Abstract
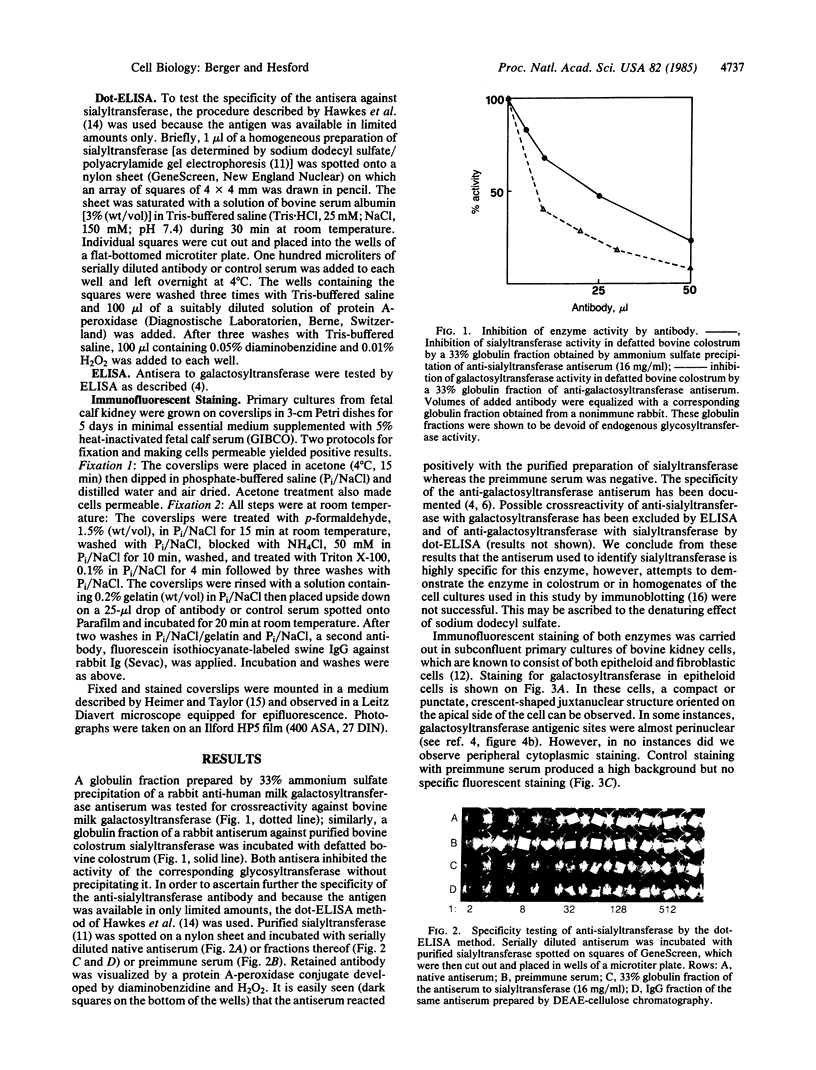
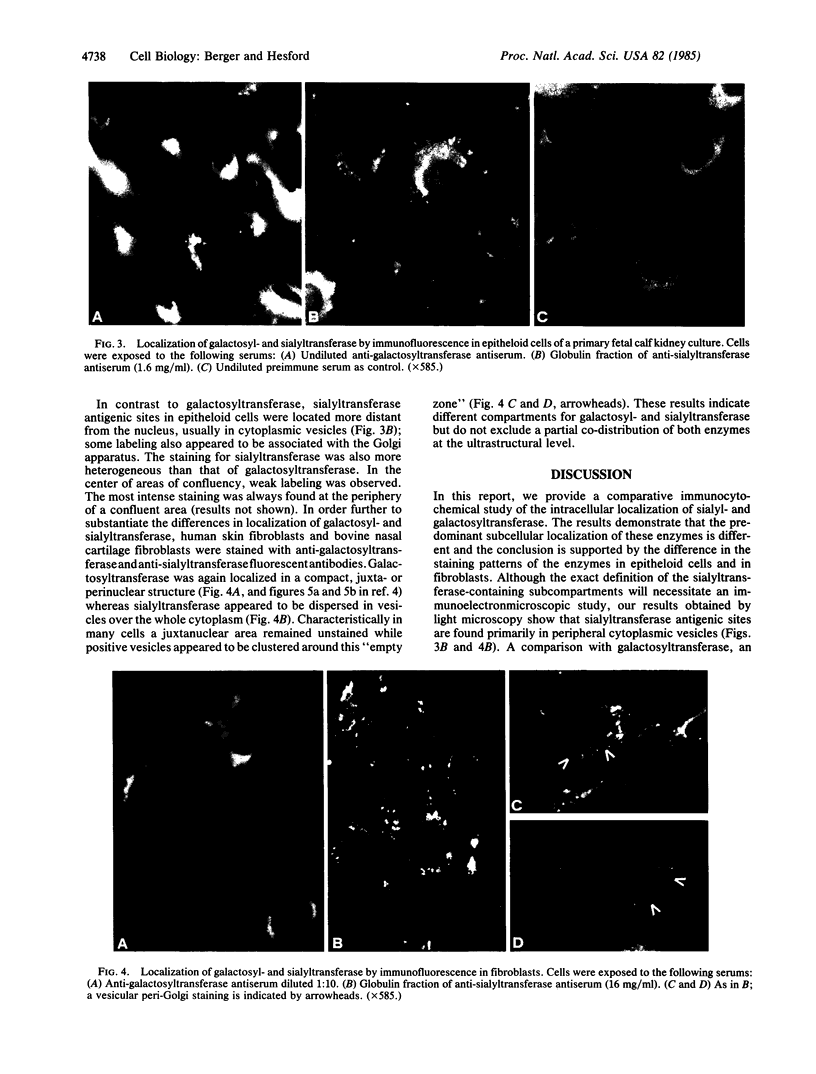
Polyclonal rabbit antisera against soluble human milk galactosyltransferase and bovine colostrum sialyltransferase were used to localize by indirect immunofluorescence the respective intracellular enzymes in primary cultures from bovine fetal kidneys and established cell lines of human and bovine fibroblasts. Staining for galactosyltransferase was juxtanuclear and crescent shaped in epitheloid cells; a similar staining, occasionally perinuclear and sparsely distributed in the cytoplasm, was found in fibroblasts. In contrast, staining for sialyltransferase in epitheloid kidney cells derived from the same primary culture was observed predominantly in cytoplasmic vesicles that were spread over the whole cytoplasm. Sialyltransferase-positive vesicles had a similar distribution in fibroblasts and often appeared concentrated around an unstained Golgi area. Thus, in both cell types galactosyl- and sialyltransferase were localized in different subcellular compartments. Since both galactosyl- and sialyltransferase participate in formation of the terminal glycan NeuAc(alpha 2----6)Gal(beta 1----4)GlcNAc(Neu, neuraminic acid) present in many N-glycosidic complex types of glycans, different subcellular compartments for these enzymes support a model of functional compartmentalization of the Golgi apparatus that is compatible with an assembly-line model for glycan chain elongation and termination.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G., O'Shaughnessy D. The site of incorporation of sialic acid residues into glycoproteins and the subsequent fates of these molecules in various rat and mouse cell types as shown by radioautography after injection of [3H]N-acetylmannosamine. I. Observations in hepatocytes. J Cell Biol. 1981 Jan;88(1):1–15. doi: 10.1083/jcb.88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. G., Buddecke E., Kamerling J. P., Kobata A., Paulson J. C., Vliegenthart J. F. Structure, biosynthesis and functions of glycoprotein glycans. Experientia. 1982 Oct 15;38(10):1129–1162. doi: 10.1007/BF01959725. [DOI] [PubMed] [Google Scholar]
