Abstract
The advent of medical nutrition therapy and nutritional physiology affords the opportunity to link diet to specific cardiovascular mechanisms, suggesting novel treatments for cardiovascular disease. This study tests the hypothesis that beetroot juice increases the plasma nitric oxide (NO) concentration, which is associated with improvements in cardiorespiratory function at rest and during submaximal aerobic exercise. The subjects were 12 healthy, young adult, normotensive African-American females, with a body mass of 61 ± 2 kg, body fat of 28% ± 4%, and peak oxygen consumption of 26 ± 3 mL·kg−1·min−1. The subjects were studied at rest and during cycle ergometer exercise at 40%, 60%, and 80% of peak oxygen consumption. Plasma NO concentration, respiratory quotient (RQ), minute ventilation, systolic and diastolic blood pressure (SBP and DBP), heart rate, and oxygen consumption were compared between isocaloric, isovolumetric placebo control orange juice and experimental beetroot juice treatments on separate days. The beetroot juice treatment increased plasma NO concentration and decreased oxygen consumption, SBP, and the heart rate-SBP product at rest and at 40%, 60%, and 80% of peak oxygen consumption in the absence of significant effects on RQ, minute ventilation, heart rate, and DBP. These findings suggest that, in healthy subjects, beetroot juice treatments increase plasma NO concentration and decrease cardiac afterload and myocardial oxygen demand at rest and during 3 submaximal levels of aerobic exercise. Future studies should determine the cellular and molecular mechanisms responsible for the improvement in cardiorespiratory function associated with dietary nitrate supplementation and whether they translate into better cardiovascular function and exercise tolerance in individuals with a compromised cardiovascular system.
Keywords: beetroot juice, blood pressure, cardiac output, oxygen consumption, myocardial oxygen demand, exercise, aerobic capacity, humans, females
Introduction
The main product of nitrate and nitrite metabolism is nitric oxide (NO) (Umbrello et al. 2013), which is a primary, endogenous vasodilator molecule that regulates blood pressure (Abrams 1996). NO is produced by nitric oxide synthase (NOS) from various sources, including endothelial cells (Brix et al. 2012), neurons (Vincent 2010), epithelial cells (Dortch-Carnes and Tosini 2013), macrophages (Andreasen et al. 2013), neutrophils (Tsuji et al. 2012), and erythrocytes (Nahavandi et al. 2006). NO is also a highly reactive free radical produced by NOS uncoupled to tetrahydrobiopterin, a critical cofactor for NOS activity (Landmesser et al. 2003) during oxidative stress (Wilkinson-Berka et al. 2013). Some of the essential hypertension in African Americans has been explained by endothelial cell dysfunction (Kalinowski et al. 2004), and it has been hypothesized that an insufficiency of NOS activity is associated with a gene variant that reduces the normal production of NO (Martinez Cantarin et al. 2010). A study supporting this hypothesis found that umbilical arteries from African Americans exhibited greater oxidative stress and inflammation during exposures to increased shear forces than did those from Caucasian Americans (Feairheller et al. 2011a, 2011b). One approach to overcoming NO deficiency is dietary supplementation of nitrate and increasing the bioavailability of NO by using foods such as beetroot juice (Miller et al. 2012). Treatments based on dietary supplementation of nitrate with beetroot juice have been reported to increase exercise tolerance and to be associated with lower blood pressure and less oxygen consumption (V̇O2), compared with a placebo control treatment, in type 2 diabetics (Gilchrist et al. 2013), healthy 60- to 70-year-old men and women (Kelly et al. 2013), and 30-year-old trained male cyclists (Cermak et al. 2012). Therefore, the current study was designed to test the hypothesis that dietary nitrate supplementation using beetroot juice increases the plasma NO concentration and improves cardiorespiratory functions at rest and during submaximal aerobic exercise.
Materials and methods
This study used a cross-over design in which participants were randomly assigned to receive either a placebo control orange juice or a beetroot juice treatment on separate days.
Subjects
Twelve healthy, young adult women who were physically active, but not exercise trained, volunteered to participate in this study. None of the subjects were regular users of tobacco products or consumers of alcohol. They were all disease free and had no medical conditions. The procedures outlined in the study were approved by the Institutional Human Participants Review Board of Howard University. After the experimental procedures and study risks were explained, all the subjects gave their written informed consent before commencement of the study. Percentage of body fat was measured by dual energy X-ray absorptiometry (GE Lunar, Madison, Wis., USA).
