The Prg4 locus encodes the proteoglycan lubricin that protects against osteoarthritis. Ogawa et al. discover that running induces maximal expression of Prg4 in the superficial zone of knee joint articular cartilage in a COX-2-dependent fashion. This correlated with increased CREB-dependent gene expression. Fluid flow shear stress increases secretion of extracellular PGE2, PTHrP, and ATP, which together promote CREB-dependent induction of Prg4. These results suggest that mechanical motion may induce Prg4 expression through the same signaling pathways activated in vitro by fluid flow shear stress.
Keywords: CREB, PGE2, PTHrP, Prg4/lubricin, articular cartilage, extracellular ATP
Abstract
Lubricin is a secreted proteoglycan encoded by the Prg4 locus that is abundantly expressed by superficial zone articular chondrocytes and has been noted to both be sensitive to mechanical loading and protect against the development of osteoarthritis. In this study, we document that running induces maximal expression of Prg4 in the superficial zone of knee joint articular cartilage in a COX-2-dependent fashion, which correlates with augmented levels of phospho-S133 CREB and increased nuclear localization of CREB-regulated transcriptional coactivators (CRTCs) in this tissue. Furthermore, we found that fluid flow shear stress (FFSS) increases secretion of extracellular PGE2, PTHrP, and ATP (by epiphyseal chondrocytes), which together engage both PKA- and Ca++-regulated signaling pathways that work in combination to promote CREB-dependent induction of Prg4, specifically in superficial zone articular chondrocytes. Because running and FFSS both boost Prg4 expression in a COX-2-dependent fashion, our results suggest that mechanical motion may induce Prg4 expression in the superficial zone of articular cartilage by engaging the same signaling pathways activated in vitro by FFSS that promote CREB-dependent gene expression in this tissue.
While a great deal is known about the signals that regulate the formation and maturation of growth plate cartilage (for review, see Lefebvre and Bhattaram 2010), considerably less is known about the signals that regulate these processes in articular cartilage (for review, see Pacifici et al. 2005). Growth plate and articular chondrocytes arise from distinct progenitor populations such that articular chondrocytes share a common origin with synovial cells that line the joint cavity (Koyama et al. 2008). The superficial-most layer of articular cartilage is distinguished from deeper layers of articular cartilage (and from cartilage cells in the growth plate) by expression of lubricin. Lubricin is a secreted proteoglycan encoded by the Prg4 locus that is highly expressed by both superficial zone articular chondrocytes and synoviocytes (Marcelino et al. 1999). Prg4 also encodes the related gene products superficial zone protein (SZP), megakaryocyte-stimulating factor (MSF), and hemangiopoietin (HAPO), representing alternative spliced transcripts and/or post-translationally modified proteins (for review, see Rhee et al. 2005). It is thought that lubricin and SZP are critical to maintain appropriate boundary lubrication of diarthrodial joints (Swann et al. 1985). Individuals with two mutant alleles of PRG4 have the camptodactyly-arthropathy-coxa vara-pericarditis syndrome (CACP) (Marcelino et al. 1999) and display noninflammatory synoviocyte hyperplasia and subintimal fibrosis of the synovial capsule (Bahabri et al. 1998). Mice lacking Prg4 (Prg4−/−) appear normal at birth but subsequently display synovial hyperplasia, subintimal fibrosis, and an abnormal articular cartilage surface (Rhee et al. 2005). In contrast, heterozygous Prg4 mutant (Prg4+/−) mice do not display these joint pathologies, consistent with the recessive nature of CACP (Rhee et al. 2005).
Col2-driven overexpression of lubricin in the articular cartilage has recently been shown to protect this tissue from degradation following surgically induced joint destabilization (Ruan et al. 2013), suggesting that lubricin may somehow counter the signaling pathways that lead to cartilage destruction. Hence, an understanding of the signals that regulate lubricin expression in the articular cartilage could potentially provide a therapeutic opportunity to modulate expression of this gene and thus stem the degradation of articular cartilage observed during osteoarthritis. While Wnt/β-catenin signaling has been found to be essential for expression of Prg4/lubricin during early development (Koyama et al. 2008; Yasuhara et al. 2011), the signals that maintain the expression of this locus in adult articular cartilage remain obscure. Studies have pointed to a role for both TGF-β signaling (Lee et al. 2008) and mechanical loading (Nugent et al. 2006a,b; Jones et al. 2009) in the maintenance of Prg4/lubricin expression in either newborn or adult articular cartilage. In this study, we both document that voluntary wheel running induces the expression of the Prg4 locus in the superficial zone of knee joint articular cartilage and identify multiple signaling pathways downstream from fluid flow shear stress (FFSS) that promote the expression of Prg4 in this tissue in a CREB-dependent manner.
Results
Wheel running increases recombination driven by the Prg4GFPCreERt2 allele
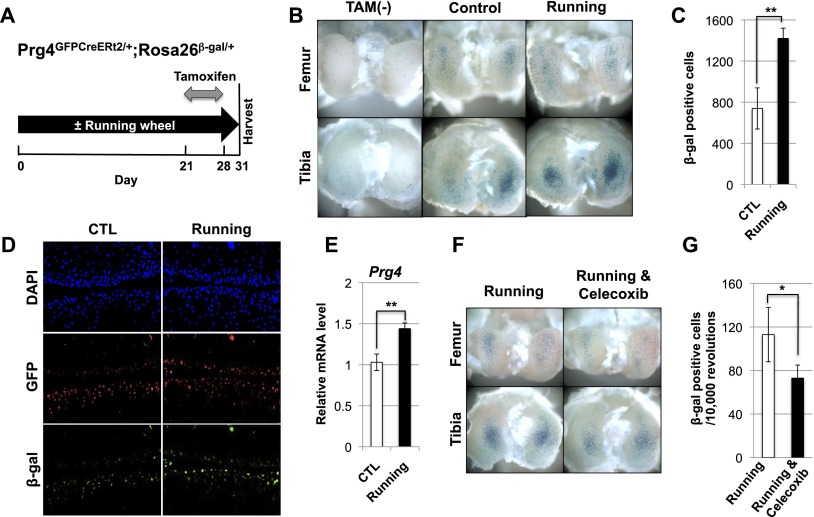
We recently developed a strain of knock-in mice that express a tamoxifen-inducible Cre recombinase from the Prg4 locus (Prg4GFPCreERt2) (E Kozhemyakina, M Zhang, A Ionescu, N Ono, A Kobayashi, H Kronenberg, ML Warman, and AB Lassar, in prep.). To examine whether voluntary wheel running could affect the expression of Prg4 in knee joints, we employed 3-mo-old Prg4GFPCreERt2/+; Rosa26floxlacZ/+ mice, which were housed individually either with or without access to a running wheel. After 3 wk of such housing, the animals were injected with tamoxifen for seven consecutive days and subsequently maintained either with or without their running wheels (experimental time course outlined in Fig. 1A). Three days following the last tamoxifen injection, the animals were sacrificed, and expression of β-galactosidase in their knee joints was determined by X-gal staining (Fig. 1A–C). Interestingly, we observed a near doubling in the number of cells expressing β-galactosidase in the knee joints of animals that had run (quantitated in Fig. 1C), with the greatest increase observed in the tibial plateau (Fig. 1B). These results indicate that running animals display higher levels of Prg4GFPCreERt2-driven recombination in knee joint articular cartilage, suggesting that signaling pathways downstream from mechanical motion may increase the expression of this locus.
Figure 1.
Wheel running increases Prg4 expression. (A) Three-month-old female Prg4GFPCreERt2/+; Rosa26floxlacZ/+ mice were housed in either the absence or presence of a running wheel for 31 d. The animals were injected with tamoxifen daily from day 22 to 28 and sacrificed for X-gal staining at day 31. (B) Whole-mount X-gal staining of the knee joints taken from either corn-oil-injected (TAM−) or tamoxifen-injected Prg4GFPCreERt2/+; Rosa26floxlacZ/+ mice that were housed either without (Control) or with (Running) a running wheel. (C) Sections of the knee joints depicted in B (n = 6 running or 6 control mice). For each mouse, the total number of β-galactosidase-expressing cells in five sections of the lateral condyles and five sections of the medial condyles in both knees was quantitated. (D) The knees of either control or running Prg4GFPCreERt2/+; Rosa26floxlacZ/+ mice (treated as outlined in A) were sectioned and immunostained with both anti-GFP and anti-β-galactosidase antibodies. (E) Endogenous expression of Prg4 relative to β-actin was determined by RT-qPCR. (F) Whole-mount X-gal staining of the knee joints of running Prg4GFPCreERt2/+; Rosa26floxlacZ/+ mice administered either a control gavage solution (Running) or such a solution containing Celecoxib (Running & Celecoxib). (G) The knees of either six control or six Celecoxib-treated Prg4GFPCreERt2/+; Rosa26floxlacZ/+ mice (all housed with running wheels) were stained with X-gal and sectioned. The total number of β-galactosidase-expressing cells in five sections of the lateral condyles and five sections of the medial condyles was quantitated and normalized to the number of rotations of the running wheels (n = 6 control mice, n = 6 Celecoxib-treated mice). Throughout this and the following figures, significance was calculated using Student's t-test. (*) P < 0.05; (**) P < 0.01. Error bar indicates standard error of the mean.
