immune responses. Thus, under pathological conditions with increased oxidative stress (e.g., ischemia/reperfusion, alcoholic fatty liver, and obesity-induced inflammation), supplementation of green tea polyphenol may help cope with these stressful conditions. A second important point is that EGCG has cell type- and environment- specific responses because gene expression and signaling molecules are differentially regulated. One prominent action of EGCG recently discovered is to increase lipolysis through autophagy-dependent and -independent mechanisms [12], [47], [62]. This suggests that EGCG plays an important role in lipid metabolism in whole body physiology as well as at the cellular level. In the future, it will be helpful to identify more specific biomarkers that respond to physiologically effective concentrations of EGCG so that pharmacodynamic studies may be performed with physiological outcomes. Further investigations are required to understand how EGCG acts differentially in different cell types. Recent molecular, cellular, and animal studies have begun to reveal detailed mechanisms linking drinking green tea and life-style adjustment with prevention of chronic diseases including cancer, diabetes, and cardiovascular disorders. Furthermore, chemical modification of an EGCG pharmacophore may modify relative therapeutic activities so that combinatorial supplementation may synergistically enhance beneficial health effects [8], [30].
Acknowledgments
This study was supported by the American Diabetes Association (1-09-JF-33; 1-12-BS-99 to J.K; 1-13-BS-150 to M.J.Q), American Heart Association (13GRNT17220057 to J.K), and UAB diabetes research center sponsored pilot and feasibility program supported by the National Institutes of Health (P60 DK-079626).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Ahn H.Y., Hadizadeh K.R., Seul C., Yun Y.P., Vetter H., Sachinidis A. Epigallocathechin-3 gallate selectively inhibits the PDGF-BB-induced intracellular signaling transduction pathway in vascular smooth muscle cells and inhibits transformation of sis-transfected NIH 3T3 fibroblasts and human glioblastoma cells (A172) Mol. Biol. Cell. 1999;10:1093–1104. doi: 10.1091/mbc.10.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aktas O., Prozorovski T., Smorodchenko A., Savaskan N.E., Lauster R., Kloetzel P.M., Infante-Duarte C., Brocke S., Zipp F. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J. Immunol. 2004;173:5794–5800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez E., Campos-Toimil M., Justiniano-Basaran H., Lugnier C., Orallo F. Study of the mechanisms involved in the vasorelaxation induced by (-)-epigallocatechin-3-gallate in rat aorta. Br. J. Pharmacol. 2006;147:269–280. doi: 10.1038/sj.bjp.0706507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aneja R., Hake P.W., Burroughs T.J., Denenberg A.G., Wong H.R., Zingarelli B. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol. Med. 2004;10:55–62. doi: 10.2119/2004-00032.aneja. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babich H., Zuckerbraun H.L., Weinerman S.M. In vitro cytotoxicity of (-)-catechin gallate, a minor polyphenol in green tea. Toxicol. Lett. 2007;171:171–180. doi: 10.1016/j.toxlet.2007.05.125. [DOI] [PubMed] [Google Scholar]
- 6.Bae Y.S., Lee J.H., Choi S.H., Kim S., Almazan F., Witztum J.L., Miller Y.I. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ. Res. 2009;104(210–218):221. doi: 10.1161/CIRCRESAHA.108.181040. (following 218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu A., Sanchez K., Leyva M.J., Wu M., Betts N.M., Aston C.E., Lyons T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010;29:31–40. doi: 10.1080/07315724.2010.10719814. [DOI] [PubMed] [Google Scholar]
- 8.Bose M., Hao X., Ju J., Husain A., Park S., Lambert J.D., Yang C.S. Inhibition of tumorigenesis in ApcMin/+mice by a combination of (-)-epigallocatechin-3-gallate and fish oil. J. Agric. Food Chem. 2007;55:7695–7700. doi: 10.1021/jf071004r. [DOI] [PubMed] [Google Scholar]
- 9.Chen A., Zhang L. The antioxidant (-)-epigallocatechin-3-gallate inhibits rat hepatic stellate cell proliferation in vitro by blocking the tyrosine phosphorylation and reducing the gene expression of platelet-derived growth factor-beta receptor. J. Biol. Chem. 2003;278:23381–23389. doi: 10.1074/jbc.M212042200. [DOI] [PubMed] [Google Scholar]
