Abstract
Breast metastases from extramammary malignancies are uncommon. The most common sources are lymphomas/leukemias and melanomas. Some of the less common sources include carcinomas of the lung, ovary, and stomach, and infrequently, carcinoid tumors, hypernephromas, carcinomas of the liver, tonsil, pleura, pancreas, cervix, perineum, endometrium and bladder. Breast metastases from extramammary malignancies have both hematogenous and lymphatic routes. According to their routes, there are common radiological features of metastatic diseases of the breast, but the features are not specific for metastases. Typical ultrasound (US) features of hematogenous metastases include single or multiple, round to oval shaped, well-circumscribed hypoechoic masses without spiculations, calcifications, or architectural distortion; these masses are commonly located superficially in subcutaneous tissue or immediately adjacent to the breast parenchyma that is relatively rich in blood supply. Typical US features of lymphatic breast metastases include diffusely and heterogeneously increased echogenicities in subcutaneous fat and glandular tissue and a thick trabecular pattern with secondary skin thickening, lymphedema, and lymph node enlargement. However, lesions show variable US features in some cases, and differentiation of these lesions from primary breast cancer or from benign lesions is difficult. In this review, we demonstrate various US appearances of breast metastases from extramammary malignancies as typical and atypical features, based on the results of US and other imaging studies performed at our institution. Awareness of the typical and atypical imaging features of these lesions may be helpful to diagnose metastatic lesions of the breast.
Keywords: Breast, Extramammary, Metastasis, Ultrasound
INTRODUCTION
The breast is the most common site of primary malignancies in adult women, but is an uncommon site for metastasis from extramammary malignancies. The incidence of metastasis to the breast varies from 1.7 to 6.6% in autopsy series, due to the inclusion or exclusion of patients with leukemia or lymphoma in different reports (1-3). The clinically observed rate of breast metastases from extramammary malignancies is rarer, ranging from 0.5 to 1.3%, due to the late appearance of extramammary malignancies in the course of malignant disease (4). This rare occurrence of metastases to the breast is suggested to be due to the presence of large areas of fibrous tissue with a relatively poor blood supply (1, 5, 6). The most common sources of extramammary metastases to the breast are lymphomas/leukemias and melanomas. Some of the less common sources include carcinomas of the lung, ovary, and stomach, and infrequently, carcinoid tumors, hypernephromas, carcinomas of the liver, tonsil, pleura, pancreas, cervix, perineum, endometrium and bladder (1, 2, 7).
No clear predisposing factors correlating with the development of breast metastasis have been identified (1, 8). However, hormones are considered to function as predisposing factors for several types of extramammary malignancies (1, 7). Estrogen may increase the vascularity and stroma of the breast, and may have a role as a predisposing factor in the development of a metastasis (1, 7). In accordance with this hypothesis, most reported cases have occurred in younger women. There have been some rare cases of the development of breast metastases in male patients with prostatic carcinomas who have been treated with estrogens (1, 9).
Breast metastases from extramammary malignancies have both hematogenous and lymphatic routes. There are common radiological features of metastatic diseases of the breast, but the features are not specific for metastatic disease. It is difficult to differentiate metastatic lesions in the breast from primary breast cancers or benign lesions. In order to avoid unnecessary surgery, it is important to be able to recognize the findings of metastatic lesions. In this review, we demonstrate various ultrasound (US) appearances of breast metastases from extramammary malignancies as typical and atypical features. This review is based on the results of US and other imaging studies performed at our institution.
Typical US Features of Hematogenous Breast Metastases
Hematogenous breast metastases from extramammary malignancies are commonly located in the upper outer quadrant and are located superficially in subcutaneous tissue or immediately adjacent to the breast parenchyma that is relatively rich in blood supply (1, 10). Metastatic breast masses tend to grow rapidly. Typical US features include single or multiple, round to oval shaped, well-circumscribed or occasionally microlobulated hypoechoic masses without spiculations, calcifications, architectural distortion, retrotumoral acoustic shadowing, or secondary skin or nipple changes (Fig. 1) (4, 6, 7, 11-13). The lack of significant desmoplastic response near the lesion, which is typical of primary tumors, explains the same size seen on clinical examinations and mammography, and the rareness of spiculation seen on mammography (1, 4, 11). These features could pose difficulty in making the diagnosis of benign breast lesions such as fibroadenomas or of well-circumscribed primary breast cancer (8). Rare tumoral calcifications found in metastases may be helpful to differentiate metastases from primary breast cancers, except for rare instances of metastasis from ovarian, thyroid, or mucin-producing gastrointestinal tract carcinomas that could contain intratumoral calcifications (1, 2, 4, 14, 15). Axillary lymph node involvement is less common in metastases than in primary breast cancers (16).