An increased number of β-galactosidase-expressing cells in the knees of Prg4GFPCreERt2/+; Rosa26floxlacZ/+ mice that had run could reflect an increased level of expression from the Prg4 locus and/or increased proliferation of Prg4-expressing cells induced by running. To address the first possibility, we evaluated β-galactosidase expression in GFP-expressing cells by immunocytochemistry (which is more quantitative than X-gal staining) in the knees of Prg4GFPCreERt2/+; Rosa26floxlacZ/+ animals that had been housed either with or without a running wheel. We observed both an increase in the number of GFP-expressing cells in the knees of animals that had run (Fig. 1D, middle panels) and markedly greater β-galactosidase fluorescence in the superficial-most cell layer of the tibial articular cartilage in running animals (Fig. 1D, bottom panels). Increased β-galactosidase expression per cell in the superficial-most layer of tibial articular cartilage suggests that running increased the expression of the Prg4GFPCreERt2 locus in these cells, thus leading to earlier recombination of the Rosa26floxlacZ allele after initiation of tamoxifen administration. Consistent with this notion, we observed that running animals expressed greater levels of Prg4 mRNA in their articular cartilage, as assayed by RT-qPCR (Fig. 1E).
To examine whether wheel running increased the proliferation of pre-existing Prg4-expressing cells, we administered tamoxifen to Prg4GFPCreERt2/+; Rosa26floxlacZ/+ mice for 7 d and then housed these animals for 31 d in either the absence or presence of a running wheel. Ten days prior to sacrifice (i.e., at 21 d with/without a running wheel), the animals were given seven consecutive daily pulses of EdU to label newly replicated DNA (experimental time course outlined in Supplemental Fig. 1A). Most importantly, this regimen of tamoxifen administration prior to running did not significantly increase the number of β-galactosidase-positive cells in the articular cartilage (Supplemental Fig. 1B,C), indicating that the apparent increase in Prg4 expression following 1 mo of running (documented in Fig. 1A–E) reflected augmented expression of this locus in a greater number of articular cartilage cells rather than an expansion of pre-existing Prg4-expressing cells. Interestingly, however, running did increase a relatively small number of EdU-positive cells (Supplemental Fig. 1D, E) in both Prg4-expressing and -nonexpressing articular cartilage cells (Supplemental Fig. 1F), suggesting that mechanical motion does increase cellular proliferation in articular cartilage to a small extent.
FFSS promotes the secretion of a signaling molecule that in turn induces Prg4 expression in chondrocytes
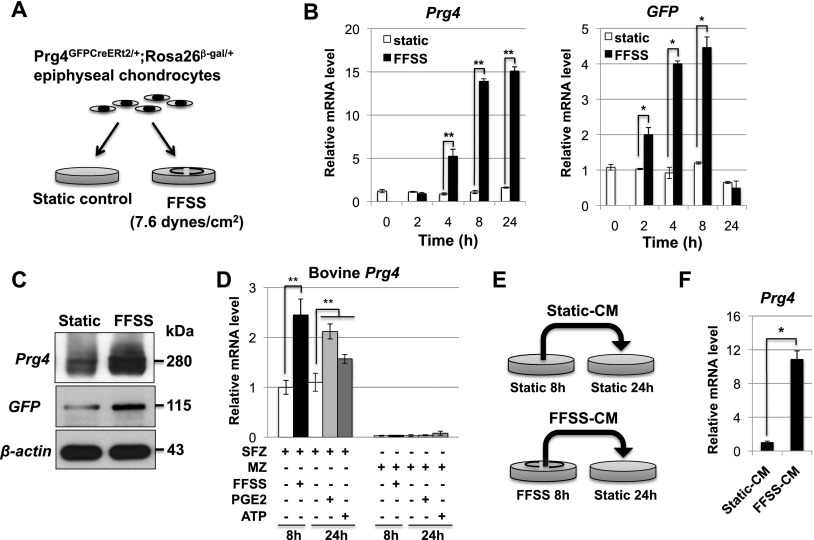
Because running increased the expression of Prg4 in superficial zone chondrocytes, we explored whether FFSS could similarly induce expression of this gene. FFSS has been shown to transduce mechanical motion in osteocytes via both connexin and pannexin channels, which release both ATP and PGE2 into the extracellular space (Bao et al. 2004; Cherian et al. 2005; Li et al. 2005; Batra et al. 2012). These signaling molecules in turn interact with their cognate receptors to alter gene expression in these cells. To examine whether mechanical motion/FFSS may act to similarly regulate Prg4 expression in articular cartilage, epiphyseal chondrocytes were isolated from 5-d-old Prg4GFPCreERt2/+; Rosa26floxlacZ/+ animals and cultured in tissue culture dishes on either a static surface or a rotary shaker, which delivered a steady laminar, nonpulsatile shear stress of ∼7.6 dyn/cm2. The cells were harvested after exposure to either static conditions or FFSS for either 2, 4, 8, or 24 h (outlined in Fig. 2A). We monitored expression of both the Prg4GFPCreERt2 and the Prg4WT alleles by assaying expression of either GFP or Prg4, respectively, by RT-qPCR. We found that FFSS robustly induced the expression of both Prg4 alleles (Fig. 2B) and similarly induced both the secretion of Prg4 protein into the cell culture medium and the intracellular accumulation of GFP (Fig. 2C). We also found that FFSS induced the expression of Prg4 in chondrocytes isolated from the superficial zone, but not the middle zone, of newborn bovine articular cartilage (Fig. 2D), indicating that the ability of FFSS conditions to induce the expression of Prg4 is regionally restricted in immature bovine articular cartilage. Consistent with this notion, we observed that, when cultured in the presence of tamoxifen, FFSS induced Prg4GFPCreERt2-driven recombination of the Rosa26floxlacZ allele in only a few percent (i.e., ∼3%) of epiphyseal chondrocytes that had been isolated from 5-d-old Prg4GFPCreERt2/+; Rosa26floxlacZ/+ animals (Supplemental Fig. 2). Because articular cartilage precursors that will go on to express Prg4 are only a small percentage of epiphyseal chondrocytes at this stage of development (E Kozhemyakina, M Zhang, A Ionescu, N Ono, A Kobayashi, H Kronenberg, ML Warman, and AB Lassar, in prep.), it seems likely that superficial zone articular cartilage progenitors may be uniquely competent to express Prg4 in response to FFSS.
Figure 2.
FFSS promotes the secretion of a signaling molecule that in turn induces Prg4 expression in chondrocytes. (A) Epiphyseal chondrocytes were isolated from 5-d-old Prg4GFPCreERt2/+; Rosa26floxlacZ/+ animals and cultured in tissue culture dishes on either a static surface or a rotary shaker (which delivered ∼7.6 dyn/cm2). (B) Expression of both the Prg4WT and the Prg4GFPCreERt2 alleles in epiphyseal chondrocytes cultured under either static or FFSS conditions were assayed relative to β-actin by RT-qPCR. (C) Either conditioned medium (top panel) or total cell lysates (middle and bottom panels) were collected from epiphyseal chondrocytes cultured under either static or FFSS conditions for 24 h, and expression of the indicated proteins was assayed by Western blot. (D) Either FFSS, PGE2, or extracellular ATP was administered (for the indicated time period) to either superficial zone (SFZ) or middle zone (MZ) newborn bovine articular chondrocytes. Prg4 expression was assayed relative to that of 18S rRNA by RT-qPCR. (E,F) Medium conditioned by murine epiphyseal chondrocytes cultured under either static or FFSS conditions for 8 h was applied to new plates of chondrocytes cultured under static conditions for 24 h. Expression of endogenous Prg4 was assayed relative to β-actin by RT-qPCR. Throughout this figure: (*) P < 0.05; (**) P < 0.01. Error bar indicates standard error of the mean.
To determine whether FFSS induced the secretion of a signaling molecule that in turn induced Prg4 expression, we isolated conditioned medium from murine epiphyseal chondrocytes cultured under either static conditions (i.e., control-conditioned medium) or on a rotary shaker (i.e., FFSS-conditioned medium). The two conditioned mediums were then applied to epiphyseal chondrocytes cultured under static conditions (outlined in Fig. 2E). We found that, while FFSS-conditioned medium robustly induced the expression of endogenous Prg4 in chondrocytes cultured under static conditions, control-conditioned medium failed to elicit this effect (Fig. 2F). Thus, FFSS promotes the secretion of a signaling molecule that in turn induces Prg4 expression.