Nitrate supplementation
The subjects were randomly assigned to consume 500 mL of either beetroot juice (Biotta Inc., Carmel, Ind., USA) or the placebo control beverage, orange juice. Our rationale for the 500-mL dose of beetroot juice was based on the acute blood pressure lowering and antiplatelet activity reported at this dose (Webb et al. 2008). We selected orange juice as the control because 500 mL had the same constituents as beetroot juice: 220 cal, 52 g carbohydrates, negligible fat (1 g), 4 g protein, and 140 mg sodium. Orange juice is also similar to beetroot juice in texture and antioxidant content (Pellegrini et al. 2003) and has about 5% of the sodium content of beetroot juice (Niu et al. 2008). Biotta beetroot juice has an average listed content of nitrate of 1500 mg·L−1 (Kenjale et al. 2011), whereas the nitrate content of orange juice is negligible (Smiechowska 2003). The subjects arrived at the laboratory in a fasting condition on 2 separate days, approximately 1–2 weeks apart. They had been instructed to refrain from exercise for 12 h and upon arrival at the laboratory were placed in a sitting position to ingest the 500 mL beetroot juice or the placebo control orange juice. To digest and metabolize the beverages, the subjects ingested them 120 min before the test (Kapil et al. 2010; Vanhatalo et al. 2010; Kenjale et al. 2011; Lansley et al. 2011). During the postprandial period, before testing, the subjects remained under supervised resting conditions in the laboratory and did not ingest food or fluid. There were no tolerance issues or untoward effects reported for either the beetroot juice or the control orange juice supplementation. Ingesting beetroot juice did have the benign side effect of producing red urine and stools, consistent with previous studies (Webb et al. 2008; Bailey et al. 2009; Kenjale et al. 2011); this strong, albeit benign, effect necessitated conducting this study without blinding.
Blood samples
Two hours after the beetroot juice and orange juice treatments, blood samples were collected for measuring the plasma NO concentration. Using a sterile lancet, capillary blood (100 μL) was collected from the fingertip. The blood samples were mixed immediately with a nitrite preservation solution containing 0.8 mol·L−1 ferricyanide, 10 mmol·L−1 N-ethylmaleimide, and 1% NP-40 (Boston Bio-Products, Ashland, Mass., USA). Following the addition of the blood to the preservation solution, the sample was deproteinated with trichloroacetic acid (Sigma–Aldrich, St. Louis, Mo., USA) and centrifuged at 15 000g for 3 min. The resulting blood plasma solution was then placed into a tube of 1 mol·L−1 ferricyanide sulfuric acid with potassium iodine (Sigma–Aldrich). The plasma NO concentration was measured in duplicate by an electrochemical method using the inNO-T nitric oxide analyzer (Innovative Instruments Inc., Tampa, Fla., USA). Before each recording, the inNO-T amino-700 electrode was calibrated using a standard nitrite solution (100 μmol·L−1).
Ergometry tests
All the subjects performed a progressive exercise test of peak oxygen consumption (V̇O2peak) on an electronically braked leg cycle ergometer (Lode Corival, Groningen, the Netherlands). The initial workload began at a level of 20 W for 3 min and was increased by equal work intensities every 3 min to volitional fatigue. Before the study, the cycle ergometer was calibrated for power outputs of 10–1000 W. During the test, blood pressure was determined noninvasively using the SunTech 4240 automated sphyg-momanometric device (SunTech Medical Inc., Morrisville, N.C., USA), which measures blood pressure by gating the R-wave with Korotkoff sounds. Heart rate was measured by an electrocardiogram, with 3 electrodes placed at the RL, LA, and V5 anatomical sites connected to the automated blood pressure monitor. During the exercise tests, V̇O2, minute ventilation (V̇E), the carbon dioxide excretion rate, and the respiratory exchange quotient (RQ) were measured breath by breath by a computerized metabolic system (Physio-Dyne Max II, Quogue, N.Y., USA). The V̇O2 measured during the last minute of the progressive exercise test was defined as V̇O2peak. Prior to each test, the gas analyzers and respiratory flow meters were calibrated with high-precision calibration gases (20.99% ± 0.01% oxygen and 5.00% ± 0.01% carbon dioxide; Scott Medical Products, Plumsteadville, Pa., USA) and a 3-L calibration syringe (Hans Rudolph, Shawnee, Kans., USA), respectively. After the V̇O2 test, the subjects ingested the experimental supplement or control and performed 2 separate submaximal ergometry tests under identical conditions on different days. The subjects had 5 min of sitting rest, and then baseline measures of heart rate, systolic and diastolic blood pressure (SBP and DBP), and V̇O2 were recorded. The subjects then completed 3 bouts of exercise at the constant submaximal workloads corresponding to 40%, 60%, and 80% of their predetermined V̇O2peak values, with every workload lasting 5 min. Heart rate, blood pressure, and V̇O2 were measured at minutes 4 and 5 of each exercise workload, with the mean values used for analysis, and the rate-pressure product (heart rate × SBP) was computed. All the submaximal ergometry tests were performed during the luteal phase of the menstrual cycle to eliminate any confounding influences of hormonal changes on blood pressure.