- 10.Chen D., Pamu S., Cui Q., Chan T.H., Dou Q.P. Novel epigallocatechin gallate (EGCG) analogs activate AMP-activated protein kinase pathway and target cancer stem cells. Bioorg. Med. Chem. 2012;20:3031–3037. doi: 10.1016/j.bmc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L., Xin X., Yuan Q., Su D., Liu W. Phytochemical properties and antioxidant capacities of various colored berries. J. Sci. Food Agric. 2013 doi: 10.1002/jsfa.6216. [DOI] [PubMed] [Google Scholar]
- 12.Chen N., Bezzina R., Hinch E., Lewandowski P.A., Cameron-Smith D., Mathai M.L., Jois M., Sinclair A.J., Begg D.P., Wark J.D., Weisinger H.S., Weisinger R.S. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr. Res. 2009;29:784–793. doi: 10.1016/j.nutres.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Chou C.W., Huang W.J., Tien L.T., Wang S.J. (-)-Epigallocatechin gallate, the most active polyphenolic catechin in green tea, presynaptically facilitates Ca2+-dependent glutamate release via activation of protein kinase C in rat cerebral cortex. Synapse. 2007;61:889–902. doi: 10.1002/syn.20444. [DOI] [PubMed] [Google Scholar]
- 14.Chyu K.Y., Babbidge S.M., Zhao X., Dandillaya R., Rietveld A.G., Yano J., Dimayuga P., Cercek B., Shah P.K. Differential effects of green tea-derived catechin on developing versus established atherosclerosis in apolipoprotein E-null mice. Circulation. 2004;109:2448–2453. doi: 10.1161/01.CIR.0000128034.70732.C2. [DOI] [PubMed] [Google Scholar]
- 15.Collins Q.F., Liu H.Y., Pi J., Liu Z., Quon M.J., Cao W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5'-AMP-activated protein kinase. J. Biol. Chem. 2007;282:30143–30149. doi: 10.1074/jbc.M702390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corcoran M.P., McKay D.L., Blumberg J.B. Flavonoid basics: chemistry, sources, mechanisms of action, and safety. J. Nutr. Gerontol. Geriatr. 2012;31:176–189. doi: 10.1080/21551197.2012.698219. [DOI] [PubMed] [Google Scholar]
- 17.Cuervo A.M. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14:70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Cuervo A.M., Bergamini E., Brunk U.T., Droge W., Ffrench M., Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 19.de Boer V.C., de Goffau M.C., Arts I.C., Hollman P.C., Keijer J. SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech. Ageing Dev. 2006;127:618–627. doi: 10.1016/j.mad.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Devika P.T., Stanely Mainzen Prince P. (-)Epigallocatechin-gallate (EGCG) prevents mitochondrial damage in isoproterenol-induced cardiac toxicity in albino Wistar rats: a transmission electron microscopic and in vitro study. Pharmacol. Res.: Off. J. Ital. Pharmacol Soc. 2008;57:351–357. doi: 10.1016/j.phrs.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Dong Z., Ma W., Huang C., Yang C.S. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (-)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997;57:4414–4419. [PubMed] [Google Scholar]
- 22.Elbling L., Herbacek I., Weiss R.M., Jantschitsch C., Micksche M., Gerner C., Pangratz H., Grusch M., Knasmuller S., Berger W. Hydrogen peroxide mediates EGCG-induced antioxidant protection in human keratinocytes. Free Radic. Biol. Med. 2010;49:1444–1452. doi: 10.1016/j.freeradbiomed.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Elbling L., Weiss R.M., Teufelhofer O., Uhl M., Knasmueller S., Schulte-Hermann R., Berger W., Micksche M. Green tea extract and (-)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol. 2005;19:807–809. doi: 10.1096/fj.04-2915fje. [DOI] [PubMed] [Google Scholar]
- 24.Ermakova S., Choi B.Y., Choi H.S., Kang B.S., Bode A.M., Dong Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J. Biol. Chem. 2005;280:16882–16890. doi: 10.1074/jbc.M414185200. [DOI] [PubMed] [Google Scholar]
- 25.Fang M., Chen D., Yang C.S. Dietary polyphenols may affect DNA methylation. J. Nutr. 2007;137:223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- 26.Fang M.Z., Wang Y., Ai N., Hou Z., Sun Y., Lu H., Welsh W., Yang C.S. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–7570. [PubMed] [Google Scholar]
