Abstract
The assessment of metabolic function in cells isolated from human blood for treatment and diagnosis of disease is a new and important area of translational research. It is now becoming clear that a broad range of pathologies which present clinically with symptoms predominantly in one organ, such as the brain or kidney, also modulate mitochondrial energetics in platelets and leukocytes allowing these cells to serve as “the canary in the coal mine” for bioenergetic dysfunction. This opens up the possibility that circulating platelets and leukocytes can sense metabolic stress in patients and serve as biomarkers of mitochondrial dysfunction in human pathologies such as diabetes, neurodegeneration and cardiovascular disease. In this overview we will describe how the utilization of glycolysis and oxidative phosphorylation differs in platelets and leukocytes and discuss how they can be used in patient populations. Since it is clear that the metabolic programs between leukocytes and platelets are fundamentally distinct the measurement of mitochondrial function in distinct cell populations is necessary for translational research.
Abbreviations: ROS/RNS, reactive oxygen species/reactive nitrogen species; OCR, oxygen consumption rate; ECAR, extracellular acidification rate; XF, extracellular flux analyzer
Keywords: Reserve capacity, Oxidative stress, Metabolic shift, Biomarker, Leukocytes, Platelets
Graphical abstract
Highlights
-
•
Monocytes, lymphocytes, neutrophils and platelets have distinct bioenergetic programs that regulate energy production.
-
•
Platelets and monocytes exhibit a high level of aerobic glycolysis and mitochondrial respiration.
-
•
Lymphocytes have a low glycolytic capacity while neutrophils have little or no detectable oxidative phosphorylation.
-
•
The levels of mitochondrial complex IV and III subunits differ substantially between lymphocytes, monocytes and platelets.
Introduction
Circulating leukocytes and platelets are programmed for distinct roles in normal physiology which include mediating the inflammatory process, thrombosis, clearance of foreign bodies and sensing and responding to systemic biological signals in the circulation. The dynamic functions of peripheral blood leukocytes and platelets require an integrated metabolic machinery to meet energetic demand during normal physiology which is likely to involve both glycolysis and mitochondrial oxidative phosphorylation. The role of both these important ATP generating pathways in supporting the biological function of platelets and leukocytes has been recognized but these findings have not been integrated into an overall understanding of these cell types in human subjects. This review analyzes the similarities and differences in the glycolytic and oxidative metabolic profiles in leukocytes and platelets from human subjects and discusses the implications of these findings for the utilization of these cell types for translational research.
Biological functions and metabolic programs of platelets and leukocytes
The myeloid lineage supports the greatest variety of differentiated circulating cells which include erythrocytes, platelets, neutrophils, and monocytes. Monocytes are phagocytic cells with a uni-lobular nucleus that have an important role in the innate immune system [1], [2], [3]. Once secreted from the bone marrow into the blood, these cells survey the body for sites of inflammation. On encountering inflammatory stress signals the monocytes must rapidly activate and migrate to areas of injury where they can differentiate into the pro-inflammatory (M1) or anti-inflammatory (M2) phenotype [3]. In the M1 state the activated monocyte–macrophage cell undergoes a metabolic switch from oxidative phosphorylation to glycolysis [4]. This change is important to provide substrates for biosynthetic programs, maintain mitochondrial membrane potential and also provide ATP to the cell [5]. Inhibition of oxidative phosphorylation also increases reactive oxygen species (ROS) production which exerts bactericidal activities [5]. During the resolution of inflammation, the macrophages transform into the alternatively activated M2 phenotype and a more oxidative phosphorylation phenotype [6]. Thus the metabolic programs of monocyte/macrophage populations are highly plastic and adapt to facilitate the changing function of these cells in the inflammatory process. Whether early changes in metabolic phenotype associated with exposure to pro-inflammatory conditions can be detected in the pre-differentiated monocyte in the circulation is not clear. Typically, differentiation of the M1/M2 macrophages occurs at the site of inflammation not in the circulation. From the translational perspective the pre-differentiated monocyte is the dominant form in the circulation. Monocytes are then a potentially good sensor of metabolic stressors such as hyperlipidemia or hyperglycemia in the circulation of patients.
Lymphocytes are derived from the lymphoid lineage and are uni-nucleated cells that play an important role in adaptive immunity [7]. This heterogeneous population of cells is normally in a quiescent state and primarily uses mitochondria to meet their energetic demands [8]. Activation of lymphocytes is associated with a switch to a metabolic phenotype with an increase in both glycolytic function and mitochondrial oxygen consumption [9]. This is essential for their diverse immunological functions, which includes clonal expansion and the production of cytokines and antibodies [10], [11], [12], [13]. From a translational perspective, the abundance, heterogeneity, and reactivity of these cells make them ideal for investigating the relationship of bioenergetics with the disease processes associated with inflammation.