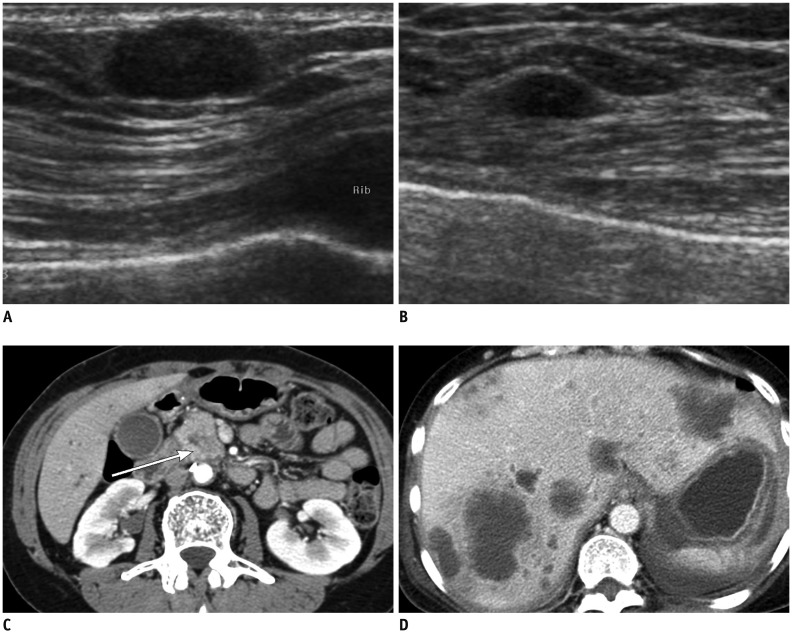
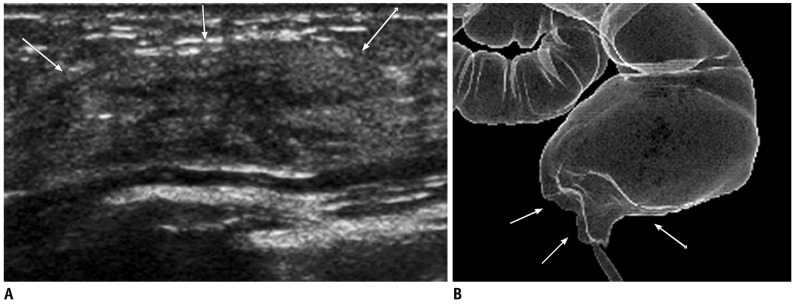
Fig. 1.
55-year-old woman with metastatic pancreatic head cancer.
A, B. Breast ultrasound images show well circumscribed, oval-shape hypoechoic masses in superficial area of right breast (A) and in middle of left breast (B), suggesting metastasis. C, D. Contrast enhanced abdomen CT scan shows ill-defined low density mass in pancreatic head (arrow in C) with multiple liver metastases (D).
Lymphoma or leukemia can also show single or multiple solid masses with a circumscribed or ill-defined margin (Fig. 2) (17, 18). Primary involvement of the breast by lymphoreticular malignancies is rare, due to the relatively small amount of lymphoid tissue within the breast as compared with the gut or lung (14, 19). Metastatic involvement of lymphoma or leukemia occurs more frequently than primary breast lymphoma or leukemia (20).
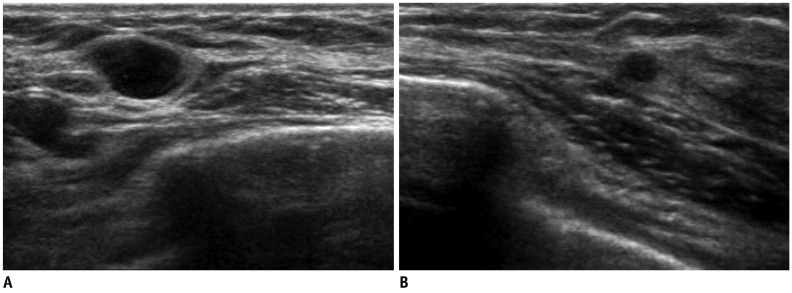
Fig. 2.