- 27.Feng W., Cherednichenko G., Ward C.W., Padilla I.T., Cabrales E., Lopez J.R., Eltit J.M., Allen P.D., Pessah I.N. Green tea catechins are potent sensitizers of ryanodine receptor type 1 (RyR1) Biochem. Pharmacol. 2010;80:512–521. doi: 10.1016/j.bcp.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimura Y., Sumida M., Sugihara K., Tsukamoto S., Yamada K., Tachibana H. Green tea polyphenol EGCG sensing motif on the 67-kDa laminin receptor. PloS One. 2012;7:e37942. doi: 10.1371/journal.pone.0037942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fung S.T., Ho C.K., Choi S.W., Chung W.Y., Benzie I.F. Comparison of catechin profiles in human plasma and urine after single dosing and regular intake of green tea (Camellia sinensis) Br. J. Nutr. 2013;109:2199–2207. doi: 10.1017/S0007114512004370. [DOI] [PubMed] [Google Scholar]
- 30.Giunta B., Hou H., Zhu Y., Salemi J., Ruscin A., Shytle R.D., Tan J. Fish oil enhances anti-amyloidogenic properties of green tea EGCG in Tg2576 mice. Neurosci. Lett. 2010;471:134–138. doi: 10.1016/j.neulet.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto K., Sakagami H. Induction of apoptosis by epigallocatechin gallate and autophagy inhibitors in a mouse macrophage-like cell line. Anticancer Res. 2008;28:1713–1718. [PubMed] [Google Scholar]
- 32.Hellermann G.R., Solomonson L.P. Calmodulin promotes dimerization of the oxygenase domain of human endothelial nitric-oxide synthase. J. Biol. Chem. 1997;272:12030–12034. doi: 10.1074/jbc.272.18.12030. [DOI] [PubMed] [Google Scholar]
- 33.Hong Byun E., Fujimura Y., Yamada K., Tachibana H. TLR4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin-3-gallate through 67-kDa laminin receptor. J. Immunol. 2010;185:33–45. doi: 10.4049/jimmunol.0903742. [DOI] [PubMed] [Google Scholar]
- 34.Hong J., Lu H., Meng X., Ryu J.H., Hara Y., Yang C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–7246. [PubMed] [Google Scholar]
- 35.Hotta Y., Huang L., Muto T., Yajima M., Miyazeki K., Ishikawa N., Fukuzawa Y., Wakida Y., Tushima H., Ando H., Nonogaki T. Positive inotropic effect of purified green tea catechin derivative in guinea pig hearts: the measurements of cellular Ca2+ and nitric oxide release. Eur. J. Pharmacol. 2006;552:123–130. doi: 10.1016/j.ejphar.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Hou Z., Sang S., You H., Lee M.J., Hong J., Chin K.V., Yang C.S. Mechanism of action of (-)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- 37.Hsu C.H., Liao Y.L., Lin S.C., Tsai T.H., Huang C.J., Chou P. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. Altern. Med. Rev.: J. Clin. Ther. 2011;16:157–163. [PubMed] [Google Scholar]
- 38.Hwang J.T., Ha J., Park I.J., Lee S.K., Baik H.W., Kim Y.M., Park O.J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007;247:115–121. doi: 10.1016/j.canlet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda I. Multifunctional effects of green tea catechins on prevention of the metabolic syndrome. Asia Pac. J. Clin. Nutr. 2008;17(Suppl 1):273–274. [PubMed] [Google Scholar]
- 40.Ikeda I., Tsuda K., Suzuki Y., Kobayashi M., Unno T., Tomoyori H., Goto H., Kawata Y., Imaizumi K., Nozawa A., Kakuda T. Tea catechins with a galloyl moiety suppress postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats. J. Nutr. 2005;135:155–159. doi: 10.1093/jn/135.2.155. [DOI] [PubMed] [Google Scholar]
- 41.Jang H.J., Ridgeway S.D., Kim J.A. Effects of the green tea polyphenol, epigallocatechin-3-gallate (EGCG), on high fat diet-induced insulin resistance and endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2013;305:E1444–1451. doi: 10.1152/ajpendo.00434.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jimenez-Lopez J.M., Cederbaum A.I. Green tea polyphenol epigallocatechin-3-gallate protects HepG2 cells against CYP2E1-dependent toxicity. Free Radic. Biol. Med. 2004;36:359–370. doi: 10.1016/j.freeradbiomed.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 43.Kargacin M.E., Emmett T.L., Kargacin G.J. Epigallocatechin-3-gallate has dual, independent effects on the cardiac sarcoplasmic reticulum/endoplasmic reticulum Ca2+ ATPase. J. Muscle Res. Cell Motil. 2011;32:89–98. doi: 10.1007/s10974-011-9256-7. [DOI] [PubMed] [Google Scholar]
