Abstract
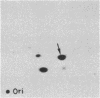
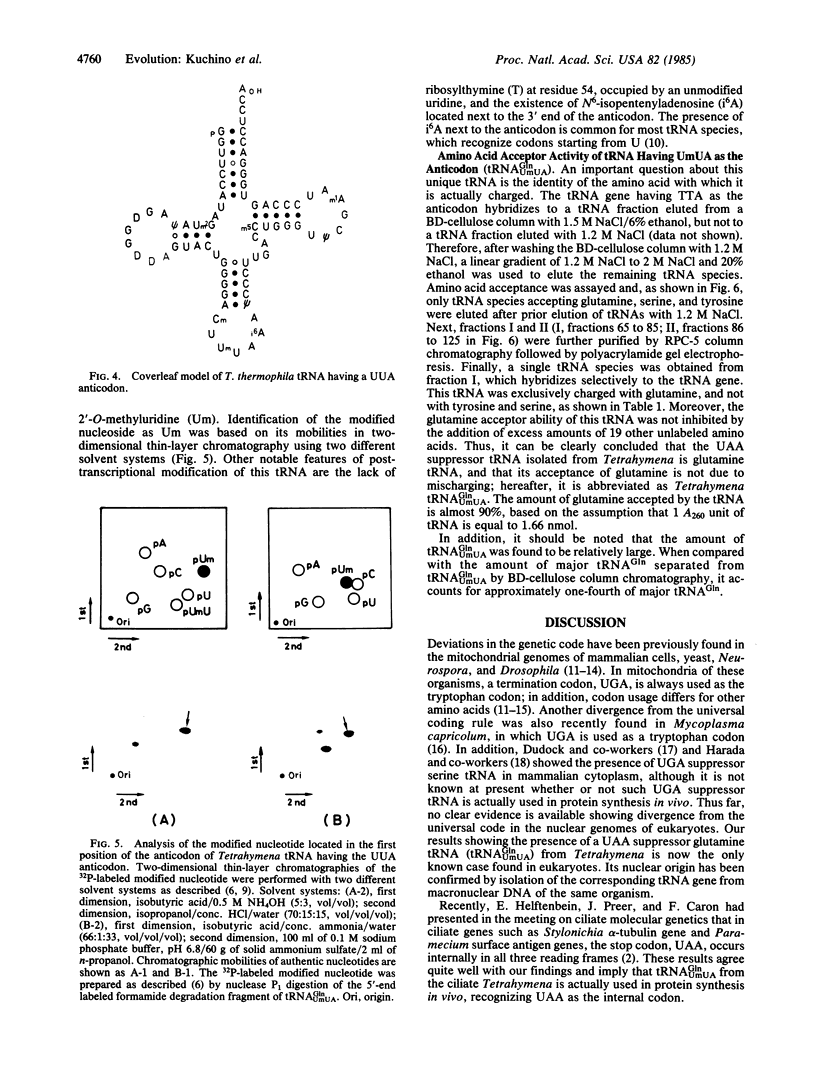
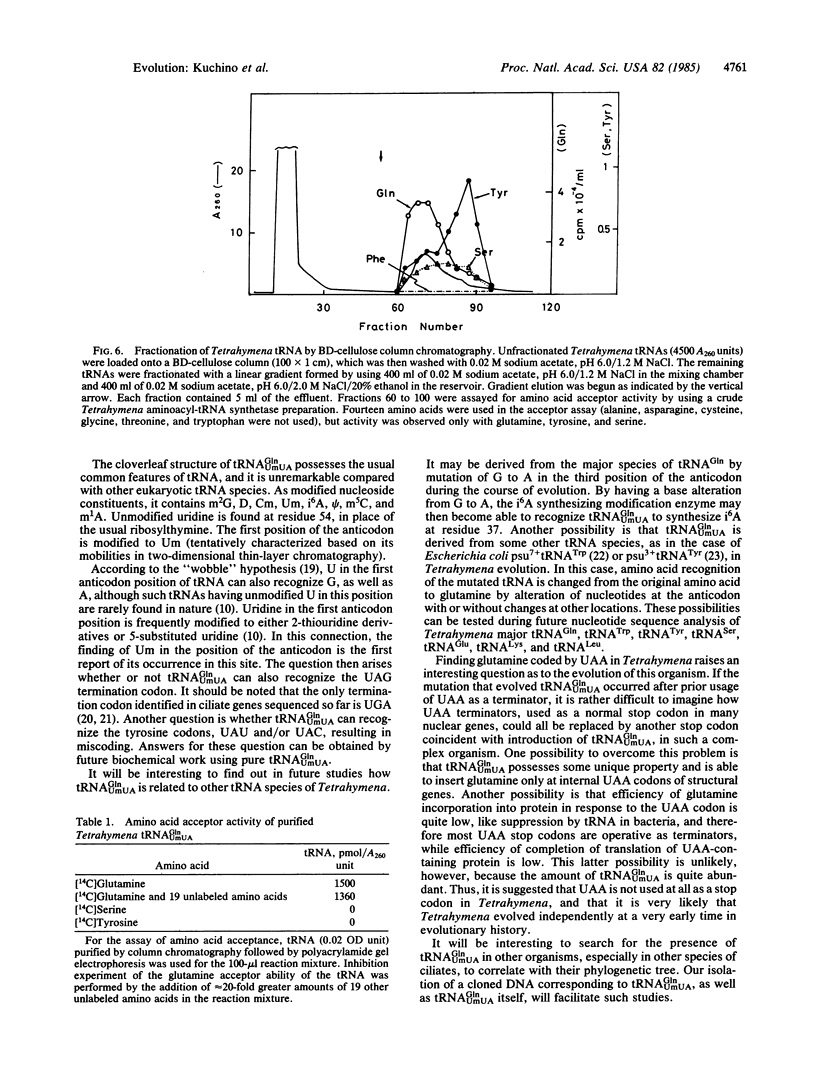
Nucleotide sequence analysis of one of several tRNA genes cloned from Tetrahymena thermophila macronuclear DNA indicated that it corresponds to a tRNA species having TTA as anticodon. Subsequently, the tRNA species corresponding to that gene was isolated and its nucleotide sequence was determined by post-labeling techniques. The nucleotide sequence was found to be pG-G-U-U-C-C-A-U-A-m2G-U-A-psi-A-G-D-G-G-D- D-A-G-U-A-C-U-G-G-G-G-A-Cm-U-Um-U-A-i6A-A-psi-C-C-C-U-U-G-A-C- m5C-U-G-G-G-U-psi-C-G-m1A-A-U-C-C-C-A-G-U-G-G-G-A-C-C-U-C-C-AOH. This tRNA sequence exactly matched the DNA sequence of the corresponding tRNA gene. The first position of anticodon is 2'-O-methyluridine (Um), forming UmUA as the anticodon, which presumably recognizes the ochre termination codon UAA. This tRNA species is aminoacylated with glutamine by a Tetrahymena crude aminoacyl tRNA synthetase fraction, suggesting that ochre termination codon is used as a glutamine codon during cytoplasmic protein synthesis in Tetrahymena.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Brenner S., Smith J. D. Identification of an ochre-suppressing anticodon. J Mol Biol. 1971 Feb 28;56(1):195–197. doi: 10.1016/0022-2836(71)90094-5. [DOI] [PubMed] [Google Scholar]
- Bannon G. A., Bowen J. K., Yao M. C., Gorovsky M. A. Tetrahymena H4 genes: structure, evolution and organization in macro- and micronuclei. Nucleic Acids Res. 1984 Feb 24;12(4):1961–1975. doi: 10.1093/nar/12.4.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell B. G., Anderson S., Bankier A. T., de Bruijn M. H., Chen E., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A. Different pattern of codon recognition by mammalian mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3164–3166. doi: 10.1073/pnas.77.6.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonitz S. G., Berlani R., Coruzzi G., Li M., Macino G., Nobrega F. G., Nobrega M. P., Thalenfeld B. E., Tzagoloff A. Codon recognition rules in yeast mitochondria. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3167–3170. doi: 10.1073/pnas.77.6.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Diamond A., Dudock B., Hatfield D. Structure and properties of a bovine liver UGA suppressor serine tRNA with a tryptophan anticodon. Cell. 1981 Aug;25(2):497–506. doi: 10.1016/0092-8674(81)90068-4. [DOI] [PubMed] [Google Scholar]