47-year-old woman with metastatic diffuse large B cell lymphoma.
A, B. US of right mid outer breast (A) and lower outer breast (B) show multiple circumscribed hypoechoic masses of metastatic lymphoma confirmed by US-guided core needle biopsy. US = ultrasound
Typical US Features of Lymphatic Breast Metastases
The appearance of lymphatic metastasis that makes it different from extramammary malignancies include diffusely and heterogeneously increased density in subcutaneous fat and glandular tissue and a thick trabecular pattern with secondary skin thickening, lymphedema, and lymph node enlargement, which are indistinguishable from those of inflammatory breast cancer (Fig. 3) (1, 14). Malignant-type microcalcifications, as is often reported in primary inflammatory breast cancer, are not common (1, 2). US shows diffuse skin thickening and obliteration of subcutaneous fat, lymphatic dilatation, without evidence of primary breast mass, that is secondary to retrograde edema from mechanical obstruction of draining lymphatics by the tumor. Typically lesions are hypoechoic, and abnormal enlargement of axillay or internal mammary lymph nodes are associated (1, 2, 21). These features of lymphatic metastases are common for metastases originating from the contralateral breast cancer, and have been reported for metastases from the stomach and ovarian carcinomas (1, 3, 14, 22, 23).
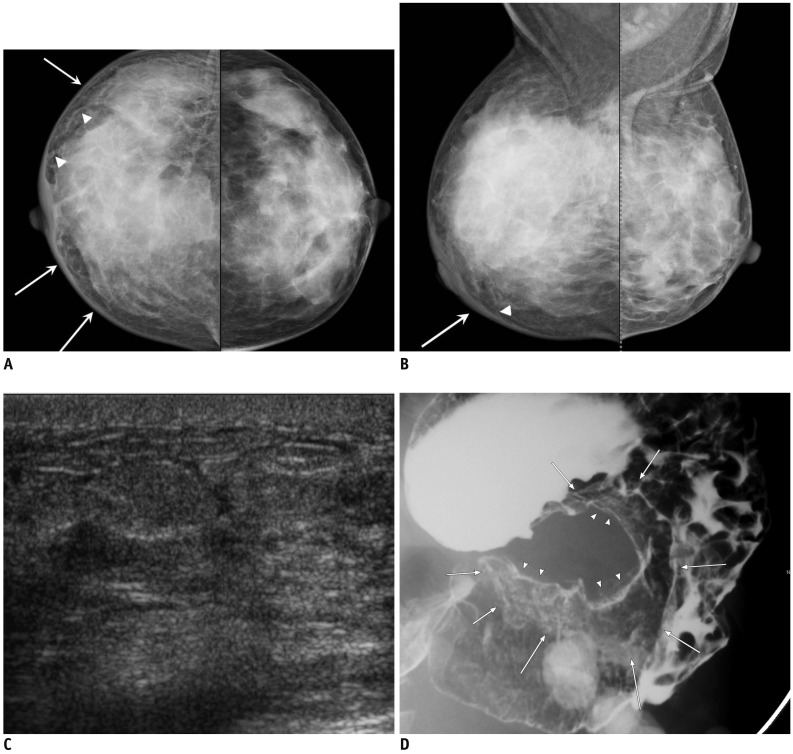
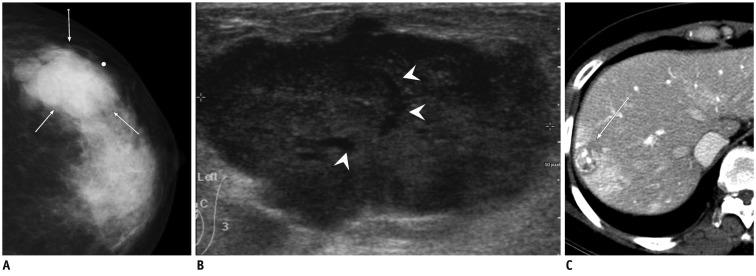
Fig. 3.