- 44.Katiyar S.K., Afaq F., Perez A., Mukhtar H. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001;22:287–294. doi: 10.1093/carcin/22.2.287. [DOI] [PubMed] [Google Scholar]
- 45.Kim H., Hiraishi A., Tsuchiya K., Sakamoto K. (-)Epigallocatechin gallate suppresses the differentiation of 3T3-L1 preadipocytes through transcription factors FoxO1 and SREBP1c. Cytotechnology. 2010;62:245–255. doi: 10.1007/s10616-010-9285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H., Sakamoto K. (-)-Epigallocatechin gallate suppresses adipocyte differentiation through the MEK/ERK and PI3K/Akt pathways. Cell Biol. Int. 2012;36:147–153. doi: 10.1042/CBI20110047. [DOI] [PubMed] [Google Scholar]
- 47.Kim H.S., Montana V., Jang H.J., Parpura V., Kim J.A. Epigallocatechin gallate (EGCG) stimulates autophagy in vascular endothelial cells: a potential role for reducing lipid accumulation. J. Biol. Chem. 2013;288:22693–22705. doi: 10.1074/jbc.M113.477505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J.A. Mechanisms underlying beneficial health effects of tea catechins to improve insulin resistance and endothelial dysfunction. Endocr. Metab. Immune Disord. Drug Targets. 2008;8:82–88. doi: 10.2174/187153008784534349. [DOI] [PubMed] [Google Scholar]
- 49.Kim J.A., Formoso G., Li Y., Potenza M.A., Marasciulo F.L., Montagnani M., Quon M.J. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J. Biol. Chem. 2007;282:13736–13745. doi: 10.1074/jbc.M609725200. [DOI] [PubMed] [Google Scholar]
- 50.Kim S.Y., Ahn B.H., Kim J., Bae Y.S., Kwak J.Y., Min G., Kwon T.K., Chang J.S., Lee Y.H., Yoon S.H., Min D.S. Phospholipase C, protein kinase C, Ca2+/calmodulin-dependent protein kinase II, and redox state are involved in epigallocatechin gallate-induced phospholipase D activation in human astroglioma cells. Eur. J. Biochem. 2004;271:3470–3480. doi: 10.1111/j.0014-2956.2004.04242.x. [DOI] [PubMed] [Google Scholar]
- 51.Kishimoto Y., Tani M., Kondo K. Pleiotropic preventive effects of dietary polyphenols in cardiovascular diseases. Eur. J. Clin. Nutr. 2013;67:532–535. doi: 10.1038/ejcn.2013.29. [DOI] [PubMed] [Google Scholar]
- 52.Klaus S., Pultz S., Thone-Reineke C., Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. 2005;29:615–623. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]
- 53.Kondo T., Ohta T., Igura K., Hara Y., Kaji K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002;180:139–144. doi: 10.1016/s0304-3835(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 54.Kumazoe M., Kim Y., Bae J., Takai M., Murata M., Suemasu Y., Sugihara K., Yamashita S., Tsukamoto S., Huang Y., Nakahara K., Yamada K., Tachibana H. Phosphodiesterase 5 inhibitor acts as a potent agent sensitizing acute myeloid leukemia cells to 67-kDa laminin receptor-dependent apoptosis. FEBS Lett. 2013;587:3052–3057. doi: 10.1016/j.febslet.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 55.Kumazoe M., Sugihara K., Tsukamoto S., Huang Y., Tsurudome Y., Suzuki T., Suemasu Y., Ueda N., Yamashita S., Kim Y., Yamada K., Tachibana H. 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. J. Clin. Invest. 2013;123:787–799. doi: 10.1172/JCI64768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuriyama S., Shimazu T., Ohmori K., Kikuchi N., Nakaya N., Nishino Y., Tsubono Y., Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA: J. Am. Med. Assoc. 2006;296:1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 57.Lambert J.D., Lee M.J., Diamond L., Ju J., Hong J., Bose M., Newmark H.L., Yang C.S. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab. Dispos.: Biol. Fate Chem. 2006;34:8–11. doi: 10.1124/dmd.104.003434. [DOI] [PubMed] [Google Scholar]
- 58.Lee H., Bae S., Yoon Y. The anti-adipogenic effects of (-)epigallocatechin gallate are dependent on the WNT/beta-catenin pathway. J. Nutr. Biochem. 2013;24:1232–1240. doi: 10.1016/j.jnutbio.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Lee I.T., Lin C.C., Lee C.Y., Hsieh P.W., Yang C.M. Protective effects of (-)-epigallocatechin-3-gallate against TNF-alpha-induced lung inflammation via ROS-dependent ICAM-1 inhibition. J. Nutr. Biochem. 2013;24:124–136. doi: 10.1016/j.jnutbio.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Lee M.J., Maliakal P., Chen L., Meng X., Bondoc F.Y., Prabhu S., Lambert G., Mohr S., Yang C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol., Biomark. Prev.: Publ. Am. Assoc. Cancer Res., Cospons. Am. Soc. Prev. Oncol. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 61.Lee M.J., Wang Z.Y., Li H., Chen L., Sun Y., Gobbo S., Balentine D.A., Yang C.S. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol., Biomark. Prev.: Publ. Am. Assoc. Cancer Res., Cospons. Am. Soc. Prev. Oncol. 1995;4:393–399. [PubMed] [Google Scholar]