Neutrophils serve an essential function in the innate immune system and are the first line of defense during bacterial infection. Neutrophils eliminate and destroy microorganisms by phagocytosis, generation of ROS, the extrusion of genomic DNA as Neutrophil Extracellular Traps (NETs), and by the release of cytotoxic granules [14], [15]. Neutrophils have very few mitochondria which do not play a role in energy metabolism, but maintain their mitochondrial membrane potential for apoptotic signaling [16], [17], [18]. The energy required for neutrophil chemotaxis and activity is derived from glycolysis [19]. The translational applications of the oxidative burst in neutrophils have been well studied, but less is known regarding the regulatory role of glycolysis under normal and pathological conditions in these cells [20].
Platelets are cytoplasmic fragments that are released by megakaryocytes in the bone marrow and stored in the spleen. These anuclear cells play an important role in hemostasis and are essential for thrombus formation at sites of injury. With a lifetime of 5–7 days in the circulation and no nucleus their metabolic program must be stable over this time period and be available for the energy requiring processes engaged when they are activated. At a basal state both oxidative phosphorylation and glycolysis play a role in energy production in platelets [21], [22]. Platelet aggregation results in an increase in glycolytic metabolism but it has been shown that a robust oxidative phosphorylation system is required to enable optimal levels of platelet functionality [23]. Platelets have been used widely in translational research in a broad range of pathological conditions including neurological disorders and diabetes [24]. In the next section we will demonstrate how the basal cellular bioenergetics are different between these cell types and the implications these findings have for translational research which use these cells as sensors of pathological changes in mitochondrial dysfunction.
Leukocytes and platelets as systemic biomarkers of metabolic stress
Many chronic pathological conditions such as metabolic syndrome, cancer and atherosclerosis are associated with an inflammatory response with the release of proinflammatory mediators particularly the cytokines. Leukocytes and platelets respond to these pro-inflammatory mediators in the systemic circulation through an activation process which changes the cellular phenotype as discussed in the previous section. Several investigators have tested the concept that leukocytes and platelets can act as biomarkers of mitochondrial dysfunction associated with several diseases including diabetes, neurodegenerative diseases, atherosclerosis and cancer [24], [25], [26], [27], [28]. For example, patients with septic shock demonstrated a strong association between decreased mitochondrial function, specifically loss of ATP synthase activity, in peripheral blood mononuclear cells and increased mortality [25]. It has also been shown that platelets from patients with type 2 diabetes have lower mitochondrial membrane potential and higher ATP content compared to controls [29]. A study of mononuclear cells in type 2 diabetes showed that the mitochondrial mass was decreased and that the mitochondria were hyperpolarized [30]. Mitochondrial complex I activity was found to be decreased in aged platelets [31] and those obtained from patients with Alzheimer's disease had higher mitochondrial membrane potential than controls [32]. Furthermore, platelets derived from normal individuals with a maternal history of Alzheimer's had lower cytochrome c oxidase activity [33]. It has been reported that leukocytes from patients with leukemia have higher numbers of circular dimer mitochondrial DNA compared to healthy controls, suggesting that leukocyte mitochondrial function is also important in cancer [34]. Mitochondria isolated from mononuclear cells in patients with fibromyalgia exhibited lower membrane potential and levels of coenzyme Q10 but increased superoxide production [35].
New approaches to measuring cellular bioenergetics in leukocytes and platelets
Development of sensitive assays using an extracellular flux analyzer (XF) has advanced the translational application of bioenergetics by making it possible to determine mitochondrial function in leukocytes and platelets isolated from peripheral blood [22], [36]. The XF analyzer measures oxygen consumption rate which can be ascribed to mitochondrial respiration as well as the change in pH which can be related to glycolysis [37], [38], [39]. Assays using inhibitors of mitochondrial respiratory complexes and glycolysis have developed sensitive protocols for the determination of cellular bioenergetics in leukocytes and platelets [22], [24], [27].
