Abstract
Background
This study utilized diffusion tensor imaging (DTI) to analyze white matter tractography in anterior limb of the internal capsule (ALIC), fornix, and uncinate fasciculus (UF) of individuals with 22q11.2 Deletion Syndrome and controls. Aberrations in these tracts have been previously associated with schizophrenia. With up to 25% of individuals with 22q11.2DS developing schizophrenia in adulthood, we hypothesized reduction in structural integrity of these tracts, including an association with prodromal symptoms of psychosis. We further predicted association between allelic variation in a functional polymorphism of the NoGo-66 receptor gene and 22q11.2DS white matter integrity.
Methods
Tractography was conducted using fiber assignment by streamline tracking algorithm in DTI studio. Subjects were genotyped for the rs701428 SNP of the Nogo-66 receptor gene, and assessed for presence of prodromal symptoms.
Results
We found significant group differences between 22q11.2DS and controls in DTI metrics for all three tracts. DTI metrics of ALIC and UF were associated with prodromal symptoms in 22q11.2DS. Further, ALIC DTI metrics were associated with allelic variation of the rs701428 SNP of the NoGo-66 receptor gene in 22q11.2DS.
Conclusions
Alterations in DTI metrics suggest white matter microstructural anomalies of the ALIC, fornix, and UF in 22q11.2DS. Structural differences in ALIC appear to be associated with the Nogo-66 receptor gene, which has been linked to myelin-mediated axonal growth inhibition. Moreover, the association between psychosis symptoms and ALIC and UF metrics suggests that the Nogo-66 receptor gene may represent a susceptibility gene for psychosis through its disruption of white matter microstructure and myelin-associated axonal growth.
Keywords: Vel-ocardio-facial Syndrome, RTN4R gene, rs701428, Diffusion Tensor Imaging (DTI), anterior limb of internal capsule, uncinate fasciculus
1. Introduction
The 22q11.2 deletion syndrome (22q11.2 DS), also known as velo-cardio-facial syndrome (VCFS), is a neurogenetic disorder involving the interstitial deletion of over 40 genes on the q11.2 band of one copy of chromosome 22. Phenotypic features of this syndrome include cardiac malformations, palatal anomalies, learning difficulties, emotion dysregulation, and social withdrawal (Shprintzen 2008; Swillen et al. 1997; Antshel et al. 2008). Strikingly, up to 25% of individuals with this syndrome develop psychosis as they transition to adulthood (Shprintzen et al. 1992; Murphy et al. 1999; Bassett and Chow 1999; Arnold et al. 2001; Green et al. 2009).
Several of the genes at the 22q11.2 locus are highly expressed in brain (Maynard et al. 2003) and known to affect neurodevelopment. One such gene is the Nogo-66 receptor gene, which codes for a glycosylphosphatidylinositol (GPI) linked protein. Nogo-66 receptor, also termed the NoGo receptor or Reticulon 4 receptor (RTN4R) (Sinibaldi et al. 2004), is associated with inhibition of axonal growth (Fournier et al. 2001). The Nogo-66 receptor is localized to axons and binds three functionally different inhibitory growth factors associated with myelin inhibition, including myelin-associated glycoprotein (MAG), oligodendrocyte-myelin glycoprotein (OMgp), and Nogo-A (RTN4). These factors inhibit growth of neurites and establishment of nerve terminals. Disruption of the Nogo-66 receptor pathway can result in insufficient inhibition of neuronal sprouting and associated processes by which myelin mediates axonal growth(Fournier et al. 2001; Fournier et al. 2002; Uranova et al. 2001). This, in turn, has been hypothesized to be associated with symptoms of schizophrenia (Karoutzou et al. 2008; Segal et al. 2007; Budel et al. 2008). Although a direct association between specific variants of the gene and schizophrenia has not been consistently identified, a recent study has demonstrated, in Caucasian populations, a nominal association between psychiatric status and the rs701428 variant (Budel et al., 2008), which is thought to contribute to the regulation of expression of the gene (Taylor et al., 2006). This variant is also included in two significant haplotype associations with disease status (Budel et al., 2008).
Recent diffusion tensor imaging (DTI) studies suggest that the microstructure of several fronto-temporal and fronto-subcortical white matter tracts is aberrant in idiopathic schizophrenia (Cummings 1995; Levy et al. 1997; Manoach et al. 2000; Levitt et al. 2002), including the anterior limb of the internal capsule (ALIC), the fornix and the uncinate fasciculus (UF). The ALIC contains fibers connecting the thalamus to frontal lobe and corticopontine fibers (Rosenberger et al. 2012). Bilateral reductions in fractional anisotropy (FA) and increases in radial diffusivity (RD) have been reported in the ALIC of individuals with schizophrenia (Levitt et al. 2010; Rosenberger et al. 2012), and have been associated with prefrontal–based neuropsychological impairments such as episodic memory (Levitt et al. 2010). The fornix connects the hippocampus to subcortical structures, including mammillary bodies, anterior nucleus of the thalamus, nucleus accumbens, and ventral tegmental area, as well as prefrontal cortex. Reduced FA in the fornix of individuals with schizophrenia has been associated with impairments in visual and verbal memory (Fitzsimmons et al. 2009; Nestor et al. 2007; Kuroki et al. 2006). The UF connects orbital prefrontal cortex to temporal lobe, playing a role in the connectivity between cortical cognition, memory function, and emotional function. Asymmetry and alterations in FA of the UF have been observed in individuals with schizophrenia (Kawashima et al. 2009; Price et al. 2008; Kubicki et al. 2002) and by our group in 22q11.2 DS, based on an atlas-based white matter analysis (Radoeva et al. 2012). Taken together, these studies support mounting evidence for alterations in fronto-temporal and fronto-subcortical connectivity in individuals in schizophrenia.
Since individuals with 22q11.2 DS are at significantly elevated risk for developing schizophrenia, we hypothesized that the structural integrity of white matter in these structures would be altered in individuals with 22q11.2DS relative to their unaffected siblings and age-matched peers, and that the white matter anomalies would be associated with an increased risk for psychosis. Moreover, since individuals with 22q11.2 DS carry only one copy of the NoGo/RTN4R gene, we further hypothesized that in the participants with 22q11.2 DS, allelic variation in the rs701428 polymorphism of this gene would be associated with the white matter integrity of these tracts of interest.