- Berger E. G., Mandel T., Schilt U. Immunohistochemical localization of galactosyltransferase in human fibroblasts and HeLa cells. J Histochem Cytochem. 1981 Mar;29(3):364–370. doi: 10.1177/29.3.6787115. [DOI] [PubMed] [Google Scholar]
- Bretz R., Bretz H., Palade G. E. Distribution of terminal glycosyltransferases in hepatic Golgi fractions. J Cell Biol. 1980 Jan;84(1):87–101. doi: 10.1083/jcb.84.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Brands R., Rothman J. E. Attachment of terminal N-acetylglucosamine to asparagine-linked oligosaccharides occurs in central cisternae of the Golgi stack. Cell. 1985 Feb;40(2):463–472. doi: 10.1016/0092-8674(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. C., Kozdrowski I., Wyss S. R., Berger E. G. The charge heterogeneity of soluble human galactosyltransferases isolated from milk, amniotic fluid and malignant ascites. Eur J Biochem. 1979 Feb 1;93(3):453–460. doi: 10.1111/j.1432-1033.1979.tb12843.x. [DOI] [PubMed] [Google Scholar]
- Goldfischer S. The internal reticular apparatus of Camillo Golgi: a complex, heterogeneous organelle, enriched in acid, neutral, and alkaline phosphatases, and involved in glycosylation, secretion, membrane flow, lysosome formation, and intracellular digestion. J Histochem Cytochem. 1982 Jul;30(7):717–733. doi: 10.1177/30.7.6286754. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Heimer G. V., Taylor C. E. Improved mountant for immunofluorescence preparations. J Clin Pathol. 1974 Mar;27(3):254–256. doi: 10.1136/jcp.27.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatra B. S., Herries D. G., Brew K. Some kinetic properties of human-milk galactosyl transferase. Eur J Biochem. 1974 May 15;44(2):537–560. doi: 10.1111/j.1432-1033.1974.tb03513.x. [DOI] [PubMed] [Google Scholar]
- Klohs W. D., Bernacki R. J., Korytnyk W. Effects of nucleotides and nucleotide:analogs on human serum sialyltransferase. Cancer Res. 1979 Apr;39(4):1231–1238. [PubMed] [Google Scholar]