Statistics
Results are expressed as means ± SD. Using SPSS software (SPSS Inc., Chicago, Ill., USA), a 2-way repeated-measures ANOVA was performed to evaluate the difference between the supplement and control conditions; factor 1 was specified as control vs. beetroot juice treatment, and factor 2 as exercise level. Pearson’s product-moment correlation analyses were performed across the plasma NO concentration and other study outcome measures. The significance level was set at p < 0.05.
Results
Table 1 presents the demographic and physiological characteristics of the study subjects, showing that they were young adult females with a normal to high percentage of body fat and low V̇O2peak, indicating sedentary lifestyles.
Table 1.
Descriptive characteristics of the study subjects.
| Variables | Subjects (n = 12) |
|---|---|
| Age (y) | 20.7±0.8 |
| Height (cm) | 160.9±3.9 |
| Weight (kg) | 61.0±2.4 |
| Body fat (%) | 27.9±3.7 |
| V̇O2peak (mL·kg−1·min−1) | 26.1±3.3 |
Note: Data are presented as means ± SD. V̇O2peak, peak oxygen consumption.
Figure 1 shows that the beetroot juice treatment increased the plasma NO concentration from (mean ± SD) 4.5 ± 3.4 nmol·L−1 to 17.8 ± 8.8 nmol·L−1 (p < 0.001). Correlations between plasma NO concentration and the aforementioned variables were found to be not significant (p > 0.1).
Fig. 1.
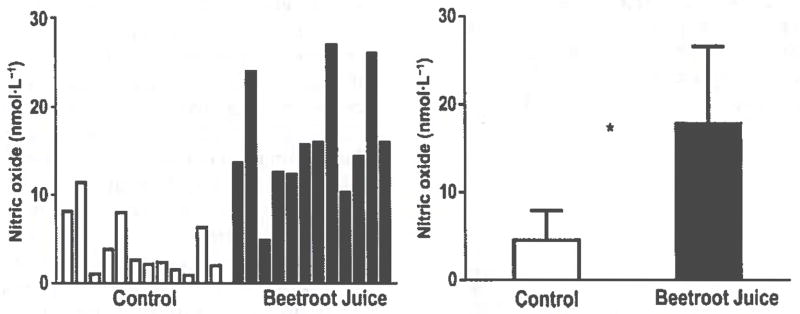
Effects of beetroot juice treatment on plasma nitric oxide. Left panel: Bars represent individual subject values of plasma nitric oxide concentrations, expressed in nmol·L−1, for a placebo control orange juice treatment and an isocaloric, isovolumetric beetroot juice treatment on separate days in 12 healthy, normotensive, young adult, African-American, female university students. The individual values for the 12 subjects are depicted as sequential bars for each treatment. *, Difference between the mean ± SD placebo control orange juice treatment (4.5 ± 3.4 nmol·L−1) and the beetroot juice treatment (17.8 ± 8.8 nmol·L−1) was statistically significant at p < 0.001.
Table 2 shows that, compared with the placebo orange juice control treatment, the beetroot juice treatment decreased SBP at rest and at the constant exercise workloads corresponding to 40%, 60%, and 80% of the subjects’ predetermined V̇O2peak (p < 0.05). The beetroot juice treatment had no significant effect on RQ, V̇E, heart rate, or DBP.