- 62.Lee M.S., Kim C.T., Kim Y. Green tea (-)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. Ann. Nutr. Metab. 2009;54:151–157. doi: 10.1159/000214834. [DOI] [PubMed] [Google Scholar]
- 63.Li C.P., Yao J., Tao Z.F., Li X.M., Jiang Q., Yan B. Epigallocatechin-gallate (EGCG) regulates autophagy in human retinal pigment epithelial cells: a potential role for reducing UVB light-induced retinal damage. Biochem. Biophys. Res. Commun. 2013;438:739–745. doi: 10.1016/j.bbrc.2013.07.097. [DOI] [PubMed] [Google Scholar]
- 64.Li G.X., Chen Y.K., Hou Z., Xiao H., Jin H., Lu G., Lee M.J., Liu B., Guan F., Yang Z., Yu A., Yang C.S. Pro-oxidative activities and dose-response relationship of (-)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: a comparative study in vivo and in vitro. Carcinogenesis. 2010;31:902–910. doi: 10.1093/carcin/bgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W., Zhu S., Li J., Assa A., Jundoria A., Xu J., Fan S., Eissa N.T., Tracey K.J., Sama A.E., Wang H. EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochem. Pharmacol. 2011;81:1152–1163. doi: 10.1016/j.bcp.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y., Zhao S., Zhang W., Zhao P., He B., Wu N., Han P. Epigallocatechin-3-O-gallate (EGCG) attenuates FFAs-induced peripheral insulin resistance through AMPK pathway and insulin signaling pathway in vivo. Diabetes Res. Clin. Pract. 2011;93:205–214. doi: 10.1016/j.diabres.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 67.Liang Y.C., Lin-shiau S.Y., Chen C.F., Lin J.K. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (-)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J. Cell. Biochem. 1997;67:55–65. doi: 10.1002/(sici)1097-4644(19971001)67:1<55::aid-jcb6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 68.Lorenz M., Wessler S., Follmann E., Michaelis W., Dusterhoft T., Baumann G., Stangl K., Stangl V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J. Biol. Chem. 2004;279:6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 69.Massey A.C., Kaushik S., Cuervo A.M. Lysosomal chat maintains the balance. Autophagy. 2006;2:325–327. doi: 10.4161/auto.3090. [DOI] [PubMed] [Google Scholar]
- 70.Matsuo N., Yamada K., Yamashita K., Shoji K., Mori M., Sugano M. Inhibitory effect of tea polyphenols on histamine and leukotriene B4 release from rat peritoneal exudate cells. In vitro Cell. Dev. Biol. Anim. 1996;32:340–344. doi: 10.1007/BF02722960. [DOI] [PubMed] [Google Scholar]
- 71.Menard S., Castronovo V., Tagliabue E., Sobel M.E. New insights into the metastasis-associated 67 kD laminin receptor. J. Cell. Biochem. 1997;67:155–165. [PubMed] [Google Scholar]
- 72.Meng Q., Velalar C.N., Ruan R. Regulating the age-related oxidative damage, mitochondrial integrity, and antioxidative enzyme activity in Fischer 344 rats by supplementation of the antioxidant epigallocatechin-3-gallate. Rejuvenation Res. 2008;11:649–660. doi: 10.1089/rej.2007.0645. [DOI] [PubMed] [Google Scholar]
- 73.Michel J.B., Feron O., Sacks D., Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J. Biol. Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 74.Moon H.S., Chung C.S., Lee H.G., Kim T.G., Choi Y.J., Cho C.S. Inhibitory effect of (-)-epigallocatechin-3-gallate on lipid accumulation of 3T3-L1 cells. Obesity. 2007;15:2571–2582. doi: 10.1038/oby.2007.309. [DOI] [PubMed] [Google Scholar]
- 75.Murase T., Misawa K., Haramizu S., Hase T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem. Pharmacol. 2009;78:78–84. doi: 10.1016/j.bcp.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 76.Mythri R.B., Bharath M.M. Curcumin: a potential neuroprotective agent in Parkinson's disease. Curr. Pharm. Des. 2012;18:91–99. doi: 10.2174/138161212798918995. [DOI] [PubMed] [Google Scholar]