Cellular mitochondrial physiology and glycolysis in platelets and leukocytes
In a recent study we characterized the bioenergetic profiles in human platelets, monocytes, leukocytes and neutrophils to determine how they selectively utilize glycolysis and oxidative phosphorylation [22]. Here we will use data obtained from the measurement of oxygen consumption and extracellular acidification after the sequential addition of mitochondrial inhibitors using the methods described previously [22]. In Fig. 1A we show the relative proportion of maximal mitochondrial oxygen consumption dedicated to the major bioenergetic functions in monocytes, lymphocytes, and platelets. Platelets dedicate over 50% of their mitochondrial function to ATP synthesis which is approximately double that used by monocytes or lymphocytes. The difference between maximal oxygen consumption induced by uncoupling and the basal respiration is termed the reserve or spare respiratory capacity and can potentially be used by the cell for responses to oxidative stress, additional work or movement of ions into the mitochondria [37], [40]. Reserve capacity is greatest in the monocytes and lymphocytes while the platelet reserve capacity is only approximately 20% of maximal mitochondrial function. The differences in the mitochondrial oxygen utilization by these cell types indicate that they differ in the metabolic programs governing energy generation. We surmised that these differences would be reflected in the mitochondrial protein composition between each cell type. As shown in Fig. 1B where the mitochondrial proteins were quantified by western blot the citrate synthase levels normalized to total cell protein were similar between platelets, lymphocytes and monocytes. In contrast, Complex IV subunit I protein, the catalytic portion of cytochrome c oxidase, is present at higher levels in monocytes than in lymphocytes, whereas the Reiske iron sulfur protein of complex III is found at higher levels in lymphocytes. Both Complex III and IV proteins are low in platelets. These data show that there are indeed differences in mitochondrial electron transport chain proteins in these cells which will impact on the regulation and function of mitochondrial metabolism. With respect to using these cell types as biomarkers of bioenergetic function these findings have important implications. For example, detection of a pathology associated with decreased cytochrome c oxidase will be most readily detected in platelets which have the lowest levels of this enzyme and will be less evident in monocytes. It also follows that measurement of mitochondrial function in a crude preparation of peripheral blood mononuclear cells (PBMCs) will be a weighted average of the bioenergetic activity of diverse cell types which cannot then serve as an optimal sensor of bioenergetic health [41].
Fig. 1.
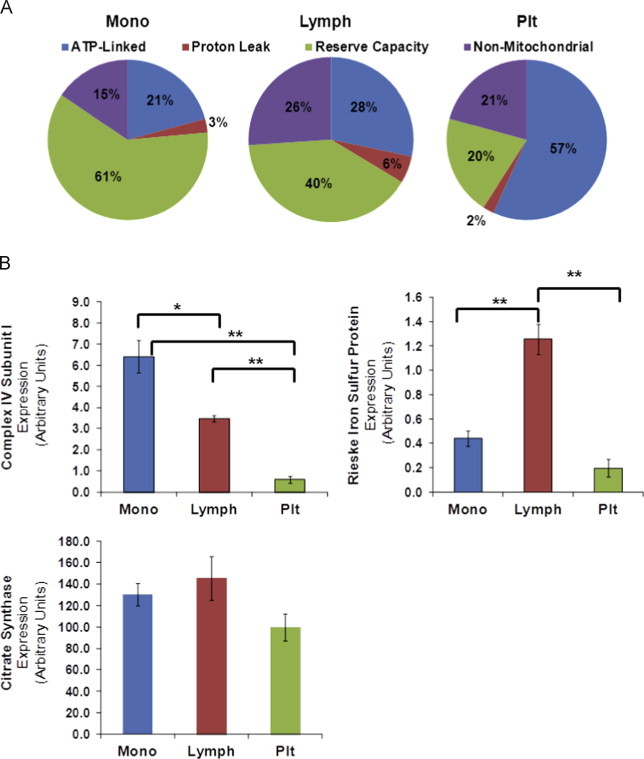
Distinct mitochondrial metabolism in leukocytes and platelets. Monocytes, lymphocytes and platelets were isolated from blood collected from healthy donors as described in [22]. The cells were seeded on a seahorse XF96 plate to assess bioenergetic function with a seahorse extracellular flux analyzer. Basal oxygen consumption was determined, followed by sequential injections of oligomycin, FCCP and antimycin A. The OCR was normalized to total protein based on number of cells plated per well. (A) Indices of bioenergetics were determined in monocytes, lymphocytes and platelets from individual donors. n=9–10. (B) Mitochondrial respiratory chain complexes and TCA cycle protein expression in each cell type were determined by western blotting for cytochrome c oxidase subunit I and Reiske iron–sulfur protein and citrate synthase. Isolated cells were solubilized in RIPA buffer and 50 µg of protein was resolved in SDS PAGE gels followed by western blotting. Data presented as mean±±SEM n=3 independent donors. *p<0.05 and **p<0.005.