Table 2.
Cardiorespiratory effects of the beetroot juice treatment.
| Variable | Baseline | 40% V̇O2peak | 60% V̇O2peak | 80% V̇O2peak |
|---|---|---|---|---|
| Control | ||||
| HR (beats·min−1) | 85.8±11.5 | 104±10.8 | 132±10.4 | 154.5±10.1 |
| V̇E (L·min−1) | 9.2±2.4 | 19.9±2.0 | 30.0±5.8 | 39.9±9.3 |
| RQ | 0.82±0.03 | 0.81±0.03 | 0.92±0.04 | 0.94±0.02 |
| SBP (mm Hg) | 120±10 | 129±12 | 148±15 | 162±17 |
| DBP (mm Hg) | 90±9 | 88±7 | 85±9 | 88±14 |
| Beetroot juice | ||||
| HR (beats·min−1) | 83.2±11.0 | 102±10.5 | 130.4±11.5 | 153.0±11.1 |
| V̇E (L·min−1) | 8.2±2.1 | 18.4±2.3 | 29.3±5.2 | 39.2±9.6 |
| RQ | 0.79±0.03 | 0.77±0.05 | 0.91±0.05 | 0.94±0.03 |
| SBP (mm Hg) | 115±8* | 121±15* | 142±11* | 152±16* |
| DBP(mmHg) | 86±5 | 80±8 | 85±8 | 84±8 |
Note: Data are presented as means ± SD. V̇O2peak, peak oxygen consumption; HR, heart rate; V̇OE, pulmonary ventilation; RQ, respiratory quotient; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Significantly different from control (p < 0.05).
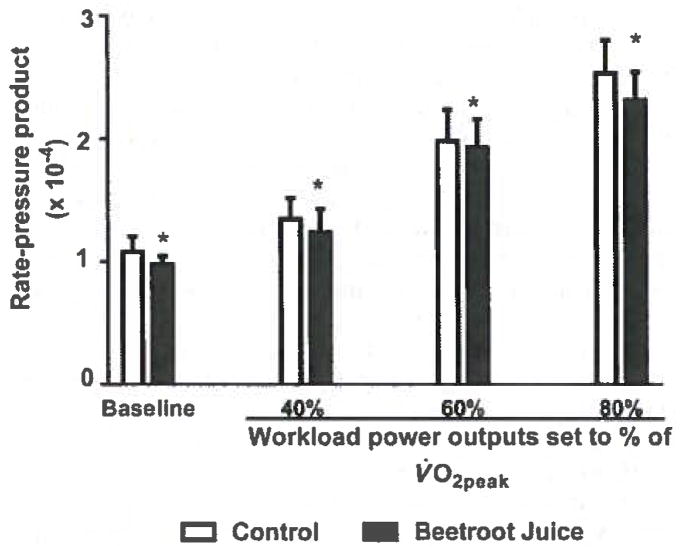
Figure 2 demonstrates that the beetroot juice treatment decreased the heart rate-SBP product at rest and at the constant exercise workloads corresponding to 40%, 60%, and 80% of the predetermined V̇O2peak (p < 0.01). Figure 3 shows that the beetroot juice treatment had no effect on V̇O2 at rest but decreased V̇O2 measured at the constant workloads corresponding to 40%, 60%. and 80% of the predetermined V̇O2peak (p < 0.05).
Fig. 2.
Effects of beetroot juice treatment on the rate-pressure product. Bars represent means ± SD of the heart rate × systolic blood pressure product, expressed in beats·min−1·mm Hg−1 × 10−4, for a placebo control orange juice treatment and an isocaloric, isovolumetric beetroot juice treatment on separate days in 12 normotensive, young adult, African-American, female university students. The treatments are compared at rest (baseline) and at constant workload power outputs corresponding to 3 submaximal levels of aerobic exercise (40%, 60%, and 80% of each subject’s predetermined peak oxygen consumption (V̇O2peak)). *, Difference between the control and beetroot juice treatments was statistically significant at p < 0.01.
Fig. 3.