FFSS promotes the secretion of PGE2 by chondrocytes, which is necessary for maximal expression of Prg4
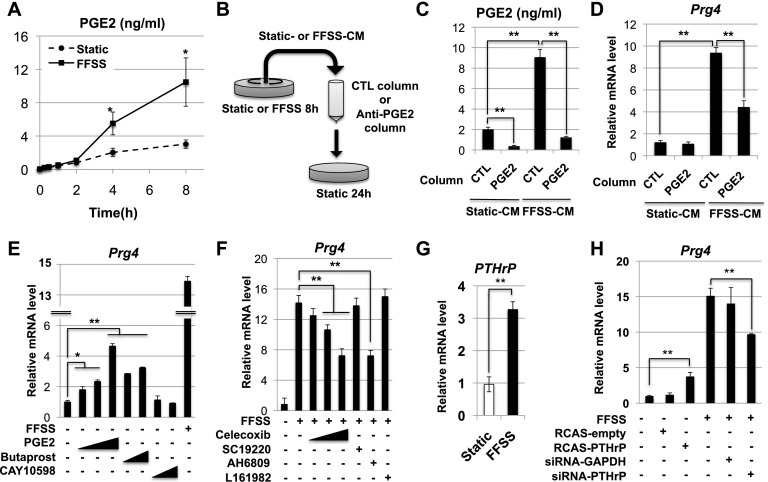
Because FFSS induces the secretion of PGE2 in osteocytes (Cherian et al. 2005; Li et al. 2005), we examined whether such is the case in chondrocytes. Indeed, we found that administration of FFSS to epiphyseal chondrocytes increased the secretion of PGE2 into the medium (Fig. 3A). Most importantly, depletion of >90% of PGE2 from FFSS-conditioned medium attenuated the ability of this medium to induce the expression of Prg4 in static chondrocytes by ∼50% (Fig. 3B–D). Thus, PGE2 is likely to be one component in FFSS that induces the expression of Prg4. Indeed, administration of PGE2 to chondrocytes cultured under static conditions was able to induce the expression of Prg4 but to a lesser extent than FFSS culture conditions (Fig. 3E), again suggesting that PGE2 works synergistically with other components secreted into FFSS-conditioned medium to induce Prg4 expression. PGE2 has four distinct G-protein-coupled receptors: EP1 is coupled to Gq/11(α), EP2 and EP4 are coupled to Gs(α), and EP3 is coupled to Gi(α) (for review, see Sugimoto and Narumiya 2007). We found that, while administration of the EP2 agonist Butaprost could also induce the expression of Prg4 in static epiphyseal chondrocytes, administration of the EP4 agonist CAY10598 did not elicit this effect (Fig. 3E). Consistent with the notion that PGE2/EP2 signaling is necessary for maximal induction of Prg4 in response to FFSS, we observed that treatment of epiphyseal chondrocytes with either the COX-2 inhibitor Celecoxib (which blocks PGE2 synthesis) or the EP1/2 antagonist AH6809 blocked the induction of Prg4 by FFSS in these cells by ∼50% (Fig. 3F). In contrast, administration of either an EP1 antagonist (SC19220) or an EP4 antagonist (L161982) did not block the ability of FFSS to induce Prg4 expression (Fig. 3F). Thus, FFSS-induced secretion of PGE2 and its paracrine interaction with EP2 account for ∼50% of FFSS-induced expression of Prg4 expression in chondrocytes.
Figure 3.
FFSS induces Prg4 expression via both PGE2- and PTHrP-dependent pathways. (A) Concentration of PGE2 secreted into conditioned medium harvested from epiphyseal chondrocytes cultured under either FFSS or static conditions for the indicated time periods. (B) Either control- or FFSS-conditioned medium was poured over a column containing either control IgG or anti-PGE2. Flow-through material that was not bound to the column was applied to new epiphyseal chondrocytes cultured under static conditions. (C,D) Depletion of >90% of PGE2 from FFSS-conditioned medium (C) attenuated the ability of this medium to induce the expression of Prg4 in static chondrocytes by ∼50% (D). (E) Epiphyseal chondrocytes were cultured under either FFSS or static conditions with increasing concentrations of either PGE2 (0.01, 0.1, and 1 μM), the EP2 agonist Butaprost (1 and 10 μM), or the EP4 agonist CAY10598 (1 and 10 μM). Prg4 expression was assayed relative to β-actin by RT-qPCR. (F) Epiphyseal chondrocytes were cultured under either static or FFSS conditions with increasing concentrations of either the COX-2 inhibitor Celecoxib (0.1, 1, 10 μM), the EP1 antagonist SC19220 (10 μM), the EP1/2 antagonist AH6809 (1 μM), or the EP4 antagonist L161982 (10 μM). Prg4 expression was assayed relative to β-actin by RT-qPCR. (G) Epiphyseal chondrocytes were isolated from 5-d-old mice and cultured under either FFSS or static conditions for 8 h. Gene expression was assayed relative to β-actin by RT-qPCR. (H) Epiphyseal chondrocytes were cultured under either FFSS or static conditions after transfection with either an RCAS empty expression vehicle, RCAS-encoding PTHrP, siRNA targeting GAPDH, or siRNA targeting PTHrP. Gene expression of Prg4 (relative to β-actin) was assayed by RT-qPCR. Throughout this figure: (*) P < 0.05; (**) P < 0.01. Error bar indicates standard error of the mean.
Running induces maximal Prg4 expression in the knee joint via a COX-2-dependent pathway
In addition to increasing secretion of PGE2 into the medium (Fig. 3A), FFSS also significantly increased (by sixfold) the expression of Ptgs2 (Supplemental Fig. 3A), which encodes COX-2, a critical enzyme controlling synthesis of PGE2. Interestingly, wheel running similarly increased the accumulation of PGE2 in the superficial zone of knee joint articular cartilage (Supplemental Fig. 3B, red arrows). To examine whether PGE2 synthesis is necessary for wheel running to induce the expression of Prg4 in knee joints, we housed 3-mo-old Prg4GFPCreERt2/+; Rosa26floxlacZ/+ mice in the presence of running wheels (as described above) and administered either a control gavage solution or such a solution containing Celecoxib, a COX-2 antagonist, on a daily basis. Celecoxib administration markedly suppressed Prg4GFPCreERt2-mediated recombination to ∼65% of that observed in control running animals (Fig. 1F,G), suggesting that mechanical motion boosts maximal Prg4 expression in articular chondrocytes via a COX-2-dependent pathway.
FFSS enhances the expression of PTHrP, which also promotes the expression of Prg4 in epiphyseal chondrocytes
Interestingly, we observed that FFSS administration to epiphyseal chondrocytes increased the expression of PTHrP (Fig. 3G), which signals via the Gs(α)-coupled PTH/PTHrP receptor PTh1r (for review, see Sakamoto et al. 2005). To investigate whether PTHrP could similarly induce the expression of Prg4 in chondrocytes, we transfected static epiphyseal chondrocytes with either an empty expression vehicle (RCAS) or this expression vehicle encoding PTHrP (RCAS-PTHrP). We observed that overexpression of PTHrP induced Prg4 expression in static epiphyseal chondrocytes and, conversely, that knockdown of PTHrP with siRNA attenuated the ability of FFSS to induce Prg4 expression in these cells by ∼30% (Fig. 3H). Taken together, our findings suggest that FFSS promotes expression of Prg4 in chondrocytes via induction of both PGE2 and PTHrP secretion.
FFSS promotes secretion of extracellular ATP, which is also necessary to induce maximal expression of Prg4 expression in articular chondrocytes
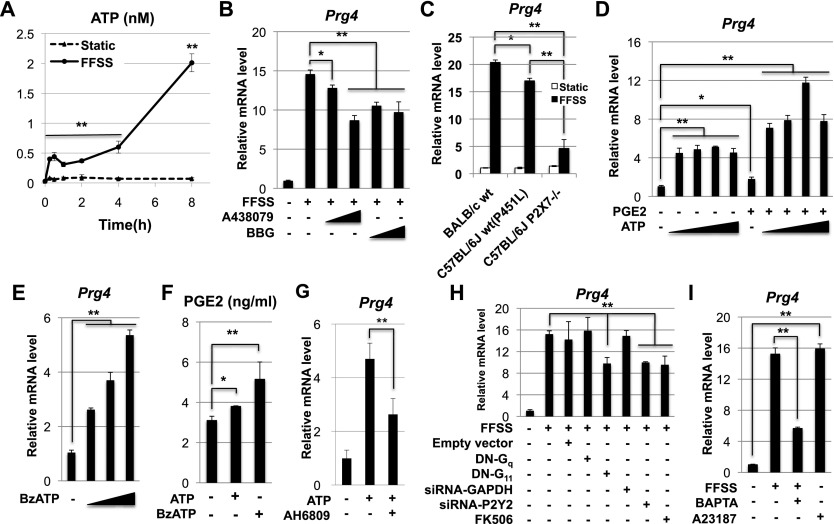
As discussed above, FFSS has been shown to promote the secretion of both ATP and PGE2 in osteocytes (Bao et al. 2004; Cherian et al. 2005; Li et al. 2005; Batra et al. 2012). Interestingly, we found that FFSS similarly induced epiphyseal chondrocytes to secrete ATP into their medium (Fig. 4A). ATP has many types of extracellular receptors, including both ion channels (P2X receptors) and those coupled to G protein signaling (P2Y receptors). Because the P2X7 receptor for ATP has previously been demonstrated to play a role in both sensing mechanical loading in bones and transducing FFSS signaling in osteocytes (Bao et al. 2004; Li et al. 2005; Thi et al. 2012), we evaluated its role in promoting the expression of Prg4 in chondrocytes in response to FFSS. Administration of the P2X7 antagonists A438079 or Brilliant Blue-G (BBG) significantly decreased induction of Prg4 in chondrocytes by FFSS (Fig. 4B), suggesting that the P2X7 ATP receptor plays a role in this process. Consistent with this notion, we found that FFSS-mediated induction of Prg4 expression in epiphyseal chondrocytes isolated from P2X7 knockout mice (in a C57BL/6J background) was significantly below that induced in chondrocytes isolated from wild-type C57BL/6J animals (Fig. 4C). While C57BL/6J mice contain an allelic mutation (P451L) in the predicted death domain of the P2X7 receptor that confers a reduced sensitivity to ATP-induced pore formation (Adriouch et al. 2002), BALB/c mice carry P451 at this position (as do rats and humans), which confers greater sensitivity to ATP-induced pore formation (Adriouch et al. 2002). Interestingly, FFSS administration to epiphyseal chondrocytes induced a higher level of Prg4 expression in chondrocytes isolated from BALB/c animals (which contain P451 in P2X7) than in chondrocytes isolated from C57BL/6J animals (which contain P451L in P2X7) (Fig. 4C). Together, these findings indicate that the relative induction of Prg4 by FFSS correlates with the relative sensitivity of the P2X7 receptor to ATP-induced pore formation.