44-year-old woman with metastatic signet ring cell carcinoma from stomach.
A, B. Craniocaudal and mediolateral oblique mammograms show skin thickening (arrows), coarse trabecular pattern and thickening of Cooper's ligaments (arrowheads) in right breast. C. Ultrasound of right breast shows diffuse parenchymal heterogeneity with skin thickening and lymphatic dilatation. D. Barium study of stomach reveals large ulceroinfiltrative mass (arrows and arrowheads) from upper body to prepyloric antrum.
Bilateral diffuse parenchymal breast involvement with or without lymph node enlargement is one of the US features of lymphoreticular malignancies (Fig. 4) (17, 18).
Fig. 4.
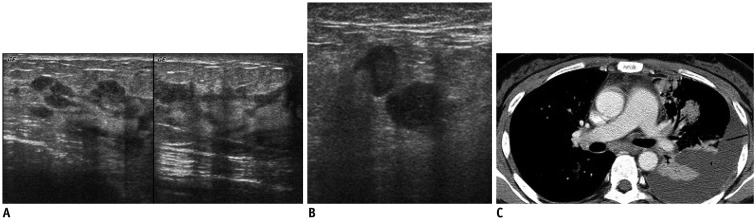
34-year-old man with metastatic NK/T cell lymphoma.
A. US shows diffuse hyperechoic infiltrations with thickening of skin and subcutaneous fat tissue and dilated lymphatic channels. B. PET-CT shows increased FDG uptake in corresponding area. US-guided core needle biopsy of right lower outer breast area revealed NK/T cell lymphoma. US = ultrasound
Sabaté et al. (19) suggested a more diffuse infiltrative parenchymal pattern when the lesions are presented as high-grade lymphomas.
Atypical Mass Lesions of Breast Metastases
Ultrasound features of breast metastases from extramammary malignancies can vary in addition to the above-mentioned typical appearances.
Findings observed for primary breast cancer including spiculation, calcification, and surrounding parenchymal distortion may appear for breast metastases from extramammary malignancies and lesions are often indistinguishable from each other (Fig. 5). Therefore, it is important to recognize the possibility of the presence of a metastastic tumor as well as the presence of primary breast cancer in patients with a known malignancy. Breast metastases from extramammary malignancies are mostly seen in patients with disseminated malignant disease (9, 24). However, the breast may be the first metastatic site and a breast lesion the first sign of extramammary malignant disease (1, 7, 24). Pre-surgical pathological confirmation with the use of core needle biopsy is necessary in patients with breast lesions that are indistinguishable from primary breast cancer.
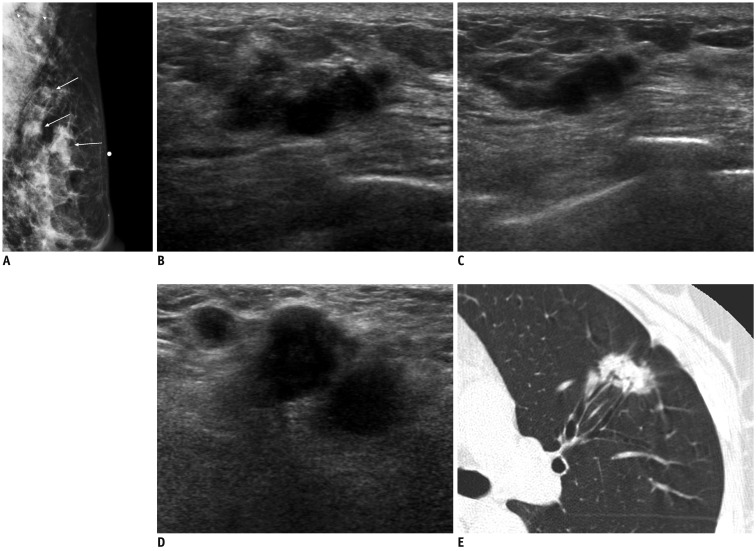
Fig. 5.
58-year-old woman with metastatic adenocarcinoma from lung.