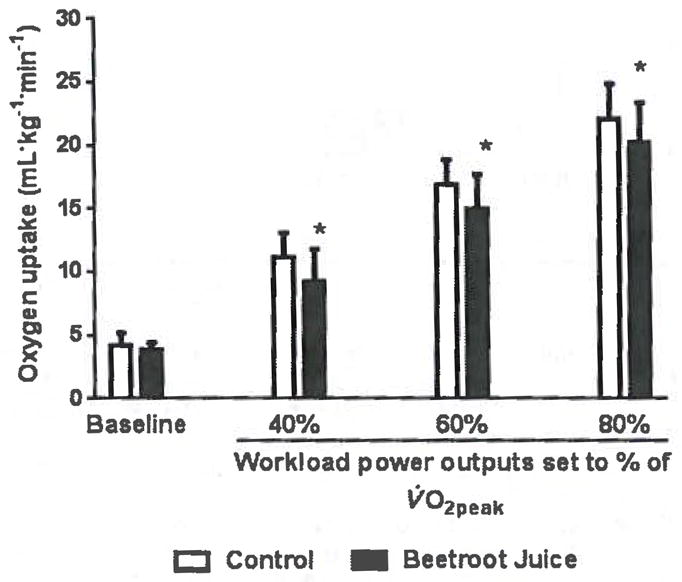
Effects of beetroot juice treatment on oxygen consumption. Bars represent means ± SD of oxygen consumption, expressed in mL·kg−1·min−1, for a placebo control orange juice treatment and an isocaloric, isovolumetric beetroot juice treatment on separate days in 12 normotensive, young adult, African-American, female university students. The treatments are compared at rest (baseline) and at constant workload power outputs corresponding to 3 submaximal levels of aerobic exercise (40%, 60%, and 80% of each subject’s predetermined peak oxygen consumption (V̇O2peak)).*, Difference between the control and beetroot juice treatments was statistically significant at p < 0.05.
Discussion
Nitrate therapy to increase production and release of NO for vasodilation, reduction of cardiac afterload, and decreased myocardial V̇O2 is a first-line drug treatment strategy for cardiovascular diseases (Belardinelli 2000). Previous studies have shown that beetroot juice and other dietary nitrate supplements also decrease blood pressure (Kelly et al. 2013) and improve whole-body oxygen utilization kinetics (Kelly et al. 2013), with increased exercise tolerance in trained cyclists (Cermak et al. 2012), despite no changes in blood pressure, endothelial function, or insulin sensitivity in type 2 diabetics (Gilchrist et al. 2013). However, we believe that the current study is the first to demonstrate these effects after dietary nitrate supplementation with beetroot juice, in addition to an indication of decreased myocardial V̇O2, in a group of healthy African-American women.
The main finding of the current study is that, compared with a placebo orange juice control treatment, a single beetroot juice treatment increased plasma NO concentrations and decreased the heart rate-SBP product, indicative of decreased myocardial oxygen demand, across a wide range of activities from rest to 80% of V̇O2peak. The relatively small, statistically significant decrements in SBP and RPP that were observed in healthy normotensive subjects after the beetroot juice treatment occurred despite the numerous homeostatic regulatory mechanisms expected to be maintaining blood pressure and myocardial oxygen demand at higher values, as observed after the placebo control orange juice treatment. If these small changes were to occur over a prolonged period of time, the small beneficial effects on cardiac dynamics could be amplified by a factor of dose x time. It was beyond the scope of this study to determine whether the small, statistically significant beetroot-juice–induced changes were clinically significant in an individual. Nevertheless, the potential impact of this study is that the small, statistically significant changes described herein, if translated into a dietary prevention strategy, may shift the average blood pressure and RPP toward a reduction in the incidence of cardiovascular disease in a population.