2. Materials and Methods
2.1 Subjects
Here, we report on data collected on 99 adolescents who are participants in a longitudinal study of biomarkers for psychosis in 22q11.2 DS (Kates et al. 2004; Kates et al. 2005; Kates et al. 2006a; Kates et al. 2006b; Kates et al. 2007a; Kates et al. 2007b; Antshel et al. 2005a; Antshel et al. 2005b; Antshel et al. 2007a; Antshel et al. 2007b; Antshel et al. 2008a; Antshel et al. 2008b). Data from 48 of these adolescents were also reported in an atlas-based, automated study of white matter microstructure (29). The institutional review board of the SUNY Upstate Medical University approved the procedures of this study, and all participants provided informed consent/assent. The participants in the larger, longitudinal study were recruited from the Center for the Diagnosis, Treatment, and Study of Velo-Cardio-Facial Syndrome at SUNY Upstate Medical University and from the community. A deletion at 22q11.2 was confirmed by fluorescence in situ hybridization (FISH). Exclusion criteria for all subjects are described in detail elsewhere (Kates et al. 2011).
This sample includes 52 individuals with 22q11.2 DS (22 male), and 47 matched controls (24 male), with average age for the 22q11.2 DS participants 18.0 (SD=2.2) and for the controls 18.1 (SD=1.6). The control group included 21 (9 male) unaffected siblings and 26 (15 male) community controls. Community controls and siblings did not differ significantly in any DTI metrics for the tracts we examined; accordingly we combined them into one control sample. The average full-scale IQ was 70 (SD=12.8) for the 22q11.2 DS adolescents and 105 (SD=16.1) for the controls. Although we do not have a large enough sample to control for potential effects of medication, we do provide, under Supplementary Methods, the medications that participants were taking at the time of their scan.
2.2 Nogo-66 Receptor (RTN4R) genotyping
The SNP rs701428 (corresponding to SNP “V34” in (Liu et al. 2002)) was genotyped via direct sequencing in 52 22q11.2 DS participants. Since individuals with this genetic disorder have the microdeletion on one copy of chromosome 22, participants were hemizygous for either the A allele or the G allele. Forward and reverse primers for the region flanking the SNP were designed using Primer 3 (http://frodo.wi.mit.edu/primer3/) to generate a single specific PCR product of 356bp. The PCR products were purified via Contech DNA Amplification Cleanup Kit. Purified DNA was then submitted for sequencing to GENEWIZ, a commercial sequencing facility. Chromatograms were downloaded and imported into Sequencher, a software of DNA sequence analysis. The genotype variant could not be obtained from the sequencing analysis in 9 participants, and was imputed based on linkage-disequilibrium with other SNPs (see Supplementary Materials for details of imputation methods).
2.3 Diffusion Tensor Imaging Acquisition
DTI scans were acquired on a 1.5T Philips Interra scanner (release 11) equipped with a Sense Head coil to improve signal strength and signal-to-noise ratio. We utilized a multi-slice, single-shot EPI (SENSE factor = 2.0), spin echo sequence to obtain 70 axial slices with no gaps between slices and 2.5 mm nominal isotropic resolution (TR/TE = 8197/76 ms, FOV = 240 × 240, data matrix = 96 × 96, zero-filled and reconstructed to 256 × 256). Diffusion weighting was applied along 15 directions with a b factor=800 s/mm2. One minimally weighted volume (b0) was acquired within each DTI dataset. The total scan time to acquire one DTI dataset (15 DW and 1 b0 images) was 2 min, 11 s. Four DTI datasets were acquired per subject. A high resolution T2 scan was also obtained to align with the DTI images.
2.4 DTI Processing and Data Analysis
The imaging data was processed using DTIStudio 3.0.2 (https://www.mristudio.org/). Utilizing a mutual information algorithm [35], all diffusion weighted images from each study were coregistered to the same reference volume, the b0 volume of the first repeat. Axial slices with severe scanning and motion artifacts were excluded via automatic outlier slice rejection in DTIStudio (with relative error > 3%), and through visual inspection. Tensor estimation was then performed. The high-resolution T2 scan of each participant was reoriented in AC-PC space, and the B0 (along with the tensor file) was realigned using linear registration to the high-resolution T2 scan. FA, AD, RD, and b0 maps were computed based on the tensor file in AC-PC space.
One-tensor streamline tractography was performed to identify the ALIC, the fornix and the UF. (Protocols are provided under Supplementary Methods.) For the ALIC seeding was started on all voxels within the seeding ROI with an FA value greater than 0.25; tractography was stopped in locations where the FA value was less than 0.25 or if the tract turning angle was above 70. For the fornix, the FA seeding threshold was greater than 0.15 and the tractography was stopped for FA below 0.15, and tract turning angle above 70. For the UF, tract reconstruction used an FA threshold greater than 0.3 for initiation of tracking, and FA threshold less than 0.18 or tract turning angle greater than 70 for discontinuation of tracking. (We were unable to reconstruct the left UF for one individual with 22q11.2 DS, the left fornix for 13 individuals (12 with 22q11.2 DS and 1 control), and the right fornix for 4 individuals (2 with 22q11.2 DS and 2 controls), resulting in the final sample size described above).
Inter-rater reliability, calculated with the intraclass correlation coefficient (ICC), for all tracts was high: for the ALIC, the ICC ranged (across DTI metrics and hemispheres) from 0.86 to 0.96; for the fornix it ranged from 0.95 to 0.99, and for the UF it ranged from 0.98 to 0.99.
2.5 Scale of Prodomal Symptoms (SOPS)
A trained, doctoral-level child psychologist or psychiatrist administered the SOPS (Miller et al. 2003) in order to identify the presence of prodromal psychotic symptoms (Kates et al. 2011). Inter-rater reliability, based on five SOPS interviews and assessed with the intra-class correlation coefficient, was 0.90. Since many of the children in our study had difficulty responding to a psychiatric interview, we reworded the questions in this scale to allow us to administer it to the child’s parent, and reduced the scale from a seven point to a five point Likert-type scale. Only the Positive Symptom Scale was used for the present analyses.