A. Mediolateral oblique mammogram shows palpable irregular mass (arrow below skin marking) and two round masses in upper outer quadrant of left breast (arrows) with enlarged lymph nodes in axilla (arrowheads). B, C. US of left upper outer breast shows irregular mass with marked hypoechogenicity, microlobulated margins, and peritumoral infiltration, similar to those of invasive ductal carcinoma (B), and smaller masses with similar features in peripheral side of main mass (C). D. US of left axilla reveals several enlarged lymph nodes with ill-defined margins, and obliteration of normal fatty hilum, suggesting metastasis. E. Chest CT scan shows irregular spiculated mass in left lower lobe, suggesting lung cancer. US = ultrasound
In unusual cases, breast metastases can be seen as heterogeneous hyperechoic masses with ill-defined margins (Fig. 6). This finding can be considered for one type of hematogenous breast metastasis.
Fig. 6.
59-year-old woman with metastatic rectal cancer.
A. Transverse US image of left axillary tail area shows heterogeneous hyperechoic mass-like lesion with ill-defined margins (arrows). B. Ray-sum image reformed from CT colonography reveals abnormal wall thickening and infiltration (arrows) involving distal rectum, suggesting rectal cancer.
There are few reports describing extramammary metastatic lesions with intratumoral cystic lesions, and little is known about their imaging features. At our institution, rapid growing, well defined or microlobulated breast masses with some cystic portions have been seen in patients with synovial sarcoma from the thigh, hepatocellular carcinoma, and insular carcinoma of the thyroid gland. These were primarily cancers that were considered as other tumors such as phyllodes tumors that have cleft-like cystic space seen on US (Fig. 7), or benign masses with multifocal cystic changes such as resolving state of hematoma (Fig. 8). In one case that was considered as a hematoma, a follow-up US after 4 months showed a markedly enlarged solid hypoechoic mass with multiple cystic foci (Fig. 8). All three cases were considered as hematogeneous metastases; these tumors are known to undergo frequent intratumoral hemorrhagic changes or necrosis with poor differentiation (25-27).
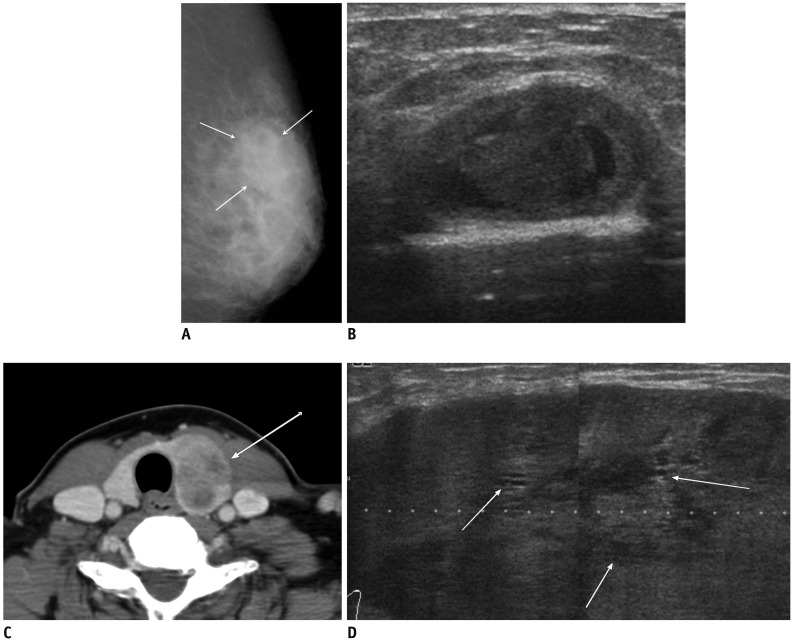
Fig. 7.
41-year-old woman with metastatic hepatocelluar carcinoma.
A. Craniocaudal mammogram shows lobular mass with relatively circumscribed margins (arrows). B. Ultrasound of left upper outer breast shows large, solid mass with microlobulated margin and internal tubular anechoic areas (arrowheads). C. Abdomen CT scan reveals hepatic mass with contrast enhancement in arterial phase, suggesting hapatocelluar carcinoma (arrow).
Fig. 8.
53-year-old woman with metastatic insular carcinoma of thyroid gland.