- Kreisel W., Volk B. A., Büchsel R., Reutter W. Different half-lives of the carbohydrate and protein moieties of a 110,000-dalton glycoprotein isolated from plasma membranes of rat liver. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1828–1831. doi: 10.1073/pnas.77.4.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The role of nucleoside diphosphatase in a uridine nucleotide cycle associated with lactose synthesis in rat mammary-gland Golgi apparatus. Biochem J. 1977 Dec 15;168(3):423–433. doi: 10.1042/bj1680423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff A. B. The endoplasmic reticulum: a cytochemist's view (a review). Proc Natl Acad Sci U S A. 1976 Aug;73(8):2781–2787. doi: 10.1073/pnas.73.8.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P. M., Novikoff A. B., Quintana N., Hauw J. J. Golgi apparatus, GERL, and lysosomes of neurons in rat dorsal root ganglia, studied by thick section and thin section cytochemistry. J Cell Biol. 1971 Sep;50(3):859–886. doi: 10.1083/jcb.50.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Regoeczi E., Chindemi P. A., Debanne M. T., Charlwood P. A. Partial resialylation of human asialotransferrin type 3 in the rat. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2226–2230. doi: 10.1073/pnas.79.7.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler J. E., Beyer T. A., Oppenheimer C. L., Paulson J. C., Prieels J. P., Rearick J. I., Hill R. L. Purification of mammalian glycosyltransferases. Methods Enzymol. 1982;83:458–514. doi: 10.1016/0076-6879(82)83043-7. [DOI] [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Schmid K., Polis A., Hunziker K., Fricke R., Yayoshi M. Partial characterization of the sialic acid-free forms of alpha-1-acid glycoprotein from human plasma. Biochem J. 1967 Aug;104(2):361–368. doi: 10.1042/bj1040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A. M. The confined function model of the Golgi complex: center for ordered processing of biosynthetic products of the rough endoplasmic reticulum. Int Rev Cytol. 1983;85:221–252. doi: 10.1016/S0074-7696(08)62374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro D. J., Tycko B., Fluss S. R., Maxfield F. R. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984 Jul;37(3):789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]