2.6 Statistical Analyses
Multiple analyses of variance (MANOVA) were conducted to investigate study group differences in DTI metrics. A separate MANOVA was run for each white matter tract of interest. For each white matter tract, the model included left and right FA, AD, and RD as dependent variables. Within the group of participants with 22q11.2 DS, MANOVA’s were also conducted to investigate the effect of allelic variation in the rs701428 SNP of the Nogo-66 receptor gene. Associations between DTI metrics and the SOPS were assessed with the Zero-Inflated Poisson (ZIP) regression analyses (Lambert 1992), since the distribution of our SOPS data included at least 50% of scores equaling zero (indicating the absence of any prodromal symptoms) (Kates et al. 2011). Due to the non-normal distribution of our SOPS data (Kolmogorov-Smirnov test = .476; p = 0.0001; skewness, z = 3.153; standard error = .29), we used the Mann Whitney U test to explore the effects of the SNP variant on the presence of positive prodromal symptoms in the sample with 22q11.2 DS.
3. Results
3.1 Study Group Differences
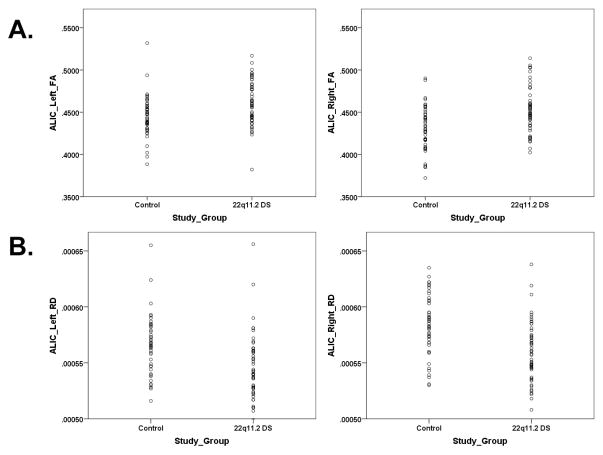
Relative to the combined group of siblings and community controls, individuals with 22q11.2DS demonstrated alterations in all three white matter tracts (see Table 1). In ALIC, participants with 22q11.2DS demonstrated significant increases in left FA (F=10.55, p<0.002, partial eta squared=0.098) and right FA (F=17.61, p<0.0001, partial eta squared=0.098, and decreases in left RD (F=13.35, p<0.0001, partial eta squared=0.121) and right RD (F=18.30, p<0.0001; partial eta squared=0.159) (see Figure 1),. In the fornix, participants with 22q11.2DS exhibited decreases in left FA (F=8.85, p<0.004, partial eta squared=0.096) and right AD (F=4.62, p<0.034, partial eta squared=0.053) (see Supplementary Figure 1). In the uncinate, participants with 22q11.2DS showed decreases in left AD (F=17.32, p<0.0001, partial eta squared=0.153), right AD (F=21.42, p<0.0001, partial eta squared=0.182), and left RD (F=5.40, p<0.022, partial eta squared=0.053) (see Supplementary Figure 2).
Table 1.
Study Group Differences in Fractional Anisotropy (FA), Axial Diffusivity (AD), and Radial Diffusivity (RD)
| White Matter Tract | 22q11.2 DS | Controls | ||
|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | |
|
|
|
|||
| ALICa,b | ||||
| Left FA | 52 | 0.461 (0.026)** | 47 | 0.445 (0.024) |
| Right FA | 52 | 0.452 (0.026)**** | 47 | 0.430 (0.026) |
| Left AD | 52 | 0.00118 (0.00004) | 47 | 0.00119 (0.00004) |
| Right AD | 52 | 0.00120 (0.00004) | 47 | 0.00120 (0.00004) |
| Left RD | 52 | 0.00055 (0.00003)**** | 47 | 0.00057 (0.00003) |
| Right RD | 52 | 0.00056 (0.00003)**** | 47 | 0.00058 (0.00003) |
| Fornixc | ||||
| Left FA | 40 | 0.338 (0.034)** | 46 | 0.359 (0.035) |
| Right FA | 50 | 0.335 (0.026) | 45 | 0.342 (0.032) |
| Left AD | 40 | 0.00209 (0.00030) | 46 | 0.00213 (0.00029) |
| Right AD | 50 | 0.00194 (0.00027)* | 45 | 0.00210 (0.00019) |
| Left RD | 40 | 0.00122 (0.00018) | 46 | 0.00119 (0.00018) |
| Right RD | 50 | 0.00115 (0.00017) | 45 | 0.00117 (0.00014) |
| Uncinated | ||||
| Left FA | 51 | 0.399 (0.025) | 47 | 0.399 (0.019) |
| Right FA | 52 | 0.387 (0.021) | 47 | 0.389 (0.018) |
| Left AD | 51 | 0.00119 (0.00003)**** | 47 | 0.00122 (0.00003) |
| Right AD | 52 | 0.00122 (0.00003)**** | 47 | 0.00125 (0.00003) |
| Left RD | 51 | 0.00061 (0.00003)* | 47 | 0.00063 (0.00003) |
| Right RD | 52 | 0.00065 (0.00002) | 47 | 0.00066 (0.00002) |
Anterior Limb of the Internal Capsule
Wilks’ Lambda = 0.775; F (6,92) = 4.45; p < 0.001; partial eta squared = 0.225); see text for results of univariate analyses of variance
Wilks’ Lambda = 0.719; F (6,78) = 5.08; p < 0.0001; partial eta squared = 0.281)
Wilks’ Lambda = 0.71; F (6,91) = 6.21; p < 0.0001; partial eta squared = 0.29)
p < 0.0001
p < 0.001 and ≥ 0.0001
p < 0.01 and ≥ 0.001
p < 0.05 and ≥ 0.01
Figure 1.
Study group differences for the left and right hemispheres of the anterior limb of the internal capsule (ALIC). DTI metrics include (A) fractional anisotropy (FA) and (B) radial diffusivity (RD).