Comparable findings using a placebo control nitrite-depleted beetroot juice treatment in which decrements in V̇O2 during moderate-intensity aerobic exercise were reported in a study of healthy, normotensive, young adult males (Wylie et al. 2013). In the current study, the subjects were young adult, overweight, sedentary, but otherwise healthy, African-American women with normal blood pressure. Compared with the placebo control trial of orange juice on a separate day, the beetroot juice treatment was effective in significantly increasing the plasma NO concentrations of the study subjects. Vitamin C in oranges is about 16-fold greater than that in beetroots, and ascorbate is reported to effectively protect endothelial tissues from oxidative-stress-induced reductions in the production and release of NO associated with apoptosis (May and Harrison 2013). Thus, our finding that the low levels of plasma NO occurred immediately after the control orange juice treatment suggests that vitamin C did not play a significant role in increasing the plasma NO concentrations of our study subjects. The plasma NO concentrations found after nitrate supplementation in our healthy African-American study group during the luteal phase of their menstrual cycles was in the same range as that reported in a study of healthy Italian women (Cicinelli et al. 1999) and that reported in an Egyptian cohort of women with hyperprolactinemia and amenorrhea (Shaarawy et al. 1997), and was about 40% of that reported for normal Egyptian women (Shaarawy et al. 1997), the last measured during the follicular menstrual phase, in the absence of dietary nitrate supplementation. These findings appear to be consistent with studies showing that the plasma NO level was higher during the follicular than during the luteal phase (Cicinelli et al. 1999; Manau et al. 1999). An age-related decline in the capacity for production of NO, presumably as a result of oxidative stress, has been demonstrated (Nyberg et al. 2012). Therefore, the lower control plasma NO levels in our African-American female subjects, compared with the women of different ethnicities in the aforementioned studies, could be indicative of an inherited NO insufficiency or a greater amount of oxidative stress. This finding of a mean control plasma NO level in healthy African-American women that was 20%–25% that of Caucasian women appears to support the hypothesis of a predilection for hypertension based on inheritance of an NOS gene variant associated with NO deficiency in African Americans (Martinez Cantarin et al. 2010). However, some of this disparity may be explained by ethnicity-related dietary differences, which should be investigated further.
Nitrate- and (or) nitrite-induced protection against myocardial ischemia–reperfusion, cardiac arrest–resuscitation (Bryan et al. 2007), and cardiomyopathies with a reduction in indicators of oxidative stress (Zhu et al. 2011) has been demonstrated. An important target for dietary nitrates, converted to nitrites during metabolism, appears to be mitochondria. Nitrate treatment is reported to protect electron transport functions and oxidative phosphorylation, with attenuation of hydrogen peroxide production, in the cardiac myocyte mitochondria of mice subjected to doxorubicin-induced cardiomyopathy (Zhu et al. 2011). Similar results from supplementing diets with nitrates are reported for human skeletal muscle mitochondria (Larsen et al. 2011). These findings suggest that dietary nitrates can increase the efficiency of oxygen utilization in the skeletal and cardiac muscles. Unfortunately, there are no studies correlating the NO metabolite-induced improvements in oxygen utilization with enhanced ion channel, calcium handling, or intracellular signal transduction molecular activities. We have shown such mechanisms to be related to the positive inotropic effects of another endogenous regulator of vasodilation, calcitonin gene-related peptide (Al-Rubaiee et al. 2013). Mechanisms for both positive and negative dose-dependent effects of NO on the myocardium are known (Folino et al. 2011). Thus, future studies are needed to elucidate the mechanisms of improved cardiac dynamics in whole-heart, cardiacmyocyte, sarcomere, and molecular experimental models and to validate the use of dietary nitrates for cardiovascular disease prevention and treatment.
Conclusions
To the best of our knowledge, this study is the first to demonstrate that dietary supplementation of nitrate by drinking a beetroot juice beverage has the potential of decreasing V̇O2, blood pressure, and myocardial oxygen demand at 3 submaximal levels of aerobic exercise in a population of disease-free, normotensive, young adult African-American females. The beetroot juice treatment increased the plasma NO concentration, but blood pressure and myocardial oxygen demand were not correlated with the plasma NO level. These findings suggest a beneficial role for beetroot juice in the treatment of cardiovascular disease; however, the mechanisms are not likely to be caused by an increased plasma NO level. The effects of dietary nitrate supplementation on cardiac dynamics in whole-heart and cardiomyocyte sarcomere preparations will help determine the efficacy of nitrate supplementation as a medical nutrition therapy for cardiovascular disease.