Figure 4.
FFSS induces expression of Prg4 in articular chondrocytes via both ATP and PGE2 signaling pathways. (A) Concentration of ATP secreted into conditioned medium harvested from epiphyseal chondrocytes cultured under either FFSS or static conditions for the indicated time periods. (B) Administration of the P2X7 antagonists A438079 (1 and 10 μM) or Brilliant Blue-G (BBG; 1 and 10 μM) to epiphyseal chondrocytes significantly decreased induction of Prg4 by FFSS. (C) FFSS-mediated induction of Prg4 expression in epiphyseal chondrocytes isolated from either wild-type Balb/c mice (with P451 in P2X7), wild-type C57Bl/6J mice (with P451L in P2X7), or P2X7−/− mice (in a C57Bl/6J background). (D) Increasing amounts of ATP (0.01, 0.1, 1, and 10 μM) were administered to static chondrocytes in either the absence or presence of PGE2 (0.1 μM). (E) Increasing amounts (1, 10, and 100 uM) of the P2X7 agonist BzATP robustly induce the expression of Prg4 in static chondrocytes. (F) Administration of either ATP (1 mM) or BzATP (100 μM) to static chondrocytes induces secretion of PGE2 into the medium. (G) Induction of Prg4 expression in epiphyseal chondrocytes by extracellular ATP was attenuated by the EP1/2 antagonist AH6809 (1 μM). (H) FFSS-mediated induction of Prg4 in epiphyseal chondrocytes was assayed in the presence of either dominant-negative (DN) forms of either Gq(α) or G11(α), siRNAs directed against either GAPDH (control) or P2Y2, or the calcineurin antagonist FK506. (I) Addition of the Ca++ chelator BAPTA attenuated induction of Prg4 by FFSS, and, conversely, addition of the Ca++ ionophore A23187 induced the expression of Prg4 in static chondrocytes. Throughout this figure, Prg4 expression was assayed relative to β-actin by RT-qPCR. (*) P < 0.05; (**) P < 0.01. Error bar indicates standard error of the mean.
To evaluate whether ATP and PGE2 signals work in combination to induce the expression of Prg4, we administered submaximal levels of each compound to chondrocytes cultured under static conditions and found that these agents could indeed synergistically induce the expression of Prg4 (Fig. 4D). We found that both ATP and BzATP, which is a potent P2X7 agonist (for review, see Burnstock 2012), each promoted Prg4 expression (Fig. 4D,E) and also increased secretion of PGE2 into the medium (Fig. 4F). This later finding is consistent with the notion that ATP activation of P2X7 opens the pannexin 1 channel, which in turn releases PGE2 into the medium (Li et al. 2005). Interestingly, induction of Prg4 expression by extracellular ATP is attenuated by 50% in the presence of the EP1/2 antagonist AH6809 (Fig. 4G), again suggesting that ATP and PGE2 work in combination to induce expression of Prg4. Most importantly, just like FFSS administration, either exogenous PGE2 or ATP can induce Prg4 expression only in superficial zone (as opposed to middle zone) bovine articular chondrocytes (Fig. 2D), attesting to the unique competence of superficial zone articular chondrocytes to express this gene in response to PGE2 and/or ATP signaling pathways.
Ca++ signaling is critical for maximal expression of Prg4
Both the ligand-gated P2X7 ATP receptor and the Gq(α)/G11(α)-coupled P2Y2 ATP receptor have been demonstrated to be expressed in human primary chondrocytes (Millward-Sadler et al. 2004; Knight et al. 2009) and, upon ATP binding, to induce increased levels of intracellular Ca++ (Millward-Sadler et al. 2004; Grol et al. 2012). Thus, we investigated whether expression of dominant-negative (DN) forms of either Gq(α) or G11(α) or siRNA knockdown of P2Y2 would affect FFSS-mediated induction of Prg4. Indeed, we found that expression of either DN-G11(α) or siRNA against P2Y2 attenuated FFSS-induced Prg4 expression by ∼35% (Fig. 4H). Consistent with the idea that FFSS induces Prg4 expression in a Ca++-dependent fashion, we observed that addition of the Ca++ chelator BAPTA to the cell culture medium significantly attenuated induction of Prg4 by FFSS (Fig. 4I) and, conversely, that addition of the Ca++ ionophore A23187 robustly induced the expression of Prg4 in static chondrocytes (Fig. 4I). In addition, we observed that inhibition of the calcium-regulated phosphatase calcineurin by administration of FK506, attenuated FFSS-induced Prg4 expression by ∼40% (Fig. 4H), indicating that calcineurin activity is critical for maximal induction of Prg4 by FFSS.
Knockdown of pannexin 1 attenuates the ability of FFSS to induce expression of Prg4
Mechanical loading/FFSS signal transduction in osteocytes is mediated by ATP/PGE2 release from the cell via either the pannexin 1 channel (which is coupled to the P2X7 ATP receptor) (Bao et al. 2004; Li et al. 2005; Thi et al. 2012) or the connexin 43 hemichannel (Cherian et al. 2005). Because either extracellular ATP or PGE2 is capable of inducing Prg4 expression in epiphyseal chondrocytes (Figs. 3, 4), we investigated whether FFSS-mediated induction of Prg4 expression in these cells similarly relied on pannexin 1. Indeed, we found that siRNA-mediated knockdown of mouse pannexin 1 significantly attenuated induction of Prg4 expression in murine epiphyseal chondrocytes by FFSS (Supplemental Fig. 4A), which was rescued by overexpression of human pannexin 1 (Supplemental Fig. 4E). Consistent with prior work in osteoblasts, we observed that siRNA-mediated knockdown of pannexin 1 reduced the secretion of both ATP and PGE2 into the medium by chondrocytes that had been cultured under FFSS conditions (Supplemental Fig. 4B,C). In addition, we observed that siRNA-mediated knockdown of connexin 43 could also attenuate FFSS-induced Prg4 expression and decreased secretion of both ATP and PGE2 in epiphyseal chondrocytes (data not shown). Together, these results support the notion that induction of Prg4 expression in chondrocytes in response to FFSS is mediated via ATP/PGE2 release from the cell by the pannexin 1 channel and/or the connexin 43 hemichannel.