3.2 Effects of Genotype
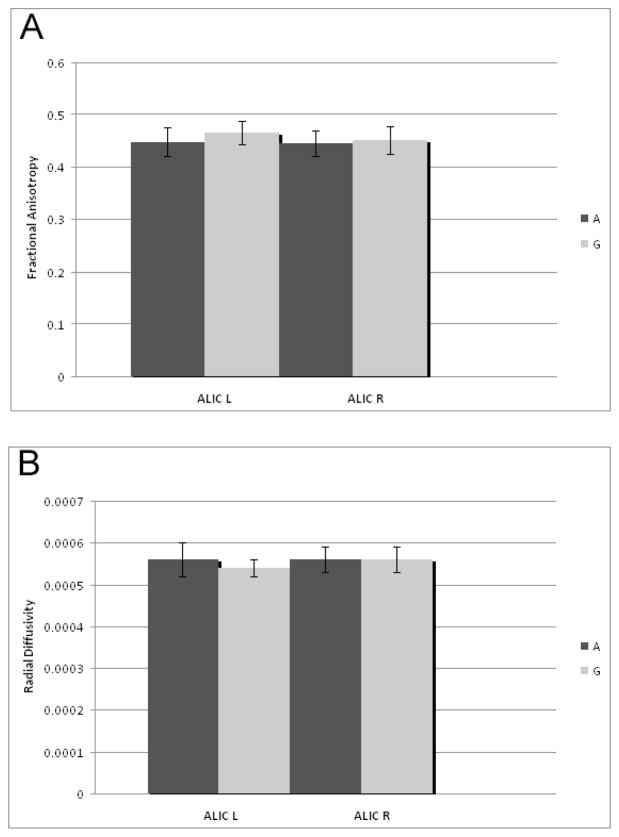
Within the sample of individuals with 22q11.2DS, we investigated the effect of allelic variation of the rs701428 SNP of the NoGo-66 Receptor gene on white matter integrity (See Table 2, Figure 2). Only the Wilks’ Lambda for the ALIC approached significance (0.695; p = 0.055). Accordingly, this SNP accounted for variability in ALIC’s left FA (F=4.44, p<0.042, partial eta squared=0.107) and left RD (F=4.33, p<0.045, partial eta squared=0.105), such that FA was higher and RD was lower in individuals who were hemizygous for the G allele. Genotype was not significantly associated with number of positive prodromal symptoms.
Table 2.
Allelic (Nogo-66/RTN4R, rs701428) Differences in Fractional Anisotropy (FA), Axial Diffusivity (AD), and Radial Diffusivity (RD) of Anterior Limb of the Internal Capsule (ALIC) in participants with 22q11.2 DS
| White Matter Tract | A Allele | G Allele | ||
|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | |
|
|
|
|||
| ALIC | ||||
| Left FA | 11 | 0.447 (0.027)* | 28 | 0.465 (0.022) |
| Right FA | 11 | 0.444 (0.024) | 28 | 0.451 (0.026) |
| Left AD | 11 | 0.00118 (0.00004) | 28 | 0.00118 (0.00004) |
| Right AD | 11 | 0.00118 (0.00004) | 28 | 0.00120 (0.00004) |
| Left RD | 11 | 0.00056 (0.00004)* | 28 | 0.00054 (0.00002) |
| Right RD | 11 | 0.00056 (0.00003) | 28 | 0.00056 (0.00003) |
p < 0.05 and ≥ 0.01
Figure 2.
Effect of allelic variation of the rs701428 SNP of the NoGo-66 receptor gene on DTI metrics in the ALIC for the left (L) and right (R) hemispheres. DTI metrics include (A) fractional anisotropy, and (B) radial diffusivity. Only individuals diagnosed with 22q11DS are included. Error bars reflect the standard deviation from the mean.
3.3 Association between DTI Metrics and Prodromal Symptoms
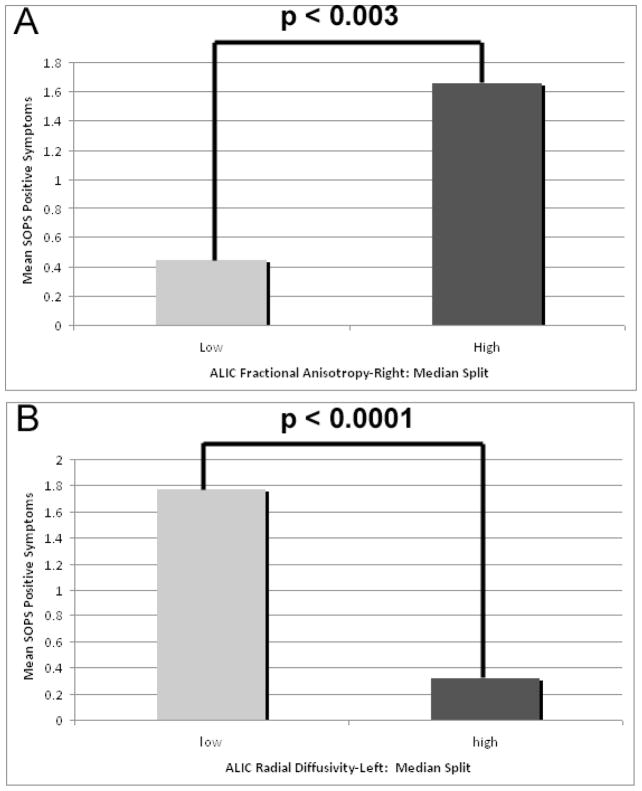
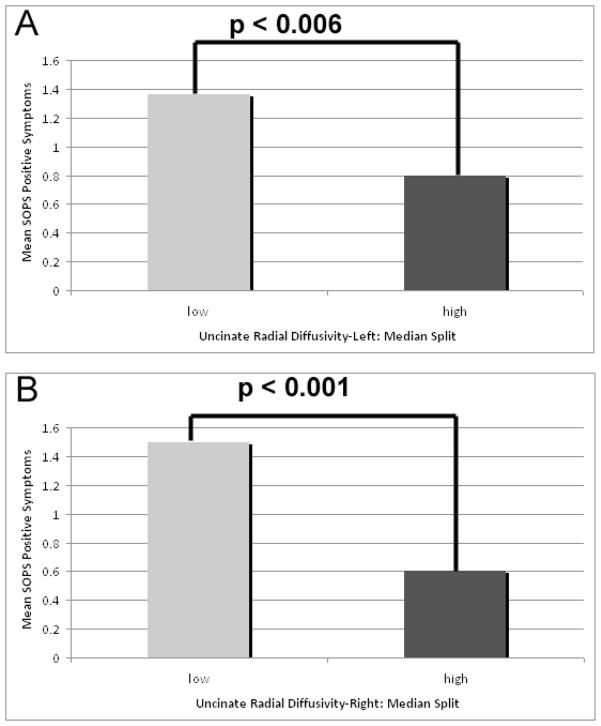
Zero-inflated Poisson regression analyses indicated that in the 22q11.2DS group, DTI metrics were associated with positive prodromal symptoms of psychosis, as measured by the SOPS. Positive prodromal symptoms were associated with the ALIC’s right FA (z=2.93; p<0.003) and left RD (z=−3.96; p<0.0001) (Figure 3). Positive prodromal symptoms were also associated with the uncinate’s left RD (z=−2.74; p<0.006) and right RD (z=−3.22; p<0.001)(Figure 4).