A. Mediolateral oblique mammogram shows focal asymmetry in left upper outer breast (arrows). B. US of left upper outer breast shows circumscribed benign-looking mass with multifocal cystic changes, mimicking resolving hematoma in middle of breast parenchyma. C. Contrast enhanced neck CT scan reveals large heterogeneous low density mass in left thyroid gland (arrow). D. Follow-up US after 4 months shows markedly enlarged hypoechoic mass with circumscribed margins and multiple small internal cystic portions (arrows). US = ultrasound
Sometimes cyst-like marked hypoechoic areas within the tumor can be caused by high cellularity. In mass-forming cases of lymphoreticular malignancy, focal hypoechoic masses or almost anechoic masses with pseudocystic configuration representing compact cellularity can be noted in this series (Figs. 9, 10) (23, 28, 29).
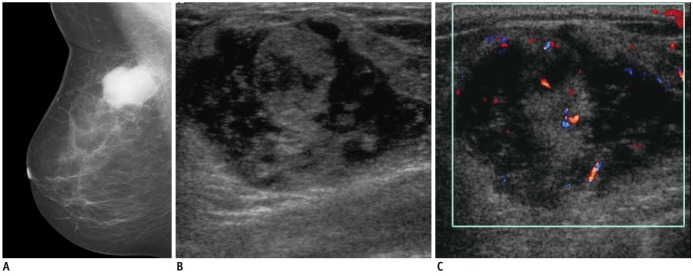
Fig. 9.
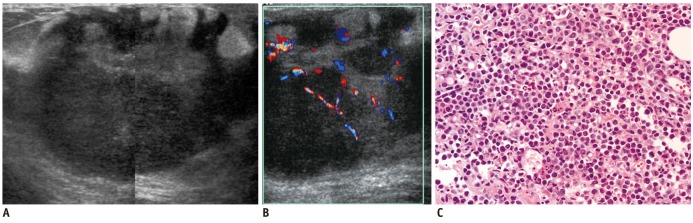
58-year-woman with multiple myeloma.
A. Mediolateral mammogram shows relatively well-circumscribed hyperdense mass. B. US of right upper center breast shows heterogeneous hypoehoic mass with microlobulated margins and bulging contour to subcutaneous layer. C. Color Doppler US shows increased vascularity within tumor. US = ultrasound
Fig. 10.
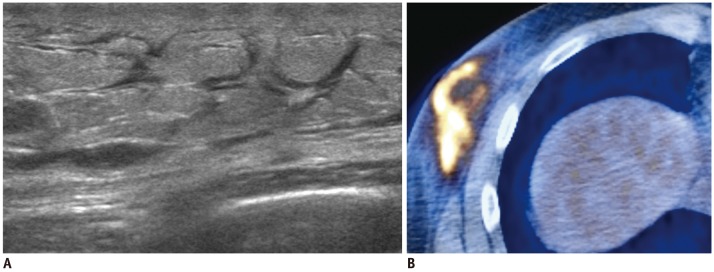
13-year-old girl with acute myeloid leukemia.
A. US of left upper outer breast shows large, well-circumscribed oval shape mass with marked hypoechogenicity with nodular hyperechoic portion. This feature mimics intracystic solid mass. B. On color Doppler US, marked hypoechoic area was revealed to be solid tumor with increased vascularity. C. Very hypoechoic area was high cellular area of chloroma as revealed by core needle biopsy specimen (hematoxylin & eosin staining, × 400) (B). US = ultrasound
Atypical Non-Mass Lesions of Breast Metastases
One patient with lung cancer showed multiple small Hypoechoic masses with segmental, ductal distribution from the nipple, and axillary lymph node enlargement. Histopathological examination with the use of core needle biopsy confirmed the diagnosis of breast metastasis from lung cancer (Fig. 11). These features with segmental distribution may mimic those of ductal carcinoma in situ (DCIS) or papillary lesions; however, no associated microcalcifications can help to distinguish breast metastasis from DCIS or papillary lesions. These findings may correspond to thoseof one type of lymphangitic or hemato-lymphangitic metastasis.
Fig. 11.
40-year-old woman with metastatic lung cancer.