- 77.Nakagawa H., Hasumi K., Woo J.T., Nagai K., Wachi M. Generation of hydrogen peroxide primarily contributes to the induction of Fe(II)-dependent apoptosis in Jurkat cells by (-)-epigallocatechin gallate. Carcinogenesis. 2004;25:1567–1574. doi: 10.1093/carcin/bgh168. [DOI] [PubMed] [Google Scholar]
- 78.Nakagawa H., Wachi M., Woo J.T., Kato M., Kasai S., Takahashi F., Lee I.S., Nagai K. Fenton reaction is primarily involved in a mechanism of (-)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem. Biophys. Res. Commun. 2002;292:94–101. doi: 10.1006/bbrc.2002.6622. [DOI] [PubMed] [Google Scholar]
- 79.O’Neill L.A., Hardie D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 80.Ogg S., Paradis S., Gottlieb S., Patterson G.I., Lee L., Tissenbaum H.A., Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 81.Oh C.J., Yang E.S., Shin S.W., Choi S.H., Park C.I., Yang C.H., Park J.W. Epigallocatechin gallate, a constituent of green tea, regulates high glucose-induced apoptosis. Arch. Pharm. Res. 2008;31:34–40. doi: 10.1007/s12272-008-1117-6. [DOI] [PubMed] [Google Scholar]
- 82.Ok W.J., Cho H.J., Kim H.H., Lee D.H., Kang H.Y., Kwon H.W., Rhee M.H., Kim M., Park H.J. Epigallocatechin-3-gallate has an anti-platelet effect in a cyclic AMP-dependent manner. J. Atheroscler. Thromb. 2012;19:337–348. doi: 10.5551/jat.10363. [DOI] [PubMed] [Google Scholar]
- 83.Orsolic N., Sirovina D., Gajski G., Garaj-Vrhovac V., Jembrek M.J., Kosalec I. Assessment of DNA damage and lipid peroxidation in diabetic mice: effects of propolis and epigallocatechin gallate (EGCG) Mutat. Res. 2013 doi: 10.1016/j.mrgentox.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 84.Pallauf K., Rimbach G. Autophagy, polyphenols and healthy ageing. Ageing Res. Rev. 2013;12:237–252. doi: 10.1016/j.arr.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 85.Park I.J., Lee Y.K., Hwang J.T., Kwon D.Y., Ha J., Park O.J. Green tea catechin controls apoptosis in colon cancer cells by attenuation of H2O2-stimulated COX-2 expression via the AMPK signaling pathway at low-dose H2O2. Ann. N.Y. Acad. Sci. 2009;1171:538–544. doi: 10.1111/j.1749-6632.2009.04698.x. [DOI] [PubMed] [Google Scholar]
- 86.Picchi A., Gao X., Belmadani S., Potter B.J., Focardi M., Chilian W.M., Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ. Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 87.Potenza M.A., Marasciulo F.L., Tarquinio M., Tiravanti E., Colantuono G., Federici A., Kim J.A., Quon M.J., Montagnani M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1378–1387. doi: 10.1152/ajpendo.00698.2006. [DOI] [PubMed] [Google Scholar]
- 88.Pullikotil P., Chen H., Muniyappa R., Greenberg C.C., Yang S., Reiter C.E., Lee J.W., Chung J.H., Quon M.J. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-alpha. J. Nutr. Biochem. 2012;23:1134–1145. doi: 10.1016/j.jnutbio.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qanungo S., Das M., Haldar S., Basu A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis. 2005;26:958–967. doi: 10.1093/carcin/bgi040. [DOI] [PubMed] [Google Scholar]
- 90.Reiter C.E., Kim J.A., Quon M.J. Green tea polyphenol epigallocatechin gallate reduces endothelin-1 expression and secretion in vascular endothelial cells: roles for AMP-activated protein kinase, Akt, and FOXO1. Endocrinology. 2010;151:103–114. doi: 10.1210/en.2009-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ren F., Zhang S., Mitchell S.H., Butler R., Young C.Y. Tea polyphenols down-regulate the expression of the androgen receptor in LNCaP prostate cancer cells. Oncogene. 2000;19:1924–1932. doi: 10.1038/sj.onc.1203511. [DOI] [PubMed] [Google Scholar]
- 92.Rizvi S.I., Zaid M.A. Impairment of sodium pump and Na/H exchanger in erythrocytes from non-insulin dependent diabetes mellitus patients: effect of tea catechins. Clin. Chim. Acta. 2005;354:59–67. doi: 10.1016/j.cccn.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 93.Rodriguez S.K., Guo W., Liu L., Band M.A., Paulson E.K., Meydani M. Green tea catechin, epigallocatechin-3-gallate, inhibits vascular endothelial growth factor angiogenic signaling by disrupting the formation of a receptor complex. Int. J. Cancer. 2006;118:1635–1644. doi: 10.1002/ijc.21545. [DOI] [PubMed] [Google Scholar]