Figure 3.
Association between ALIC DTI metrics and the positive symptom scale of the Scale of Prodromal Symptoms (SOPS). DTI metrics include (A) fractional anisotropy in the right ALIC, and (B) radial diffusivity in the left ALIC. DTI values were categorized as High or Low using a median split of the data.
Figure 4.
Association between UF DTI metrics and the positive symptom scale of the Scale of Prodromal Symptoms (SOPS). DTI metrics include (A) radial diffusivity in the left UF, and (B) radial diffusivity in the right ALIC. DTI values were categorized as High or Low using a median split of the data.
4. Discussion
The present study is the first, to our knowledge, to use manual tractography to measure white matter integrity for ALIC, fornix and UF tracts in 22q11.2 DS, and to associate the DTI metrics for these tracts with genetic variation in the Nogo-66 receptor gene as well as symptoms of psychosis. Although a recent study utilized manual tractography to investigate the fronto-occipital fasciculus and the inferior longitudinal fasciculus in 22q11.2DS (49), most previous investigations of the white matter tracts of individuals with 22q11.2DS have been limited to whole brain analyses, via atlas or voxel based morphometry (Radoeva et al. 2012; Simon et al. 2008; Barnea-Goraly et al. 2003; Kikinis et al. 2012) or whole brain tractography (Ottet et al. 2013; Villalon-Reina et al. 2013). In our study, statistically significant differences in FA and RD were seen in the ALIC between individuals with 22q11.2DS and the combined group of siblings and community controls. We also found differences in FA and AD of the fornix between groups, and in the AD and RD of the uncinate. Within the group of individuals with 22q11.2DS, DTI metrics were associated with positive prodromal symptoms of psychosis. In addition, increased FA and decreased RD of the ALIC within the 22q11.2DS group was associated with hemizygosity for the G allele in the rs701428 SNP of the Nogo-66 Receptor gene.
Our current findings are consistent with, yet extend, our previous study of whole brain, atlas-based analysis of white matter tracts in the same sample. In that study, we reported decreased FA for the UF, and decreased AD for several white matter tracts in 22q11DS subjects, suggesting an overall disruption of white matter connectivity (Radoeva et al. 2012). Moreover, previous studies of white matter integrity in other samples have revealed alterations in FA, AD, and RD in white matter tracts in 22q11.2DS, suggesting disruptions of white matter connectivity (Simon et al. 2008; Barnea-Goraly et al. 2003; Kikinis et al. 2012; Villalon-Reina et al. 2013) in individuals with this syndrome. The increases that we observed in FA in the ALIC appear to be driven by alterations in RD, suggesting a disruption in myelin development of the ALIC of individuals with 22q11.2DS (Alexander et al. 2007; Song et al. 2003). Alterations in ALIC FA are also described in a recent report by Villalon-Reina and colleagues (2013), which is the only other reported investigation of ALIC in individuals with 22q11.2 DS. Our DTI metrics also illustrate alterations in the FA of the fornix, which appear to be driven by changes in AD, suggesting axonal damage of the fornix (Song et al. 2003; Budde et al. 2009), which could represent disruptions to the internal structure or density of axons (Kumar et al. 2012). Finally, alterations in both AD and RD of the UF implicate both axonal damage to the structure and disruption in myelin development (Song et al. 2003; Budde et al. 2009). Previous mouse model studies of 22q11.2DS have implicated both altered neurogenesis and neuronal migration (Mukai et al. 2008; Meechan et al. 2009). Such findings could underlie the altered DTI metrics seen in the present study that suggest both axonal and myelination abnormalities in 22q11.2DS.
As we note above, the specific white matter tracts analyzed through our study have been previously linked to the pathophysiology of schizophrenia (see also review, (Kubicki et al. 2007). Since abnormal fronto-temporal connectivity has been implicated in the development of schizophrenia, the white matter tract alterations we report in the ALIC, fornix, and UF could be associated with the increased vulnerability to schizophrenia and symptoms of psychosis in 22q11.2DS. Previous research has shown FA alterations for individuals with schizophrenia in all three white matter tracts analyzed in the present study. Moreover, DTI alterations of these tracts have been associated with deficits in neuropsychological function (Fitzsimmons et al. 2009; Nestor et al. 2007; Kuroki et al. 2006) in schizophrenia, including immediate (Kubicki et al., 2002) and working memory (Mamah et al., 2010), verbal abstract reasoning (Kubicki et al., 2002) and executive function (Mamah et al., 2010). Though these studies have presented decreased FA in schizophrenia, others have demonstrated focal increases in FA within regions of temporal connectivity in association to symptoms of schizophrenia (Hubl et al 2004; Shergill et al. 2007; Rotarska-Jagiela et al. 2009; Alba-Ferrara and de Erausquin, 2013). Therefore, the associations that we observed between DTI metrics of the ALIC and the UF and positive prodromal symptoms further supporting the involvement of these white matter tracts in the pathophysiology of psychosis symptoms in 22q11.2DS. Accordingly, we suggest that the presence of these structural anomalies in this population may play a role in the development of schizophrenia. This is also consistent with a previous study implicating the involvement of structural connectivity as a basis for the development of schizophrenia in 22q11.2DS (Ottet et al. 2013).