FFSS engages a PKA/CREB signaling pathway to promote Prg4 expression
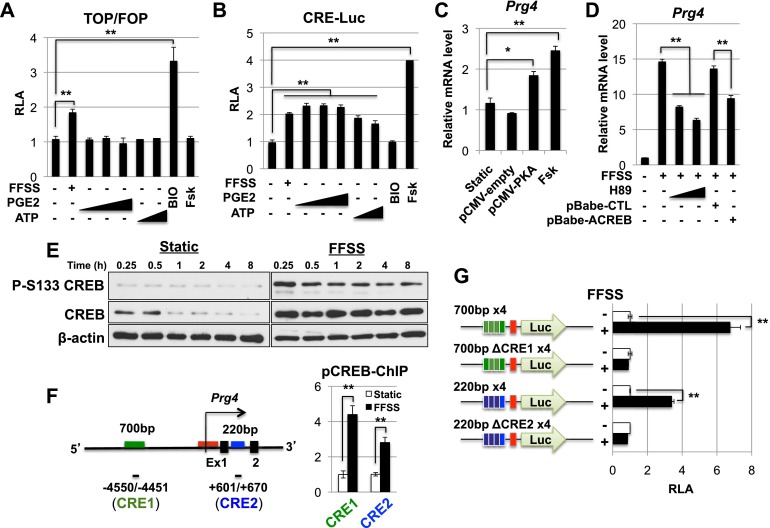
In osteoblasts, administration of FFSS can induce signaling pathways that stabilize β-catenin and thereby promote the activity of LEF/TCF transcription factors (Xia et al. 2010) and/or induce the activity of protein kinase A (Cherian et al. 2003). Notably, while culture of epiphyseal chondrocytes under FFSS conditions resulted in increased activation of either TOP-luciferase (a LEF/TCF reporter) (Fig. 5A) or CRE-luciferase (a CREB reporter) (Fig. 5B), administration of either exogenous PGE2 or ATP induced expression of only the CRE-luciferase reporter (Fig. 5A,B). Thus, extracellular PGE2 and ATP activate signaling pathways that specifically promote the activity of CREB transcription factors in chondrocytes. To evaluate whether activation of PKA was sufficient to induce the expression of Prg4, we either treated epiphyseal chondrocytes with forskolin (which increases the activity of adenylate cyclase) or transfected these cells with an activated form of PKA. We observed that activation of either PKA or adenylate cyclase was sufficient to induce the expression of Prg4 in epiphyseal chondrocytes cultured under static conditions (Fig. 5C). To examine whether PKA signaling is necessary for FFSS to enhance Prg4 expression in epiphyseal chondrocytes, we examined whether administration of the PKA antagonist H89 would affect Prg4 induction by FFSS. Indeed, we found that H89 administration significantly attenuated the ability of FFSS to induce Prg4 expression by ∼60% (Fig. 5D). One potential pathway for PKA signaling to induce gene transcription is via PKA-mediated phosphorylation of the transcription factor CREB (on Ser133). To examine whether CREB activity is necessary for FFSS to induce Prg4 expression, we transfected epiphyseal chondrocytes with an expression vehicle encoding ACREB, a dominant-negative form of CREB that has been previously used to block CREB activity both in vitro (Ahn et al. 1998) and in vivo (Long et al. 2001). Indeed, transfection of an expression vehicle encoding ACREB attenuated the ability of FFSS to induce Prg4 expression (Fig. 5D). Consistent with a role for CREB in promoting the induction of Prg4, we found that FFSS increased steady-state levels of both total and phospho-S133 CREB in epiphyseal chondrocytes (Fig. 5E) and also increased binding of phospho-S133 CREB to chromatin encompassing either of two CREB-binding sites (both of which contain the CRE consensus core-binding sequence CGTCA) located either upstream of the transcriptional start site (TSS) of Prg4 (i.e., CRE1, at −4503 base pair [bp] relative to the TSS) or in the first intron of this gene (i.e., CRE2, at +679 bp relative to the TSS) (Fig. 5F). In addition, we found that FFSS could induce the expression (in transfected epiphyseal chondrocytes) of a luciferase reporter containing the Prg4 basal promoter driven by four repeats of either a 700-bp sequence encompassing the CRE1-binding site or a 220-bp sequence encompassing the downstream CRE2-binding site (Fig. 5G). In both cases, mutation of the CRE consensus core-binding site (which eliminated binding of CREB to either of these sequences) (Supplemental Fig. 5) completely eliminated induction of these reporters by FFSS (Fig. 5G).
Figure 5.
FFSS induces Prg4 expression via the PKA/CREB signaling pathway. (A) Epiphyseal chondrocytes were cotransfected with a luciferase reporter driven by either reiterated consensus TCF-binding sites (TOP-firefly luciferase) or mutated TCF-binding sites (FOP-firefly luciferase) plus SV40 Renilla luciferase. The chondrocytes were cultured in the presence of either FFSS, exogenous PGE2 (0.01, 0.1, and 1 μM), ATP (0.1 and 1 mM), the Wnt agonist BIO (10 μM), or forskolin (FSK; 10 μM). The ratio of TOP/FOP luciferase activity (normalized to that of SV40 Renilla luciferse) is shown for each treatment regimen. (B) Epiphyseal chondrocytes were cotransfected with a luciferase reporter driven by reiterated consensus CRE-binding sites (CRE-firefly luciferase) plus SV40 Renilla luciferase. The chondrocytes were cultured in the presence of either FFSS, exogenous PGE2 (0.01, 0.1, and 1 μM), ATP (0.1 and 1 mM), the Wnt agonist BIO (10 μM), or FSK (1 μM). Relative luciferase activity (RLA; normalized to that of SV40 Renilla luciferase) is shown for each treatment regimen. (C) Transfection of epiphyseal chondrocytes (cultured under static conditions) with an expression vehicle encoding an activated form of PKA or treatment with FSK was sufficient to induce expression of Prg4. Prg4 expression was assayed relative to β-actin by RT-qPCR. (D) Administration of the PKA antagonist H89 or transfection with an expression vehicle encoding ACREB attenuated the ability of FFSS to induce Prg4 expression in epiphyseal chondrocytes. Prg4 expression was assayed relative to β-actin by RT-qPCR. (E) FFSS increases steady levels of both total and phospho-S133 CREB in epiphyseal chondrocytes. (F) FFSS increases binding of phospho-CREB to two conserved CRE-binding sites (CRE1 and CRE2) that surround the TSS of Prg4 in epiphyseal chondrocytes, as detected by chromatin immunoprecipitation. (G) Epiphyseal chondrocytes were cotransfected with a firefly luciferase reporter driven by the Prg4 basal promoter plus four repeats of Prg4 sequences encompassing either wild-type or mutant CRE1- or CRE2-binding sites plus SV40-Renilla luciferase. The cells were cultured under either static or FFSS conditions, and RLA is displayed. In both cases, mutation of the CRE consensus core-binding site completely eliminated induction of these reporters by FFSS. Throughout this figure: (*) P < 0.05; (**) P < 0.01. Error bar indicates standard error of the mean.
Either FFSS or running induces both an increase of phospho-S133 CREB and nuclear translocation of the CREB-regulated transcriptional coactivators 1/2 (CRTC1/2) in chondrocytes
In addition to P300/CBP, CREB interacts with another coactivator family, termed either transducers of regulated CREB activity (TORCs) or CRTCs (for review, see Altarejos and Montminy 2011). The CRTC family consists of three members (CRTC1, CRTC2, and CRTC3). Nuclear translocation and subsequent interaction of the CRTC proteins with CREB is blocked by phosphorylation of CRTC proteins by salt-inducible kinases 1/2 (SIK1/2), which phosphorylate CRTC proteins (on S151 in CRTC1 and on S171 in CRTC2) to generate an essential 14-3-3-binding site, thus inducing interaction of these proteins with cytoplasmic 14-3-3 proteins (for review, see Altarejos and Montminy 2011). SIK-mediated phosphorylation of CRTC proteins is regulated by either CaMK 1/IV, which can phosphorylate SIK2, resulting in subsequent SIK2 ubiquitination and proteolysis (Sasaki et al. 2011), or PKA-mediated phosphorylation of SIK family members, which blocks the activity of these proteins (for review, see Altarejos and Montminy 2011). In addition, the Ca++-inducible phosphatase calcineurin can also remove the SIK2 phosphorylation mark from CRTC proteins (for review, see Altarejos and Montminy 2011) and thereby promote the nuclear translocation of CRTC proteins and their subsequent interaction with CREB. Thus, either increased intracellular Ca++/activated calmodulin/CaMK or elevated PKA signaling blocks CRTC protein phosphorylation by SIK2 and renders CRTC1/2 proteins able to translocate into the nucleus and interact with CREB (schematically outlined in Fig. 7, below).
Figure 7.
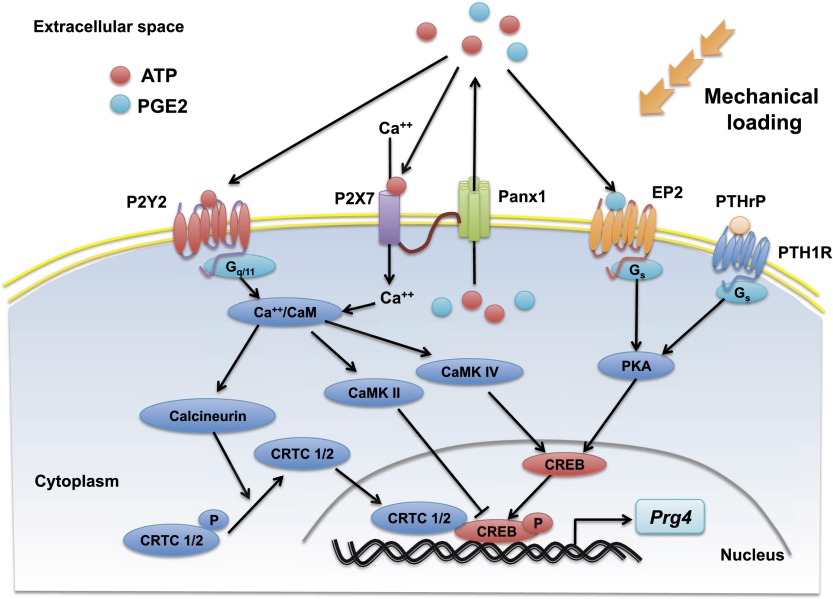
Mechanical motion/FFSS induce Prg4 expression in articular cartilage via PGE2, PTHrP, and ATP signaling pathways. Our results suggest that both running (i.e., mechanical loading in vivo) and FFSS applied to articular cartilage in vitro increase extracellular levels of PGE2, PTHrP, and ATP in this tissue. PGE2 and ATP escape from the cell via the P2X7-coupled pannexin 1 channel. PGE2 and PTHrP work via the Gs(α)-coupled EP2 receptor and the PTH1 receptor, respectively, to activate PKA and induce phosphorylation of CREB-S133 (for review, see Altarejos and Montminy 2011). Extracellular ATP works via both the P2X7 (Grol et al. 2012) and the P2Y2 receptors to elevate intracellular Ca++, which in turn induces dephosphorylation and nuclear translocation of CRTC family members CRTC1/2. Phospho-S133 CREB works in combination with nuclear CRTC1/2 to induce expression of Prg4 in articular chondrocytes.