A. US of left breast shows multiple hypoechoic masses with segmental distribution from nipple and dilated segmental ducts with irregular wall thickening, mimicking DCIS or intraductal papillary lesions. B. US of left axilla shows enlarged lymph nodes, mimicking metastasis from breast cancer. C. Chest CT scan revealed multiple masses and consolidations in left upper lobe with large amount of pleural effusion caused by pleural seeding. Biopsy from breast masses revealed metastatic adenocarcinoma from lung which was positive for TTF-1 and negative for BRST-2. US = ultrasound, DCIS = ductal carcinoma in situ, TTF = thyroid transcription factor-1, BSRT = Gross Cystic Disease, Fluid Protein-15 (GCDFP-15)
CONCLUSION
Breast metastases from extramammary malignancies show variable US features. Therefore, the possibility of metastatic lesion should be considered in evaluating breast lesions in patients with primary malignancy in other organs. Awareness of typical and atypical imaging features of such lesions may be helpful to diagnose metastatic lesions in the breast.
References
- 1.Lee SH, Park JM, Kook SH, Han BK, Moon WK. Metastatic tumors to the breast: mammographic and ultrasonographic findings. J Ultrasound Med. 2000;19:257–262. doi: 10.7863/jum.2000.19.4.257. [DOI] [PubMed] [Google Scholar]
- 2.McCrea ES, Johnston C, Haney PJ. Metastases to the breast. AJR Am J Roentgenol. 1983;141:685–690. doi: 10.2214/ajr.141.4.685. [DOI] [PubMed] [Google Scholar]
- 3.Muttarak M, Nimmonrat A, Chaiwun B. Metastatic carcinoma to the male and female breast. Australas Radiol. 1998;42:16–19. doi: 10.1111/j.1440-1673.1998.tb00556.x. [DOI] [PubMed] [Google Scholar]
- 4.Bohman LG, Bassett LW, Gold RH, Voet R. Breast metastases from extramammary malignancies. Radiology. 1982;144:309–312. doi: 10.1148/radiology.144.2.7089284. [DOI] [PubMed] [Google Scholar]
- 5.Silverman JF, Feldman PS, Covell JL, Frable WJ. Fine needle aspiration cytology of neoplasms metastatic to the breast. Acta Cytol. 1987;31:291–300. [PubMed] [Google Scholar]
- 6.Yeh CN, Lin CH, Chen MF. Clinical and ultrasonographic characteristics of breast metastases from extramammary malignancies. Am Surg. 2004;70:287–290. [PubMed] [Google Scholar]
- 7.Vergier B, Trojani M, de Mascarel I, Coindre JM, Le Treut A. Metastases to the breast: differential diagnosis from primary breast carcinoma. J Surg Oncol. 1991;48:112–116. doi: 10.1002/jso.2930480208. [DOI] [PubMed] [Google Scholar]
- 8.Amichetti M, Perani B, Boi S. Metastases to the breast from extramammary malignancies. Oncology. 1990;47:257–260. doi: 10.1159/000226826. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen M, Andersen JA, Henriksen FW, Kristensen PB, Lorentzen M, Ravn V, et al. Metastases to the breast from extramammary carcinomas. Acta Pathol Microbiol Scand A. 1981;89:251–256. doi: 10.1111/j.1699-0463.1981.tb00218.x. [DOI] [PubMed] [Google Scholar]
- 10.Hajdu SI, Urban JA. Cancers metastatic to the breast. Cancer. 1972;29:1691–1696. doi: 10.1002/1097-0142(197206)29:6<1691::aid-cncr2820290637>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Yang WT, Metreweli C. Sonography of nonmammary malignancies of the breast. AJR Am J Roentgenol. 1999;172:343–348. doi: 10.2214/ajr.172.2.9930779. [DOI] [PubMed] [Google Scholar]
- 12.Bartella L, Kaye J, Perry NM, Malhotra A, Evans D, Ryan D, et al. Metastases to the breast revisited: radiological-histopathological correlation. Clin Radiol. 2003;58:524–531. doi: 10.1016/s0009-9260(03)00068-0. [DOI] [PubMed] [Google Scholar]