Differential glycolytic and oxidative metabolism in leukocytes and platelets
To gain further insight into the relative utilization of glycolysis and oxidative phosphorylation in circulating platelets and monocytes we show the plot of OCR vs ECAR under basal conditions and with inhibition of mitochondrial ATP synthesis by oligomycin (Fig. 2A). Platelets have the highest basal OCR and show the largest increase on inhibition of mitochondrial ATP synthase. Monocytes have a slightly lower basal OCR compared to platelets and a more modest increase in glycolysis on addition of oligomycin. Lymphocytes are predominantly utilizing oxidative phosphorylation under basal conditions and have a limited capacity to increase glycolytic flux on addition of oligomycin. Neutrophils have little or no dependence on oxidative phosphorylation and not surprisingly glycolysis is not increased when the mitochondrial ATP synthase is inhibited although it is highly induced on activation of the oxidative burst [22]. In Fig. 2B these data are represented as the proportional difference in the OCR/ECAR ratio for each cell type across the full spectrum of glycolytic and mitochondrial metabolism. Interestingly, both platelets and monocytes exhibit a high degree of aerobic glycolysis whereas lymphocytes are almost entirely oxidative and neutrophils entirely glycolytic. Platelets appear to be the most metabolically active circulating “cells” under basal conditions which may be related to their relatively small size and high surface area associated with the extensive open canicular system of the cell. It is critical for platelets to maintain their calcium and other ion balance during circulation to prevent inadvertent activation, and many of these channels require ATP to function. The neutrophils are an interesting contrast as their mitochondria perform other roles, such as redox signaling and controlling apoptosis, which are more important for the function of these cells [42].
Fig. 2.
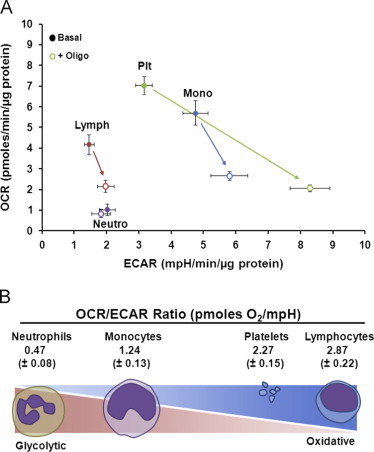
Distinct glycolytic metabolism in leukocytes and platelets. Oxygen consumption rate and extracellular acidification rate (ECAR) were measured in isolated monocytes, lymphocytes, platelets and neutrophils from healthy donors. (A) The basal and oligomycin sensitive mitochondrial bioenergetics (OCR) and glycolytic (ECAR) rates are plotted for each cell type. (B) The basal OCR/ECAR ratio is shown plotted at the approximate position showing the relative use of glycolysis (100% on the left) and oxidative phosphorylation (100% on the right). Data presented as mean±SEM n=9–10 independent healthy donors.
Future outlook
These data clearly indicate how the metabolic programs are distinct in the circulating leukocytes and platelets. In translational research the platelets and monocytes can then act as differential sensors of the metabolic and inflammatory stresses associated with cardiovascular disease, neurodegeneration, diabetes or other chronic pathologies. Lymphocytes in the circulation represent mixed populations due to clonal expansion and as such their bioenergetics may be an index of the status of inflammation or infection. Neutrophils are predominantly glycolytic and changes in oxidative burst capacity rather than mitochondrial function will be more informative. Changes in cellular bioenergetics in these cell types can then sense both changes in their biological function in response to an underlying pathological condition and their response to chronic metabolic stress. In summary, we have shown that using an integrated approach, the glycolytic metabolism and oxidative phosphorylation can be combined to generate a unique cellular bioenergetic profile for each cell type which extends the analysis of metabolic dysfunction in translational research.
Disclosures
VDU is a member of the Seahorse Biosciences Scientific Advisory Board.