- 94.Ruderman N.B., Carling D., Prentki M., Cacicedo J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Invest. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sachinidis A., Skach R.A., Seul C., Ko Y., Hescheler J., Ahn H.Y., Fingerle J. Inhibition of the PDGF beta-receptor tyrosine phosphorylation and its downstream intracellular signal transduction pathway in rat and human vascular smooth muscle cells by different catechins. FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol. 2002;16:893–895. doi: 10.1096/fj.01-0799fje. [DOI] [PubMed] [Google Scholar]
- 96.Sah J.F., Balasubramanian S., Eckert R.L., Rorke E.A. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway. Evidence for direct inhibition of ERK1/2 and AKT kinases. J. Biol. Chem. 2004;279:12755–12762. doi: 10.1074/jbc.M312333200. [DOI] [PubMed] [Google Scholar]
- 97.Sakagami H., Arakawa H., Maeda M., Satoh K., Kadofuku T., Fukuchi K., Gomi K. Production of hydrogen peroxide and methionine sulfoxide by epigallocatechin gallate and antioxidants. Anticancer Res. 2001;21:2633–2641. [PubMed] [Google Scholar]
- 98.Sakurai N., Mochizuki K., Kameji H., Shimada M., Goda T. (-)-Epigallocatechin gallate enhances the expression of genes related to insulin sensitivity and adipocyte differentiation in 3T3-L1 adipocytes at an early stage of differentiation. Nutrition. 2009;25:1047–1056. doi: 10.1016/j.nut.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 99.Satoh M., Takemura Y., Hamada H., Sekido Y., Kubota S. EGCG induces human mesothelioma cell death by inducing reactive oxygen species and autophagy. Cancer Cell Int. 2013;13:19. doi: 10.1186/1475-2867-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schroeder E.K., Kelsey N.A., Doyle J., Breed E., Bouchard R.J., Loucks F.A., Harbison R.A., Linseman D.A. Green tea epigallocatechin 3-gallate accumulates in mitochondria and displays a selective antiapoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antioxid. Redox Signal. 2009;11:469–480. doi: 10.1089/ars.2008.2215. [DOI] [PubMed] [Google Scholar]
- 101.Shanafelt T.D., Call T.G., Zent C.S., LaPlant B., Bowen D.A., Roos M., Secreto C.R., Ghosh A.K., Kabat B.F., Lee M.J., Yang C.S., Jelinek D.F., Erlichman C., Kay N.E. Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2009;27:3808–3814. doi: 10.1200/JCO.2008.21.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shimizu M., Deguchi A., Hara Y., Moriwaki H., Weinstein I.B. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem. Biophys. Res. Commun. 2005;334:947–953. doi: 10.1016/j.bbrc.2005.06.182. [DOI] [PubMed] [Google Scholar]
- 103.Soler F., Asensio M.C., Fernandez-Belda F. Inhibition of the intracellular Ca(2+) transporter SERCA (Sarco-Endoplasmic Reticulum Ca(2+)-ATPase) by the natural polyphenol epigallocatechin-3-gallate. J. Bioenerg. Biomembr. 2012;44:597–605. doi: 10.1007/s10863-012-9462-z. [DOI] [PubMed] [Google Scholar]
- 104.Stephanou A. Role of STAT-1 and STAT-3 in ischaemia/reperfusion injury. J. Cell. Mol. Med. 2004;8:519–525. doi: 10.1111/j.1582-4934.2004.tb00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tian W.X. Inhibition of fatty acid synthase by polyphenols. Curr. Med. Chem. 2006;13:967–977. doi: 10.2174/092986706776361012. [DOI] [PubMed] [Google Scholar]
- 106.Tomas-Barberan F.A., Andres-Lacueva C. Polyphenols and health: current state and progress. J. Agric. Food Chem. 2012;60:8773–8775. doi: 10.1021/jf300671j. [DOI] [PubMed] [Google Scholar]
- 107.Toniolo A., Buccellati C., Pinna C., Gaion R.M., Sala A., Bolego C. Cyclooxygenase-1 and prostacyclin production by endothelial cells in the presence of mild oxidative stress. PloS one. 2013;8:e56683. doi: 10.1371/journal.pone.0056683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Towler M.C., Hardie D.G. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 109.Townsend P.A., Scarabelli T.M., Pasini E., Gitti G., Menegazzi M., Suzuki H., Knight R.A., Latchman D.S., Stephanou A. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol. 2004;18:1621–1623. doi: 10.1096/fj.04-1716fje. [DOI] [PubMed] [Google Scholar]