The Nogo-66 receptor gene has been previously associated with schizophrenia (Budel et al. 2008). The known involvement of the receptor protein in modulation of axonal outgrowth (Cafferty and Strittmatter 2006; Cafferty, W.B. 2006) and plasticity (McGee et al. 2005) is consistent with animal models that have shown decreased spatial learning and memory function as a result of reduced expression (van Gaalen et al. 2012). As this gene lies within the 22q11 locus, and is associated with myelin-mediated inhibition of axonal sprouting (Budel et al. 2008), our data suggest that altered dosage of this gene (by virtue of the presence of only one copy this gene in individuals with 22q11.2DS) may underlie alterations in the white matter tracts of individuals with this syndrome. To our knowledge, no previous work has implicated the Nogo-66 receptor gene in white matter anomalies in 22q11.2DS. Since the Nogo-66 receptor gene is involved in axonal development, the association that we observed between allelic variation in the Nogo-66 receptor gene and DTI metrics of the ALIC further support the notion that the confluence of, and interactions between, underlying genetic processes and white matter abnormalities could lead to symptoms of psychosis in 22q11.2DS. In other words, the Nogo-66 receptor gene may represent a susceptibility gene for psychosis through its disruption of white matter integrity and myelin – associated axonal growth in youth with this disorder.
Though previous studies have demonstrated bilateral decreased FA with cognitive function correlations in schizophrenia (Levitt et al. 2010), variation in the Nogo-66 receptor gene may provide an explanation for our observation of bilateral increased FA in ALIC of 22q11.2DS. Potentially, this gene may be expressed at a higher level in ALIC relative to other white matter regions, possibly leading to lack of myelination inhibition, affecting DTI metrics. Increased FA has been proposed to reflect underlying molecular processes that regulate neuronal modulation and plasticity, such as axonal pruning and myelination (Wolff et al. 2012). Myelination has also been considered to impede water diffusion in white matter tracts (Kubicki et al. 2005). Therefore, this genetic variation in regulation of axonal development seen in 22q11.2DS could lead to an altered anatomy of the axon, resulting in increased directionality of water diffusion suggested by an increase in FA.
In addition, our results show a positive correlation between FA values and positive prodromal symptoms of psychosis, suggesting that this increase may be pathological. Moreover, we observed that patients with the G allele at the Nogo-66 receptor locus show increased FA relative to those with the A allele, suggesting that the G allele may be a risk allele in individuals with 22q11.2 DS. Notably, across all HapMap reference populations in dbSNP, the most common genotype is heterozygous A/G, which accounts for 41–57% of the genotypes. Thus, the G allele is clearly very common in the general population. However, the homozygous G/G genotype is much less common, present in only 13–39% of the same samples. It is unknown whether the G/G genotype is associated with changes in DTI metrics in these reference populations, but it is an intriguing possibility. However, one previous report suggested that the A allele of this particular SNP was nominally associated with idiopathic schizophrenia in a Caucasian population (Budel et al. 2008). Accordingly, it is clear that additional studies should be conducted to confirm associations between the SNP rs701428 genotype and DTI analysis in 22q11.2DS and idiopathic schizophrenia.
A limitation of our study might lie in the analysis of the fornix, as individuals with 22q11.2DS have a high incidence of cavum septum pellucidum, which may alter the structural integrity of the fornix (preventing the two columns of the fornix from joining at the midline anteriorly (Radoeva et al. 2012), and, in turn, our DTI metrics. In addition, while this sample is relatively large for a study of 22q11.2 DS, our genetic subgroups were relatively small, suggesting that DTI associations with the Nogo-66 receptor gene must be viewed as preliminary. Future studies in this field should include investigations of the effect of this gene variant on white matter microstructure in control samples, as well as other white matter structures in the brains of individuals with 22q11.2DS. Investigation of the underlying molecular processes that lead to the altered neurological development and symptoms of psychosis should also be conducted.
Supplementary Material
Acknowledgments
This work was supported by funding from the National Institutes of Health (MH064824) and the Golisano Children’s Hospital, both to WRK. We also acknowledge the assistance of Cynthia Salazar who processed the DNA.
Footnotes
Contributors
WRK and ILC contributed to the conception and design of the study. MDP, ILC and ZK contributed to or conducted image processing protocols. MDP and WRK analyzed the data. MDP, MRC and ILC drafted the manuscript. All Authors participated in the critical revision of the manuscript and provided the final approval of the version to be published.
Conflicts of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alba-Ferrara LM, de Erausquin GA. What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front Integr Neurosci. 2013;7:9. doi: 10.3389/fnint.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antshel KM, Aneja A, Strunge L, Peebles J, Fremont WP, Stallone K, Abdulsabur N, Higgins AM, Shprintzen RJ, Kates WR. Autistic spectrum disorders in velo-cardio facial syndrome (22q11.2 deletion) J Autism Dev Disord. 2007;37(9):1776–1786. doi: 10.1007/s10803-006-0308-6. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Conchelos J, Lanzetta G, Fremont W, Kates WR. Behavior and corpus callosum morphology relationships in velocardiofacial syndrome (22q11.2 deletion syndrome) Psychiatry Res. 2005;138(3):235–245. doi: 10.1016/j.pscychresns.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Fremont W, Kates WR. The neurocognitive phenotype in velo-cardio-facial syndrome: a developmental perspective. Dev Disabil Res Rev. 2008;14(1):43–51. doi: 10.1002/ddrr.7. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Kates WR, Roizen N, Fremont W, Shprintzen RJ. 22q11.2 Deletion Syndrome: Genetics, Neuroanatomy and Cognitive/behavioral Features Keywords. Child Neuropsychol. 2005;11(1):5–19. doi: 10.1080/09297040590911185. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Peebles J, AbdulSabur N, Higgins AM, Roizen N, Shprintzen R, Fremont WP, Nastasi R, Kates WR. Associations between performance on the Rey-Osterrieth Complex Figure and regional brain volumes in children with and without velocardiofacial syndrome. Dev Neuropsychol. 