Acknowledgments
The authors appreciate support from the American Heart Association (SR): NIH T32 T32HL07918 (PAK), NIDDK Diabetic Complications Consortium (DiaComp, http://www.diacomp.org), Grant DK076169 (sub-award VDU), and the O'Brien Center P30 DK079337.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tugal D., Liao X., Jain M.K. Transcriptional control of macrophage polarization. Arterioscler. Thromb. Vasc. Biol. 2013;33:1135–1144. doi: 10.1161/ATVBAHA.113.301453. [DOI] [PubMed] [Google Scholar]
- 3.Zhou D., Huang C., Lin Z., Zhan S., Kong L., Fang C., Li J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell. Signal. 2013;26:192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Prados J.C., Traves P.G., Cuenca J., Rico D., Aragones J., Martin-Sanz P., Cascante M., Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill L.A., Hardie D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro H., Lutaty A., Ariel A. Macrophages, meta-inflammation, and immuno-metabolism. Sci. World J. 2011;11:2509–2529. doi: 10.1100/2011/397971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corey S.J., Minden M.D., Barber D.L., Kantarjian H., Wang J.C., Schimmer A.D. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat. Rev. Cancer. 2007;7:118–129. doi: 10.1038/nrc2047. [DOI] [PubMed] [Google Scholar]
- 8.Krauss S., Brand M.D., Buttgereit F. Signaling takes a breath – new quantitative perspectives on bioenergetics and signal transduction. Immunity. 2001;15:497–502. doi: 10.1016/s1074-7613(01)00205-9. [DOI] [PubMed] [Google Scholar]
- 9.Macintyre A.N., Rathmell J.C. Activated lymphocytes as a metabolic model for carcinogenesis. Cancer Metab. 2013;1:5. doi: 10.1186/2049-3002-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearce E.L., Poffenberger M.C., Chang C.H., Jones R.G. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearce E.L., Pearce E.J. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Windt G.J., Pearce E.L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol. Rev. 2012;249:27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Windt G.J., Everts B., Chang C.H., Curtis J.D., Freitas T.C., Amiel E., Pearce E.J., Pearce E.L. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunzer M. Traps and hyper inflammation – new ways that neutrophils promote or hinder survival. Br. J. Haematol. 2013 doi: 10.1111/bjh.12608. [DOI] [PubMed] [Google Scholar]
- 15.Parker H., Dragunow M., Hampton M.B., Kettle A.J., Winterbourn C.C. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J. Leukoc. Biol. 2012;92:841–849. doi: 10.1189/jlb.1211601. [DOI] [PubMed] [Google Scholar]
- 16.van Raam B.J., Sluiter W., de Wit E., Roos D., Verhoeven A.J., Kuijpers T.W. Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. PLoS One. 2008;3:e2013. doi: 10.1371/journal.pone.0002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maianski N.A., Geissler J., Srinivasula S.M., Alnemri E.S., Roos D., Kuijpers T.W. Functional characterization of mitochondria in neutrophils: a role restricted to apoptosis. Cell Death Differ. 2004;11:143–153. doi: 10.1038/sj.cdd.4401320. [DOI] [PubMed] [Google Scholar]
- 18.Fossati G., Moulding D.A., Spiller D.G., Moots R.J., White M.R., Edwards S.W. The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J. Immunol. 2003;170:1964–1972. doi: 10.4049/jimmunol.170.4.1964. [DOI] [PubMed] [Google Scholar]
- 19.Borregaard N., Herlin T. Energy metabolism of human neutrophils during phagocytosis. J. Clin. Investig. 1982;70:550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y., Junger W.G. Measurement of oxidative burst in neutrophils. Methods Mol. Biol. 2012;844:115–124. doi: 10.1007/978-1-61779-527-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reuter H., Gross R. Platelet metabolism. Suppl. Thromb. Haemost. 1978;63:87–95. [PubMed] [Google Scholar]
- 22.Chacko B.K., Kramer P.A., Ravi S., Johnson M.S., Hardy R.W., Ballinger S.W., Darley-Usmar V.M. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab. Investig.: J. Tech. Methods Pathol. 2013;93:690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akahori M., Uedono Y., Yamagami K., Takeyama N., Kitazawa Y., Tanaka T. Hypoxia alters the energy metabolism and aggregation of washed human platelets. Haematologia (Budap) 1995;26:191–198. [PubMed] [Google Scholar]
- 24.Zharikov S., Shiva S. Platelet mitochondrial function: from regulation of thrombosis to biomarker of disease. Biochem. Soc. Trans. 2013;41:118–123. doi: 10.1042/BST20120327. [DOI] [PubMed] [Google Scholar]