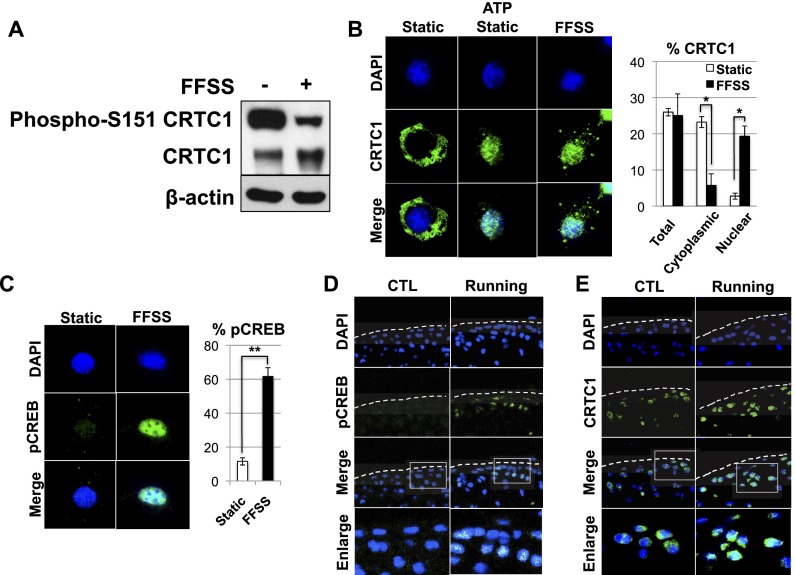
Because we found that FFSS promotes the induction of Prg4 expression in epiphyseal chondrocytes by inducing the activity of CREB, we therefore evaluated whether FFSS could induce dephosphorylation of phospho-S151 in CRTC1 and thereby induce nuclear translocation of this CREB coactivator. Indeed, we observed that FFSS administration to epiphyseal chondrocytes did indeed promote dephosphorylation of phospho-S151 in CRTC1 (Fig. 6A) and concomitantly induced a striking translocation of predominantly cytoplasmic CRTC1 (in static epiphyseal chondrocytes) into the nucleus (in FFSS-treated cells) (Fig. 6B). Interestingly, application of extracellular ATP to static chondrocytes similarly induced nuclear translocation of CRTC1 (Fig. 6B). Like CRTC1, nuclear translocation of CRTC2 was similarly promoted in epiphyseal chondrocytes by administration of either extracellular ATP or FFSS culture conditions (Supplemental Fig. 6). In addition to promoting nuclear translocation of CRTC1/2, FFSS also induced the accumulation of phospho-S133 CREB into the nucleus of cultured epiphyseal chondrocytes (Fig. 6C).
Figure 6.
FFSS/running both decrease phosphorylation of S-151 in CRTC1 and induce nuclear translocation of CRTC1 in articular cartilage. (A) Western analysis for either phospho-S-151 CRTC1, total CRTC1, or β-actin in mouse epiphyseal chondrocytes that had been cultured under either FFSS or static conditions. (B) Cellular localization of CRTC1 (by immunofluorescence) in epiphyseal chondrocytes cultured under either static conditions (in either the absence or presence of extracellular ATP) or FFSS conditions. Quantitation of cellular localization of CRTC1 is displayed on the right. (C) Cellular localization of phospho-S133 CREB (by immunofluorescence) in epiphyseal chondrocytes cultured under either static or FFSS conditions. Quantitation of chondrocytes with detectable nuclear phospho-S133 CREB is displayed on the right. (D,E) Three-month-old female Prg4GFPCreERt2/+; Rosa26floxlacZ/+ mice were housed in either the absence or presence of a running wheel for 1 mo, after which their knees were isolated, fixed, sectioned, and immunostained to assay cellular localization of either phospho-S133 CREB (D) or CRTC1 (E).
To investigate whether mechanical motion in vivo similarly promoted accumulation of nuclear phospho-S133 CREB or nuclear translocation of CRTC1, we evaluated the expression (and localization) of both phospho-S133 CREB and CRTC1 in knee joint articular cartilage of mice that had been housed for 1 mo in either the absence or presence of a running wheel. We observed that wheel running induced a striking increase in phospho-S133 CREB levels in tibial articular cartilage in the knee (Fig. 6D). Because PKA-mediated phosphorylation of CREB on Ser133 increases CREB interaction with P300/CBP (for review, see Altarejos and Montminy 2011), these findings suggest that wheel running promotes interaction of CREB with P300/CBP in articular chondrocytes. CRTC1 was predominantly located in the cytoplasm in tibial articular chondrocytes of mice housed in the absence of a running wheel, as evidenced by crescents of CRTC1 immunofluorescence in the cytoplasm surrounding DAPI-stained nuclei (Fig. 6E, left panels). In contrast, in tibial articular chondrocytes of mice housed for 1 mo with a running wheel, CRTC1 levels were increased, and CRTC1 was evident in both the cytoplasm and the nucleus (i.e., overlapping with DAPI staining) (Fig. 6E, right panels). Together, these findings indicate that running induces both an increase of phospho-S133 CREB and increased nuclear localization of CRTC1 in articular chondrocytes.
Discussion
Wheel running induces Prg4 expression via PGE2 signaling
Lifelong voluntary wheel running has been found to have a protective effect against the progression of osteoarthritis in mice that are heterozygous for mutations in the Col2a1 gene (Lapvetelainen et al. 2001). Because Prg4 expression in the articular cartilage both has been noted to be sensitive to mechanical loading (Nugent et al. 2006a,b; Jones et al. 2009) and was recently found to protect against the development of osteoarthritis (Ruan et al. 2013), we wondered whether voluntary wheel running might induce the expression of Prg4 in articular cartilage and thereby slow the progression of osteoarthritis. By employing Prg4GFPCreERt2/+; Rosa26floxlacZ/+ mice, we found that voluntary wheel running (i.e., exercise) does indeed increase expression of the Prg4 locus, most significantly in the superficial-most cells in tibial articular cartilage. In addition, we also observed a slight increase in EdU incorporation in the articular cartilage following 1 mo of wheel running, but this augmented level of cell proliferation was not sufficient to significantly increase the pre-existing Prg4-expressing cell population. Running induced an increased accumulation of PGE2 in the superficial zone of knee articular cartilage, which correlated with significantly increased levels of both phospho-S133 CREB and nuclear-localized CRTC1 in this tissue. Most notably, the increased expression of Prg4 in articular cartilage induced by wheel running was significantly attenuated by administration of the COX-2 inhibitor Celecoxib, suggesting that PGE2 signaling is necessary for maximal expression of Prg4 in the articular cartilage following exercise. Muscular motion has previously been noted to be necessary for appropriate joint formation by promoting contraction-dependent activation of β-catenin in the developing joint (Kahn et al. 2009). Seeing that activation of Gs(α)-coupled receptors can stabilize β-catenin and thereby enhance Wnt signaling (Castellone et al. 2005; Ke et al. 2012), it will be interesting to determine whether the effects of muscular motion on early joint formation is also mediated by the induction of ligands such as PGE2 and/or PTHrP (discussed below), both of which signal via Gs(α)-coupled receptors and are induced by mechanical motion in articular cartilage (Chen et al. 2008; this study).
FFSS induces Prg4 expression via PGE2/PTHrP/extracellular ATP/CREB signaling pathways
FFSS has been shown to transduce mechanical motion in osteocytes via both connexin and pannexin channels, which release both ATP and PGE2 into the extracellular space that in turn interact with their cognate receptors to alter gene expression in these cells (Bao et al. 2004; Cherian et al. 2005; Li et al. 2005; Batra et al. 2012). Indeed, mice engineered to lack the P2X7 ATP receptor display a striking reduction in the rate of periosteal bone formation (Ke et al. 2003), and administration of COX-2 inhibitors has been demonstrated to block mechanical load-induced increase in bone formation (Forwood 1996; Li et al. 2002). Thus, mechanical loading induces bone formation via both ATP and PGE2 signaling pathways. In the case of PGE2, this pathway has been demonstrated to negatively regulate osteoblast-specific expression of sclerostin (Galea et al. 2011), which is an inhibitor of bone growth. In chondrocytes, we found that FFSS increases the expression of both Ptgs2 (which encodes the PGE2 biosynthetic protein COX-2) and PTHrP, promotes the secretion of both extracellular ATP and PGE2, and induces the expression of Prg4, which is specifically expressed in superficial zone articular chondrocytes. Indeed, we observed that administration of either FFSS, PGE2, or extracellular ATP was only able to induce the expression of Prg4 in superficial, but not deeper, zone bovine articular chondrocytes and in only 3% of epiphyseal chondrocytes that had been isolated from 5-d-old Prg4GFPCreERt2/+; Rosa26floxlacZ/+ animals, thus suggesting that superficial zone articular chondrocytes (or their progenitors) express a competence factor necessary for expression of Prg4 in response to these signals.