2008;33(5):601–622. doi: 10.1080/87565640802254422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antshel KM, Stallone K, Abdulsabur N, Shprintzen R, Roizen N, Higgins AM, Kates WR. Temperament in velocardiofacial syndrome. J Intellect Disabil Res. 2007;51(Pt 3):218–227. doi: 10.1111/j.1365-2788.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- Arnold PD, Siegel-Bartelt J, Cytrynbaum C, Teshima I, Schachar R. Velo-cardio-facial syndrome: Implications of microdeletion 22q11 for schizophrenia and mood disorders. Am J Med Genet. 2001;105(4):354–362. doi: 10.1002/ajmg.1359. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Krasnow B, Ko A, Reiss A, Eliez S. Investigation of white matter structure in velocardiofacial syndrome: a diffusion tensor imaging study. Am J Psychiatry. 2003;160(10):1863–1869. doi: 10.1176/appi.ajp.160.10.1863. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EW. 22q11 Deletion Syndrome: a Genetic Subtype of Schizophrenia. Biol Psychiatry. 1999;46(7):882–891. doi: 10.1016/s0006-3223(99)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci. 2009;29(9):2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budel S, Padukkavidana T, Liu BP, Feng Z, Hu F, Johnson S, Lauren J, Park JH, McGee AW, Liao J, Stillman A, Kim JE, Yang BZ, Sodi S, Gelernter J, Zhao H, Hisama F, Arnsten AF, Strittmatter SM. Genetic variants of Nogo-66 receptor with possible association to schizophrenia block myelin inhibition of axon growth. J Neurosci. 2008;28(49):13161–13172. doi: 10.1523/JNEUROSCI.3828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci. 2006;26(47):12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. Anatomic and behavioral aspects of frontal-subcortical circuits. Ann N Y Acad Sci. 1995;769:1–13. doi: 10.1111/j.1749-6632.1995.tb38127.x. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons J, Kubicki M, Smith K, Bushell G, Estepar RS, Westin CF, Nestor PG, Niznikiewicz MA, Kikinis R, McCarley RW, Shenton ME. Diffusion tractography of the fornix in schizophrenia. Schizophr Res. 2009;107(1):39–46. doi: 10.1016/j.schres.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, Gould GC, Liu BP, Strittmatter SM. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci. 2002;22(20):8876–8883. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409(6818):341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, Weizman A, Eliez S. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61(7):658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Karoutzou G, Emrich HM, Dietrich DE. The myelin-pathogenesis puzzle in schizophrenia: a literature review. Mol Psychiatry. 2008;13(3):245–260. doi: 10.1038/sj.mp.4002096. [DOI] [PubMed] [Google Scholar]
- Kates WR, Antshel K, Willhite R, Bessette BA, AbdulSabur N, Higgins AM. Gender-moderated dorsolateral prefrontal reductions in 22q11.2 Deletion Syndrome: implications for risk for schizophrenia. Child Neuropsychol. 2005;11(1):73–85. doi: 10.1080/09297040590911211. [DOI] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Abdulsabur N, Colgan D, Funke B, Fremont W, Higgins AM, Kucherlapati R, Shprintzen RJ. A gender-moderated effect of a functional COMT polymorphism on prefrontal brain morphology and function in velo-cardio-facial syndrome (22q11.2 deletion syndrome) Am J Med Genet B Neuropsychiatr Genet. 2006;141B(3):274–280. doi: 10.1002/ajmg.b.30284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Faraone SV, Fremont WP, Higgins AM, Shprintzen RJ, Botti JA, Kelchner L, McCarthy C. Neuroanatomic predictors to prodromal psychosis in velocardiofacial syndrome (22q11.2 deletion syndrome): a longitudinal study. Biol Psychiatry. 2011;69(10):945–952. doi: 10.1016/j.biopsych.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Fremont WP, Shprintzen RJ, Strunge LA, Burnette CP, Higgins AM. Comparing phenotypes in patients with idiopathic autism to patients with velocardiofacial syndrome (22q11 DS) with and without autism. Am J Med Genet A. 2007;143A(22):2642–2650. doi: 10.1002/ajmg.a.32012. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Bessette BA, Folley BS, Strunge L, Jabs EW, Pearlson GD. Frontal and caudate alterations in velocardiofacial syndrome (deletion at chromosome 22q11.2) J Child Neurol. 2004;19(5):337–342. doi: 10.1177/088307380401900506. [DOI] [PubMed] [Google Scholar]
- Kates WR, Krauss BR, Abdulsabur N, Colgan D, Antshel KM, Higgins AM, Shprintzen RJ. The neural correlates of non-spatial working memory in velocardiofacial syndrome (22q11.2 deletion syndrome) Neuropsychologia. 2007;45(12):2863–2873. doi: 10.1016/j.neuropsychologia.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Miller AM, Abdulsabur N, Antshel KM, Conchelos J, Fremont W, Roizen N. Temporal lobe anatomy and psychiatric symptoms in velocardiofacial syndrome (22q11.2 deletion syndrome) J Am Acad Child Adolesc Psychiatry. 2006;45(5):587–595. doi: 10.1097/01.chi.0000205704.33077.4a. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Nakamura M, Bouix S, Kubicki M, Salisbury DF, Westin CF, McCarley RW, Shenton ME. Uncinate fasciculus abnormalities in recent onset schizophrenia and affective psychosis: a diffusion tensor imaging study. Schizophr Res. 2009;110(1–3):119–126. doi: 10.1016/j.schres.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikinis Z, Asami T, Bouix S, Finn CT, Ballinger T, Tworog-Dube E, Kucherlapati R, Kikinis R, Shenton ME, Kubicki M. Reduced fractional anisotropy and axial diffusivity in white matter in 22q11.2 deletion syndrome: a pilot study. Schizophr Res. 2012;141(1):35–39. doi: 10.1016/j.schres.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159(5):813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26(4):1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Nguyen HD, Macey PM, Woo MA, Harper RM. Regional brain axial and radial diffusivity changes during development. J Neurosci Res. 2012;90(2):346–355. doi: 10.1002/jnr.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki N, Kubicki M, Nestor PG, Salisbury DF, Park HJ, Levitt JJ, Woolston S, Frumin M, Niznikiewicz M, Westin CF, Maier SE, McCarley RW, Shenton ME. Fornix integrity and hippocampal volume in male schizophrenic patients. Biol Psychiatry. 2006;60(1):22–31. doi: 10.1016/j.biopsych.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. Zero-inflated Poisson regression with an application to defects in manufacturing. Technometrics. 1992;34(1):1–14. [Google Scholar]