- 25.Japiassu A.M., Santiago A.P., d'Avila J.C., Garcia-Souza L.F., Galina A., Castro Faria-Neto H.C., Bozza F.A., Oliveira M.F. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5'-triphosphate synthase activity. Crit. Care Med. 2011;39:1056–1063. doi: 10.1097/CCM.0b013e31820eda5c. [DOI] [PubMed] [Google Scholar]
- 26.Widlansky M.E., Wang J., Shenouda S.M., Hagen T.M., Smith A.R., Kizhakekuttu T.J., Kluge M.A., Weihrauch D., Gutterman D.D., Vita J.A. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl. Res. 2010;156:15–25. doi: 10.1016/j.trsl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avila C., Huang R.J., Stevens M.V., Aponte A.M., Tripodi D., Kim K.Y., Sack M.N. Platelet mitochondrial dysfunction is evident in type 2 diabetes in association with modifications of mitochondrial anti-oxidant stress proteins. Exp. Clin. Endocrinol. Diabetes. 2012;120:248–251. doi: 10.1055/s-0031-1285833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanagasi H.A., Ayribas D., Baysal K., Emre M. Mitochondrial complex I, II/III, and IV activities in familial and sporadic Parkinson's disease. Int. J. Neurosci. 2005;115:479–493. doi: 10.1080/00207450590523017. [DOI] [PubMed] [Google Scholar]
- 29.Guo X., Wu J., Du J., Ran J., Xu J. Platelets of type 2 diabetic patients are characterized by high ATP content and low mitochondrial membrane potential. Platelets. 2009;20:588–593. doi: 10.3109/09537100903288422. [DOI] [PubMed] [Google Scholar]
- 30.Widlansky M.E., Wang J., Shenouda S.M., Hagen T.M., Smith A.R., Kizhakekuttu T.J., Kluge M.A., Weihrauch D., Gutterman D.D., Vita J.A. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl. Res. 2010;156:15–25. doi: 10.1016/j.trsl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenaz G., Bovina C., Castelluccio C., Fato R., Formiggini G., Genova M.L., Marchetti M., Pich M.M., Pallotti F., Parenti Castelli G., Biagini G. Mitochondrial complex I defects in aging. Mol. Cell. Biochem. 1997;174:329–333. [PubMed] [Google Scholar]
- 32.Shi C., Guo K., Yew D.T., Yao Z., Forster E.L., Wang H., Xu J. Effects of ageing and Alzheimer's disease on mitochondrial function of human platelets. Exp. Gerontol. 2008;43:589–594. doi: 10.1016/j.exger.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Mosconi L., de Leon M., Murray J., E L., Lu J., Javier E., McHugh P., Swerdlow R.H. Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer's disease. J. Alzheimer's Dis. 2011;27:483–490. doi: 10.3233/JAD-2011-110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clayton D.A., Vinograd J. Complex mitochondrial DNA in leukemic and normal human myeloid cells. Proc. Natl. Acad. Sci. USA. 1969;62:1077–1084. doi: 10.1073/pnas.62.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordero M.D., De Miguel M., Moreno Fernandez A.M., Carmona Lopez I.M., Garrido Maraver J., Cotan D., Gomez Izquierdo L., Bonal P., Campa F., Bullon P., Navas P., Sanchez Alcazar J.A. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: implications in the pathogenesis of the disease. Arthritis Res. Ther. 2010;12:R17. doi: 10.1186/ar2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fink B.D., Herlein J.A., O'Malley Y., Sivitz W.I. Endothelial cell and platelet bioenergetics: effect of glucose and nutrient composition. PLoS One. 2012;7:e39430. doi: 10.1371/journal.pone.0039430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill B.G., Benavides G.A., Lancaster J.R., Jr, Ballinger S., Dell'Italia L., Jianhua Z., Darley-Usmar V.M. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brand M.D., Nicholls D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dranka B.P., Benavides G.A., Diers A.R., Giordano S., Zelickson B.R., Reily C., Zou L.Y., Chatham J.C., Hill B.G., Zhang J.H., Landar A., Darley-Usmar V.M. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic. Biol. Med. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dranka B.P., Hill B.G., Darley-Usmar V.M. Mitochondrial reserve capacity in endothelial cells: the impact of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maynard S., Keijzers G., Gram M., Desler C., Bendix L., Budtz-Jorgensen E., Molbo D., Croteau D.L., Osler M., Stevnsner T., Rasmussen L.J., Dela F., Avlund K., Bohr V.A. Relationships between human vitality and mitochondrial respiratory parameters, reactive oxygen species production and dNTP levels in peripheral blood mononuclear cells. Aging (Albany NY) 2013 doi: 10.18632/aging.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borregaard N., Herlin T. Energy metabolism of human neutrophils during phagocytosis. J. Clin. Investig. 1982;70:550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]