It seems likely that both the ligand-gated P2X7 ATP receptor and the Gq(α)/G11(α)-coupled P2Y2 ATP receptor play a role in FFSS-mediated induction of Prg4 expression, as knockout/knockdown of either receptor (in epiphyseal chondrocytes) attenuates this response to FFSS. In addition, FFSS apparently induces maximal Prg4 expression in a Ca++-dependent fashion, as we observed that addition of the Ca++ chelator BAPTA to the cell culture medium significantly attenuated induction of Prg4 by FFSS and, conversely, that addition of the Ca++ ionophore A23187 robustly induced the expression of Prg4 in static chondrocytes. Together, our findings suggest that FFSS-induced secretion of PGE2, PTHrP, and extracellular ATP works in synergy to promote Prg4 expression in superficial zone chondrocytes by engaging both PKA and Ca++ signaling pathways that increase levels of phospho-S133 CREB and promote dephosphorylation and nuclear accumulation of CRTC family members (summarized in Fig. 7). In addition, we observed that FFSS increased binding of phospho-S133 CREB to two CRE-binding sites located in the regulatory regions of the Prg4 gene and noted that FFSS could induce the expression of a luciferase reporter driven by the Prg4 basal promoter plus four repeats of the Prg4 regulatory sequences encoding either of these CRE-binding sites. In both cases, mutation of the CRE consensus core-binding site completely eliminated induction of these reporters by FFSS. Most importantly, expression of a dominant-negative form of CREB (ACREB) (Ahn et al. 1998; Long et al. 2001) attenuated the induction of Prg4 by FFSS. Taken together, our findings strongly support the notion that either mechanical motion (i.e., wheel running) or FFSS induces the expression of Prg4 in superficial zone articular chondrocytes by promoting CREB-dependent induction of this gene.
While we observed that administration of FFSS of ∼7.6 dyn/cm2 can induce the expression of Prg4 in either a subpopulation of murine epiphyseal chondrocytes or bovine superficial articular chondrocytes in vitro, it is unclear how this level of shear force compares with that experienced by articular chondrocytes in response to mechanical loading in vivo. On the other hand, because either administration of FFSS in vitro or wheel running both induce secretion of PGE2 and nuclear accumulation of phospho-CREB and CRTC1 and promote COX-2-dependent expression of Prg4 in articular chondrocytes, it seems likely that administration of FFSS in vivo or wheel running induces these mechano-stimulated responses via similar signaling pathways.
The chondro-protective activities of either PTh1r signaling or wheel running may be due to the induced expression of Prg4
In addition to PGE2/EP2 signaling, Prg4 expression in the articular cartilage may also be induced via the PTH/PTHrP receptor PTh1r, which, like EP2, is also coupled to Gs(α) (for review, see Sakamoto et al. 2005). PThrP is known to be expressed in articular cartilage (Chen et al. 2008), and its expression has been shown to be induced in articular chondrocytes by joint loading (Chen et al. 2008) or FFSS administration to epiphyseal chondrocytes (Supplemental Fig. 3G), thus supporting a role for the PTh1r receptor (along with the PGE2 receptor EP2) in promoting the expression of Prg4 in response to wheel running/FFSS. Consistent with this idea, we observed that overexpression of PTHrP induced Prg4 expression in static epiphyseal chondrocytes and, conversely, that knockdown of PTHrP with siRNA attenuated the ability of FFSS to induce Prg4 expression in chondrocytes. Interestingly, conditional deletion of PTHrPflox/flox from articular cartilage (as driven by GDF5-Cre) has been noted to cause increased cartilage degradation following surgically induced joint destabilization (Macica et al. 2011). In contrast, forced expression of transgenic Prg4 in articular cartilage (Ruan et al. 2013) and systemic administration of recombinant human PTH(1–34) (teriparatide) (Sampson et al. 2011) both significantly attenuated cartilage degradation following surgically induced joint destabilization. Because both wheel running (this study) and PTh1r signaling (Sampson et al. 2011) induce Prg4 expression in articular cartilage, it seems plausible that the chondro-protective activities of PTh1r signaling (Macica et al. 2011) and/or wheel running (Lapvetelainen et al. 2001) may be due to the induced expression of Prg4 in articular cartilage (which is itself chondro-protective in murine osteoarthritis models) (Ruan et al. 2013).
Materials and methods
Mouse strains, wheel-running regimens, tamoxifen and Celecoxib treatment, and EdU injection
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at Harvard Medical School. Prg4GFPCreERt2/+; Rosa26floxlacZ/+ mice (either 3-mo-old females [used in Fig. 1] or 2-mo-old males [used in Supplemental Fig.1]) (E Kozhemyakina, M Zhang, A Ionescu, N Ono, A Kobayashi, H Kronenberg, ML Warman, and AB Lassar, in prep.) were housed individually either with or without a running wheel (9.5-in diameter) connected to a digital counter (Mini Mitter) for 31 d prior to sacrifice. Tamoxifen (2 g/kg body weight; Sigma) was administered daily by intraperitoneal (i.p.) injection for either seven or 10 consecutive days as indicated in the figure legends. EdU (50 mg/kg body weight; Invitrogen) was administered daily by i.p. injection for seven consecutive days (Supplemental Fig. 1). Celecoxib (50 mg/kg body weight; LC Laboratories) was administered daily by oral gavage for 31 consecutive days (Fig. 1F,G).
Isolation of mouse epiphyseal chondrocytes and bovine articular chondrocytes
Mouse epiphyseal chondrocytes were isolated from both the femoral heads and femoral and tibial condyles of 5-d-old mice as previously described (Gosset et al. 2008). Mouse epiphyseal chondrocytes were isolated from either Prg4GFPCreERt2/+; Rosa26floxlacZ/+ (C57BL/6J background), C57BL/6J wild-type, or BALB/c wild-type mice. Unless otherwise mentioned, cell culture experiments were performed with epiphyseal chondrocytes isolated from 5-d-old Prg4GFPCreERt2/+ mice. Bovine superficial zone and deeper zone articular chondrocytes were isolated from 1- to 2-wk-old bovine calves as previously described (Kisiday et al. 2005).
Culture of chondrocytes under static or FFSS conditions
Chondrocytes (mouse epiphyseal or bovine articular) were seeded at 2 × 105 cells per square centimeter into regular six-well tissue culture plates (culture plate diameter = 3.48 cm) (Corning) and cultured in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F-12 (DMEM/F-12) containing 10% Nu-Serum IV (BD Bioscience) and penicillin/streptomycin (culture medium) (Invitrogen) with 5% CO2 in humidified air for 48 h (until cells were subsequently cultured under either static or FFSS conditions). Thirty minutes prior to exposure to subsequent static or FFSS conditions, medium was very gently changed (with 4 mL of new culture medium). For cultures exposed to FFSS, culture dishes were placed onto an orbital shaker (with a 0.5-cm radius of gyration) (IKA, KS 260 basic) that was set to rotate at five rotations per sec at 37°C, which exposed the chondrocytes to a steady laminar, nonpulsatile shear stress of ∼7.6 dyn/cm2 . An estimate of the maximal shear stress (τw) at the bottom of the dish can be calculated as τw = α √[ρη(2πf)3], where α is the radius of gyration of the shaker (in centimeters), ρ is the density of the medium (in grams per milliliter), η = 7.5 × 10−3 dyn/cm2 at 37°C, and f = frequency of rotation (rotations per second) (Pearce et al. 1996). Chondrocytes in culture dishes left undisturbed in a similar 37°C environment were used as static (no flow) controls. Small molecules used for cell culture included PGE2, Butaprost, CAY10598, SC19220, AH6809, L161982, A438079, forskolin, BIO, H89 (purchased from Cayman Chemical), ATP, BzATP, and BBG (purchased from Sigma).
Additional methods are available in the Supplemental Material.
Acknowledgments
We thank Matthew Warman (Children's Hospital, Boston) for help with generation of the Prg4GFPCreERt2/+ mice. In addition, we thank the Nikon Imaging Facility at Harvard Medical School for the use of their microscopes, and Jennifer Waters for her assistance with photomicroscopy. This work was supported by grants from National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)/National Institutes of Health (NIH) (AR055148, AR055552, and AR060735 to A.B.L.; and AR60331 to A.J.G). H.O. was supported by fellowships from the Uehara Memorial Foundation and the Japan Society for the Promotion of Science.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.231969.113.