- Grivell L. A. Mitochondrial DNA. Sci Am. 1983 Mar;248(3):78–89. doi: 10.1038/scientificamerican0383-78. [DOI] [PubMed] [Google Scholar]
- Heckman J. E., Sarnoff J., Alzner-DeWeerd B., Yin S., RajBhandary U. L. Novel features in the genetic code and codon reading patterns in Neurospora crassa mitochondria based on sequences of six mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3159–3163. doi: 10.1073/pnas.77.6.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura Y., Sakai M., Mita T., Muramatsu M. Unequal gene amplification and transcription in the macronucleus of Tetrahymena pyriformis. Biochemistry. 1979 Nov 27;18(24):5289–5294. doi: 10.1021/bi00591a004. [DOI] [PubMed] [Google Scholar]
- Kaine B. P., Spear B. B. Nucleotide sequence of a macronuclear gene for actin in Oxytricha fallax. Nature. 1982 Feb 4;295(5848):430–432. doi: 10.1038/295430a0. [DOI] [PubMed] [Google Scholar]
- Kato N., Hoshino H., Harada F. Minor serine tRNA containing anticodon NCA (C4 RNA) from human and mouse cells. Biochem Int. 1983 Nov;7(5):635–645. [PubMed] [Google Scholar]
- Kuchino Y., Mita T., Nishimura S. Nucleotide sequence of cytoplasmic initiator tRNA from Tetrahymena thermophila. Nucleic Acids Res. 1981 Sep 25;9(18):4557–4562. doi: 10.1093/nar/9.18.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Watanabe S., Harada F., Nishimura S. Primary structure of AUA-specific isoleucine transfer ribonucleic acid from Escherichia coli. Biochemistry. 1980 May 13;19(10):2085–2089. doi: 10.1021/bi00551a013. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Yamao F., Muto A., Kawauchi Y., Iwami M., Iwagami S., Azumi Y., Osawa S. UGA is read as tryptophan in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2306–2309. doi: 10.1073/pnas.82.8.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv M., Folk W. R., Berg P., Soll L. A single mutational modification of a tryptophan-specific transfer RNA permits aminoacylation by glutamine and translation of the codon UAG. J Mol Biol. 1974 Jun 25;86(2):245–260. doi: 10.1016/0022-2836(74)90016-3. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H. Drosophila melanogaster mitochondrial DNA, a novel organization and genetic code. Nature. 1983 Jul 21;304(5923):234–241. doi: 10.1038/304234a0. [DOI] [PubMed] [Google Scholar]