- 110.Valenti D., de Bari L., Manente G.A., Rossi L., Mutti L., Moro L., Vacca R.A. Negative modulation of mitochondrial oxidative phosphorylation by epigallocatechin-3 gallate leads to growth arrest and apoptosis in human malignant pleural mesothelioma cells. Biochim. Biophys. Acta. 1832;2085-2096:2013. doi: 10.1016/j.bbadis.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 111.Valenti D., De Rasmo D., Signorile A., Rossi L., de Bari L., Scala I., Granese B., Papa S., Vacca R.A. Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down's syndrome. Biochim. Biophys. Acta. 1832;542-552:2013. doi: 10.1016/j.bbadis.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 112.Van Amelsvoort J.M., Van Hof K.H., Mathot J.N., Mulder T.P., Wiersma A., Tijburg L.B. Plasma concentrations of individual tea catechins after a single oral dose in humans. Xenobiotica. 2001;31:891–901. doi: 10.1080/00498250110079149. [DOI] [PubMed] [Google Scholar]
- 113.Waltner-Law M.E., Wang X.L., Law B.K., Hall R.K., Nawano M., Granner D.K. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J. Biol. Chem. 2002;277:34933–34940. doi: 10.1074/jbc.M204672200. [DOI] [PubMed] [Google Scholar]
- 114.Weber A.A., Neuhaus T., Skach R.A., Hescheler J., Ahn H.Y., Schror K., Ko Y., Sachinidis A. Mechanisms of the inhibitory effects of epigallocatechin-3 gallate on platelet-derived growth factor-BB-induced cell signaling and mitogenesis. FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol. 2004;18:128–130. doi: 10.1096/fj.03-0007fje. [DOI] [PubMed] [Google Scholar]
- 115.Wu C.C., Hsu M.C., Hsieh C.W., Lin J.B., Lai P.H., Wung B.S. Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006;78:2889–2897. doi: 10.1016/j.lfs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 116.Wu H., Qi H., Iwasaki D., Zhu B., Shimoishi Y., Murata Y., Nakamura Y. JNK-dependent NFATc1 pathway positively regulates IL-13 gene expression induced by (-)-epigallocatechin-3-gallate in human basophilic KU812 cells. Free Radic. Biol. Med. 2009;47:1028–1038. doi: 10.1016/j.freeradbiomed.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 117.Yamamoto T., Lewis J., Wataha J., Dickinson D., Singh B., Bollag W.B., Ueta E., Osaki T., Athar M., Schuster G., Hsu S. Roles of catalase and hydrogen peroxide in green tea polyphenol-induced chemopreventive effects. J. Pharmacol. Exp. Ther. 2004;308:317–323. doi: 10.1124/jpet.103.058891. [DOI] [PubMed] [Google Scholar]
- 118.Yan J., Zhao Y., Suo S., Liu Y., Zhao B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radic. Biol. Med. 2012;52:1648–1657. doi: 10.1016/j.freeradbiomed.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 119.Yang C.S., Wang H. Cancer therapy combination: green tea and a phosphodiesterase 5 inhibitor? J. Clin. Invest. 2013;123:556–558. doi: 10.1172/JCI67589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang F., de Villiers W.J., McClain C.J., Varilek G.W. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J. Nutr. 1998;128:2334–2340. doi: 10.1093/jn/128.12.2334. [DOI] [PubMed] [Google Scholar]
- 121.Yang G.Y., Liao J., Li C., Chung J., Yurkow E.J., Ho C.T., Yang C.S. Effect of black and green tea polyphenols on c-jun phosphorylation and H(2)O(2) production in transformed and non-transformed human bronchial cell lines: possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis. 2000;21:2035–2039. doi: 10.1093/carcin/21.11.2035. [DOI] [PubMed] [Google Scholar]
- 122.Yu W., Fu Y.C., Wang W. Cellular and molecular effects of resveratrol in health and disease. J. Cell. Biochem. 2012;113:752–759. doi: 10.1002/jcb.23431. [DOI] [PubMed] [Google Scholar]
- 123.Zgheib A., Lamy S., Annabi B. Epigallocatechin gallate targeting of membrane type 1 matrix metalloproteinase-mediated Src and Janus kinase/signal transducers and activators of transcription 3 signaling inhibits transcription of colony-stimulating factors 2 and 3 in mesenchymal stromal cells. J. Biol. Chem. 2013;288:13378–13386. doi: 10.1074/jbc.M113.456533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y., Yang N.D., Zhou F., Shen T., Duan T., Zhou J., Shi Y., Zhu X.Q., Shen H.M. (-)-Epigallocatechin-3-gallate induces non-apoptotic cell death in human cancer cells via ROS-mediated lysosomal membrane permeabilization. PloS one. 2012;7:e46749. doi: 10.1371/journal.pone.0046749. [DOI] [PMC free article] [PubMed] [Google Scholar]