- Levitt JJ, Kubicki M, Nestor PG, Ersner-Hershfield H, Westin CF, Alvarado JL, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. A diffusion tensor imaging study of the anterior limb of the internal capsule in schizophrenia. Psychiatry Res. 2010;184(3):143–150. doi: 10.1016/j.pscychresns.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JJ, McCarley RW, Dickey CC, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, Ciszewski AA, Kikinis R, Jolesz FA, Shenton ME. MRI study of caudate nucleus volume and its cognitive correlates in neuroleptic-naive patients with schizotypal personality disorder. Am J Psychiatry. 2002;159(7):1190–1197. doi: 10.1176/appi.ajp.159.7.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Friedman HR, Davachi L, Goldman-Rakic PS. Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J Neurosci. 1997;17(10):3870–3882. doi: 10.1523/JNEUROSCI.17-10-03870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Abecasis GR, Heath SC, Knowles A, Demars S, Chen YJ, Roos JL, Rapoport JL, Gogos JA, Karayiorgou M. Genetic variation in the 22q11 locus and susceptibility to schizophrenia. Proc Natl Acad Sci USA. 2002;99(26):16859–16864. doi: 10.1073/pnas.232186099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR, et al. Anterior thalamic radiation integrity in schizophrenia: A diffusion-tensor imaging study. Psychiatry Res. 2010 Aug 30;183(2):144–50. doi: 10.1016/j.pscychresns.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48(2):99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- Maynard TM, Haskell GT, Peters AZ, Sikich L, Lieberman JA, LaMantia AS. A comprehensive analysis of 22q11 gene expression in the developing and adult brain. Proc Natl Acad Sci USA. 2003;100(24):14433–14438. doi: 10.1073/pnas.2235651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309(5744):2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc Natl Acad Sci USA. 2009;106(38):16434–16445. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, MacDermott AB, Karayiorgou M, Gogos JA. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci. 2008;11(11):1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56(10):940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Kuroki N, Gurrera RJ, Niznikiewicz M, Shenton ME, McCarley RW. Episodic memory and neuroimaging of hippocampus and fornix in chronic schizophrenia. Psychiatry Res. 2007;155(1):21–28. doi: 10.1016/j.pscychresns.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Ottet MC, Schaer M, Cammoun L, Schneider M, Debbane M, Thiran JP, Eliez S. Reduced fronto-temporal and limbic connectivity in the 22q11.2 deletion syndrome: vulnerability markers for developing schizophrenia? PLoS One. 2013;8(3):e58429. doi: 10.1371/journal.pone.0058429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G, Cercignani M, Parker GJ, Altmann DR, Barnes TR, Barker GJ, Joyce EM, Ron MA. White matter tracts in first-episode psychosis: a DTI tractography study of the uncinate fasciculus. Neuroimage. 2008;39(3):949–955. doi: 10.1016/j.neuroimage.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoeva PD, Coman IL, Antshel KM, Fremont W, McCarthy CS, Kotkar A, Wang D, Shprintzen RJ, Kates WR. Atlas-based white matter analysis in individuals with velo-cardio-facial syndrome (22q11.2 deletion syndrome) and unaffected siblings. Behav Brain Funct. 2012;8 doi: 10.1186/1744-9081-8-38. 38-9081-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger G, Nestor PG, Oh JS, Levitt JJ, Kindleman G, Bouix S, Fitzsimmons J, Niznikiewicz M, Westin CF, Kikinis R, McCarley RW, Shenton ME, Kubicki M. Anterior limb of the internal capsule in schizophrenia: a diffusion tensor tractography study. Brain Imaging Behav. 2012;6(3):417–425. doi: 10.1007/s11682-012-9152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A, Oertel-Knoechel V, DeMartino F, van de Ven V, Formisano E, Roebroeck A, Rami A, Schoenmeyer R, Haenschel C, Hendler T, Maurer K, Vogeley K, Linden DE. Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry Res. 2009;174(1):9–16. doi: 10.1016/j.pscychresns.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Segal D, Koschnick JR, Slegers LH, Hof PR. Oligodendrocyte pathophysiology: a new view of schizophrenia. Int J Neuropsychopharmacol. 2007;10(4):503–511. doi: 10.1017/S146114570600722X. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Kanaan RA, Chitnis XA, O’Daly O, Jones DK, Frangou S, Williams SC, Howard RJ, Barker GJ, Murray RM, McGuire P. A diffusion tensor imaging study of fasciculi in schizophrenia. Am J Psychiatry. 2007;164(3):467–473. doi: 10.1176/ajp.2007.164.3.467. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome: 30 Years of study. Dev Disabil Res Rev. 2008;14(1):3–10. doi: 10.1002/ddrr.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg R, Golding-Kushner KJ, Marion RW. Late-onset psychosis in the velo-cardio-facial syndrome. Am J Med Genet. 1992;42(1):141–142. doi: 10.1002/ajmg.1320420131. [DOI] [PubMed] [Google Scholar]
- Simon TJ, Wu Z, Avants B, Zhang H, Gee JC, Stebbins GT. Atypical cortical connectivity and visuospatial cognitive impairments are related in children with chromosome 22q11.2 deletion syndrome. Behav Brain Funct. 2008;4 doi: 10.1186/1744-9081-4-25. 25-9081-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinibaldi L, De Luca A, Bellacchio E, Conti E, Pasini A, Paloscia C, Spalletta G, Caltagirone C, Pizzuti A, Dallapiccola B. Mutations of the Nogo-66 receptor (RTN4R) gene in schizophrenia. Hum Mutat. 2004;24(6):534–535. doi: 10.1002/humu.9292. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Eyskens B, Dumoulin M, Gewillig M, Fryns JP. Intelligence and psychosocial adjustment in velocardiofacial syndrome: a study of 37 children and adolescents with VCFS. J Med Genet. 1997;34(6):453–458. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Tyekucheva S, King D, Hardison RC, Miller W, Chiaromonte F. ESPERR: Learning strong and weak signals in genomic sequence alignments to identify functional elements. Genome Research. 2006;16:1596–1604. doi: 10.1101/gr.4537706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55(5):597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Relo AL, Mueller BK, Gross G, Mezler M. NOGO-66 receptor deficient mice show slow acquisition of spatial memory task performance. Neurosci Lett. 2012;510(1):58–61. doi: 10.1016/j.neulet.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Villalon-Reina J, Jahanshad N, Beaton E, Toga AW, Thompson PM, Simon TJ. White matter microstructural abnormalities in girls with chromosome 22q11.2 deletion syndrome, Fragile X or Turner syndrome as evidenced by diffusion tensor imaging. Neuroimage. 2013;81C:441–454. doi: 10.1016/j.neuroimage.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, Evans AC, Hazlett HC, Kostopoulos P, McKinstry RC, Paterson SJ, Schultz RT, Zwaigenbaum L, Piven J IBIS Network . Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169(6):589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.