References
- Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F 2002. Cutting edge: A natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol 169: 4108–4112 [DOI] [PubMed] [Google Scholar]
- Ahn S, Olive M, Aggarwal S, Krylov D, Ginty DD, Vinson C 1998. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol 18: 967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarejos JY, Montminy M 2011. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol 12: 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahabri SA, Suwairi WM, Laxer RM, Polinkovsky A, Dalaan AA, Warman ML 1998. The camptodactyly-arthropathy-coxa vara-pericarditis syndrome: Clinical features and genetic mapping to human chromosome 1. Arthritis Rheum 41: 730–735 [DOI] [PubMed] [Google Scholar]
- Bao L, Locovei S, Dahl G 2004. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572: 65–68 [DOI] [PubMed] [Google Scholar]
- Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, DeSimone D, Bonewald LF, Lafer EM, Sprague E, et al. 2012. Mechanical stress-activated integrin α5β1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci 109: 3359–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G 2012. Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. Bioessays 34: 218–225 [DOI] [PubMed] [Google Scholar]
- Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS 2005. Prostaglandin E2 promotes colon cancer cell growth through a Gs–axin–β-catenin signaling axis. Science 310: 1504–1510 [DOI] [PubMed] [Google Scholar]
- Chen X, Macica CM, Nasiri A, Broadus AE 2008. Regulation of articular chondrocyte proliferation and differentiation by Indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum 58: 3788–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian PP, Cheng B, Gu S, Sprague E, Bonewald LF, Jiang JX 2003. Effects of mechanical strain on the function of Gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem 278: 43146–43156 [DOI] [PubMed] [Google Scholar]
- Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX 2005. Mechanical strain opens connexin 43 hemichannels in osteocytes: A novel mechanism for the release of prostaglandin. Mol Biol Cell 16: 3100–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood MR 1996. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res 11: 1688–1693 [DOI] [PubMed] [Google Scholar]
- Galea GL, Sunters A, Meakin LB, Zaman G, Sugiyama T, Lanyon LE, Price JS 2011. Sost down-regulation by mechanical strain in human osteoblastic cells involves PGE2 signaling via EP4. FEBS Lett 585: 2450–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosset M, Berenbaum F, Thirion S, Jacques C 2008. Primary culture and phenotyping of murine chondrocytes. Nat Protoc 3: 1253–1260 [DOI] [PubMed] [Google Scholar]
- Grol MW, Zelner I, Dixon SJ 2012. P2X(7)-mediated calcium influx triggers a sustained, PI3K-dependent increase in metabolic acid production by osteoblast-like cells. Am J Physiol Endocrinol Metab 302: E561–E575 [DOI] [PubMed] [Google Scholar]
- Jones AR, Chen S, Chai DH, Stevens AL, Gleghorn JP, Bonassar LJ, Grodzinsky AJ, Flannery CR 2009. Modulation of lubricin biosynthesis and tissue surface properties following cartilage mechanical injury. Arthritis Rheum 60: 133–142 [DOI] [PubMed] [Google Scholar]
- Kahn J, Shwartz Y, Blitz E, Krief S, Sharir A, Breitel DA, Rattenbach R, Relaix F, Maire P, Rountree RB, et al. 2009. Muscle contraction is necessary to maintain joint progenitor cell fate. Dev Cell 16: 734–743 [DOI] [PubMed] [Google Scholar]
- Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, et al. 2003. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol 17: 1356–1367 [DOI] [PubMed] [Google Scholar]
- Ke J, Zhang C, Harikumar KG, Zylstra-Diegel CR, Wang L, Mowry LE, Miller LJ, Williams BO, Xu HE 2012. Modulation of β-catenin signaling by glucagon receptor activation. PLoS ONE 7: e33676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiday JD, Kurz B, DiMicco MA, Grodzinsky AJ 2005. Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng 11: 141–151 [DOI] [PubMed] [Google Scholar]
- Knight MM, McGlashan SR, Garcia M, Jensen CG, Poole CA 2009. Articular chondrocytes express connexin 43 hemichannels and P2 receptors - a putative mechanoreceptor complex involving the primary cilium? J Anat 214: 275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, Okabe T, Ochiai T, Kamiya N, Rountree RB, et al. 2008. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol 316: 62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapvetelainen T, Hyttinen M, Lindblom J, Langsjo TK, Sironen R, Li SW, Arita M, Prockop DJ, Puustjarvi K, Helminen HJ 2001. More knee joint osteoarthritis (OA) in mice after inactivation of one allele of type II procollagen gene but less OA after lifelong voluntary wheel running exercise. Osteoarthritis Cartilage 9: 152–160 [DOI] [PubMed] [Google Scholar]
- Lee SY, Niikura T, Reddi AH 2008. Superficial zone protein (lubricin) in the different tissue compartments of the knee joint: Modulation by transforming growth factor β 1 and interleukin-1 β. Tissue Eng Part A 14: 1799–1808 [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Bhattaram P 2010. Vertebrate skeletogenesis. Curr Top Dev Biol 90: 291–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Burr DB, Turner CH 2002. Suppression of prostaglandin synthesis with NS-398 has different effects on endocortical and periosteal bone formation induced by mechanical loading. Calcif Tissue Int 70: 320–329 [DOI] [PubMed] [Google Scholar]
- Li J, Liu D, Ke HZ, Duncan RL, Turner CH 2005. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem 280: 42952–42959 [DOI] [PubMed] [Google Scholar]
- Long F, Schipani E, Asahara H, Kronenberg H, Montminy M 2001. The CREB family of activators is required for endochondral bone development. Development 128: 541–550 [DOI] [PubMed] [Google Scholar]
- Macica C, Liang G, Nasiri A, Broadus AE 2011. Genetic evidence of the regulatory role of parathyroid hormone-related protein in articular chondrocyte maintenance in an experimental mouse model. Arthritis Rheum 63: 3333–3343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelino J, Carpten JD, Suwairi WM, Gutierrez OM, Schwartz S, Robbins C, Sood R, Makalowska I, Baxevanis A, Johnstone B, et al. 1999. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet 23: 319–322 [DOI] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Wright MO, Flatman PW, Salter DM 2004. ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology 41: 567–575 [PubMed] [Google Scholar]
- Nugent GE, Aneloski NM, Schmidt TA, Schumacher BL, Voegtline MS, Sah RL 2006a. Dynamic shear stimulation of bovine cartilage biosynthesis of proteoglycan 4. Arthritis Rheum 54: 1888–1896 [DOI] [PubMed] [Google Scholar]
- Nugent GE, Schmidt TA, Schumacher BL, Voegtline MS, Bae WC, Jadin KD, Sah RL 2006b. Static and dynamic compression regulate cartilage metabolism of PRoteoGlycan 4 (PRG4). Biorheology 43: 191–200 [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Iwamoto M 2005. Mechanisms of synovial joint and articular cartilage formation: Recent advances, but many lingering mysteries. Birth Defects Res C Embryo Today 75: 237–248 [DOI] [PubMed] [Google Scholar]
- Pearce MJ, McIntyre TM, Prescott SM, Zimmerman GA, Whatley RE 1996. Shear stress activates cytosolic phospholipase A2 (cPLA2) and MAP kinase in human endothelial cells. Biochem Biophys Res Commun 218: 500–504 [DOI] [PubMed] [Google Scholar]
- Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, Jay GD, Stewart M, Wang H, Warman ML, et al. 2005. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest 115: 622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan MZ, Erez A, Guse K, Dawson B, Bertin T, Chen Y, Jiang MM, Yustein J, Gannon F, Lee BH 2013. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med 5: 176ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A, Chen M, Kobayashi T, Kronenberg HM, Weinstein LS 2005. Chondrocyte-specific knockout of the G protein G(s)α leads to epiphyseal and growth plate abnormalities and ectopic chondrocyte formation. J Bone Miner Res 20: 663–671 [DOI] [PubMed] [Google Scholar]
- Sampson ER, Hilton MJ, Tian Y, Chen D, Schwarz EM, Mooney RA, Bukata SV, O'Keefe RJ, Awad H, Puzas JE et al. 2011. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med 3: 101ra93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Takemori H, Yagita Y, Terasaki Y, Uebi T, Horike N, Takagi H, Susumu T, Teraoka H, Kusano K, et al. 2011. SIK2 is a key regulator for neuronal survival after ischemia via TORC1-CREB. Neuron 69: 106–119 [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S 2007. Prostaglandin E receptors. J Biol Chem 282: 11613–11617 [DOI] [PubMed] [Google Scholar]
- Swann DA, Silver FH, Slayter HS, Stafford W, Shore E 1985. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem J 225: 195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi MM, Islam S, Suadicani SO, Spray DC 2012. Connexin43 and pannexin1 channels in osteoblasts: Who is the ‘hemichannel’? J Membr Biol 245: 401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Batra N, Shi Q, Bonewald LF, Sprague E, Jiang JX 2010. Prostaglandin promotion of osteocyte gap junction function through transcriptional regulation of connexin 43 by glycogen synthase kinase 3/β-catenin signaling. Mol Cell Biol 30: 206–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara R, Ohta Y, Yuasa T, Kondo N, Hoang T, Addya S, Fortina P, Pacifici M, Iwamoto M, Enomoto-Iwamoto M 2011. Roles of β-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab Invest 91: 1739–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]