- 13.Derchi LE, Rizzatto G, Giuseppetti GM, Dini G, Garaventa A. Metastatic tumors in the breast: sonographic findings. J Ultrasound Med. 1985;4:69–74. doi: 10.7863/jum.1985.4.2.69. [DOI] [PubMed] [Google Scholar]
- 14.Yang WT, Muttarak M, Ho LW. Nonmammary malignancies of the breast: ultrasound, CT, and MRI. Semin Ultrasound CT MR. 2000;21:375–394. doi: 10.1016/s0887-2171(00)90031-3. [DOI] [PubMed] [Google Scholar]
- 15.Paulus DD, Libshitz HI. Metastasis to the breast. Radiol Clin North Am. 1982;20:561–568. [PubMed] [Google Scholar]
- 16.Cardenosa G. Breast Imaging. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 270–276. [Google Scholar]
- 17.Jackson FI, Lalani ZH. Breast lymphoma: radiologic imaging and clinical appearances. Can Assoc Radiol J. 1991;42:48–54. [PubMed] [Google Scholar]
- 18.Yang WT, Lane DL, Le-Petross HT, Abruzzo LV, Macapinlac HA. Breast lymphoma: imaging findings of 32 tumors in 27 patients. Radiology. 2007;245:692–702. doi: 10.1148/radiol.2452061726. [DOI] [PubMed] [Google Scholar]
- 19.Sabaté JM, Gómez A, Torrubia S, Camins A, Roson N, De Las Heras P, et al. Lymphoma of the breast: clinical and radiologic features with pathologic correlation in 28 patients. Breast J. 2002;8:294–304. doi: 10.1046/j.1524-4741.2002.08509.x. [DOI] [PubMed] [Google Scholar]
- 20.Paulus DD. Lymphoma of the breast. Radiol Clin North Am. 1990;28:833–840. [PubMed] [Google Scholar]
- 21.Chung SY, Oh KK. Imaging findings of metastatic disease to the breast. Yonsei Med J. 2001;42:497–502. doi: 10.3349/ymj.2001.42.5.497. [DOI] [PubMed] [Google Scholar]
- 22.Nance FC, MacVaugh H, 3rd, Fitts WT., Jr Metastatic tumor to the breast simulating bilateral primary inflammatory carcinoma. Am J Surg. 1966;112:932–935. doi: 10.1016/0002-9610(66)90154-1. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan EU, Phillips AK, Randell A, Taylor B, Garg SK. Bilateral metastatic inflammatory carcinoma in the breast from primary ovarian cancer. Obstet Gynecol. 1980;55(3 Suppl):94S–96S. doi: 10.1097/00006250-198003001-00029. [DOI] [PubMed] [Google Scholar]
- 24.Toombs BD, Kalisher L. Metastatic disease to the breast: clinical, pathologic, and radiographic features. AJR Am J Roentgenol. 1977;129:673–676. doi: 10.2214/ajr.129.4.673. [DOI] [PubMed] [Google Scholar]
- 25.Tateishi U, Hasegawa T, Beppu Y, Satake M, Moriyama N. Synovial sarcoma of the soft tissues: prognostic significance of imaging features. J Comput Assist Tomogr. 2004;28:140–148. doi: 10.1097/00004728-200401000-00024. [DOI] [PubMed] [Google Scholar]
- 26.Layfield LJ, Gopez EV. Insular carcinoma of the thyroid: report of a case with intact insulae and microfollicular structures. Diagn Cytopathol. 2000;23:409–413. doi: 10.1002/1097-0339(200012)23:6<409::aid-dc10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Brancatelli G, Federle MP, Grazioli L, Carr BI. Hepatocellular carcinoma in noncirrhotic liver: CT, clinical, and pathologic findings in 39 U.S. residents. Radiology. 2002;222:89–94. doi: 10.1148/radiol.2221010767. [DOI] [PubMed] [Google Scholar]
- 28.Pope TL, Jr, Brenbridge AN, Sloop FB, Jr, Morris JR, 3rd, Carpenter J. Primary histiocytic lymphoma of the breast: mammographic, sonographic, and pathologic correlation. J Clin Ultrasound. 1985;13:667–670. [PubMed] [Google Scholar]
- 29.Liberman L, Giess CS, Dershaw DD, Louie DC, Deutch BM. Non-Hodgkin lymphoma of the breast: imaging characteristics and correlation with histopathologic findings. Radiology. 1994;192:157–160. doi: 10.1148/radiology.192.1.8208929. [DOI] [PubMed] [Google Scholar]