Acknowledgments
This study was supported by NIH/NCRR/RCMI Grant 2G12RR003048 to Howard University.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Contributor Information
Vernon Bond, Jr., Department of Health, Human Performance and Leisure Studies and the Cancer Center Physical Medicine and Nutrition Laboratory, Howard University, Washington, DC 20059. USA
Bryan H. Curry, Department of Medicine, Division of Cardiology, Howard University Hospital, Washington, DC 20060, USA
Richard G. Adams, Department of Medicine, Division of Cardiology, Howard University Hospital, Washington, DC 20060, USA
Richard M. Millis, Department of Physiology and Biophysics, Howard University College of Medicine, Washington, DC 20059, USA
Georges E. Haddad, Department of Physiology and Biophysics, Howard University College of Medicine, Washington, DC 20059, USA
References
- Abrams J. Beneficial actions of nitrates in cardiovascular disease. Am J Cardiol. 1996;77(13):31C–37C. doi: 10.1016/S0002-9149(96)00186-5.. [DOI] [PubMed] [Google Scholar]
- Al-Rubaiee M, Gangula PR, Millis RM, Walker RK, Umoh NA, Cousins VM, et al. Inotropic and lusitropic effects of calcitonin gene-related peptide in the heart. Am J Physiol Heart Circ Physiol. 2013;304(111):H1525–H1537. doi: 10.1152/ajpheart.00874.2012.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen S, Chong SF, Wohl BM, Goldie KN, Zelikin AN. Poly(vinyl alcohol) physical hydrogel nanoparticles, not polymer solutions, exert inhibition of nitric oxide synthesis in cultured macrophages. Biomacromolecules. 2013;14(5):1687–1695. doi: 10.1021/bm400369u.. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, et al. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107(4):1144–1155. doi: 10.1152/japplphysiol.00722.2009.. [DOI] [PubMed] [Google Scholar]
- Belardinelli R. Trimetazidine and the contractile response of dysfunctional myocardium in ischaemic cardiomyopathy. Rev Port Cardiol. 2000;19(Suppl 5):V35–V39. [PubMed] [Google Scholar]
- Brix B, Mesters JR, Pellerin L, Jöhren O. Endothelial cell-derived nitric oxide enhances aerobic glycolysis in astrocytes via HIF-1α-mediated target gene activation. J Neurosci. 2012;32(28):9727–9735. doi: 10.1523/JNEUROSCI.0879-12.2012.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2007;104(48):19144–19149. doi: 10.1073/pnas.0706579104.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak NM, Gibala MJ, van Loon LJ. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int J Sport Nutr Exerc Metab. 2012;22(1):64–71. doi: 10.1123/ijsnem.22.1.64. [DOI] [PubMed] [Google Scholar]
- Cicinelli E, Ignarro LJ, Schonauer LM, Matteo MG, Galantino P, Falco N. Different plasma levels of nitric oxide in arterial and venous blood. Clin Physiol. 1999;19(5):440–442. doi: 10.1046/j.1365-2281.1999.00200.x.. [DOI] [PubMed] [Google Scholar]
- Dortch-Carnes J, Tosini G. Melatonin receptor agonist-induced reduction of SNP-released nitric oxide and cGMP production in isolated human non-pigmented ciliary epithelial cells. Exp Eye Res. 2013;107:1–10. doi: 10.1016/j.exer.2012.11.007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feairheller DL, Park JY, Sturgeon KM, Williamson ST, Diaz KM, Veerabhadrappa P, Brown MD. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Transl Sci. 2011a;4:1–37. doi: 10.1111/j.1752-8062.2011.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feairheller DL, Park JY, Rizzo V, Kim B, Brown MD. Racial differences in the responses to shear stress in human umbilical vein endothelial cells. Vasc Health Risk Manag. 2011b;7:425–431. doi: 10.2147/VHRM.S22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folino A, Rastaldo R, Cappello S, Chiribiri A, Pagliaro P, Losano G. Activity of endothelial factors on myocardial inotropy. Minerva Cardioangiol. 2011 Epub ahead of print. [PubMed] [Google Scholar]
- Gilchrist M, Winyard PG, Aizawa K, Anning C, Shore A, Benjamin N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic Biol Med. 2013;60C:89–97. doi: 10.1016/j.freeradbiomed.2013.01.024.. [DOI] [PubMed] [Google Scholar]
- Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109(21):2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A.. [DOI] [PubMed] [Google Scholar]
- Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, et al. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56(2):274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536.. [DOI] [PubMed] [Google Scholar]
- Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, Bailey SJ, et al. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol Regul Integr Comp Physiol. 2013;304(2):R73–R83. doi: 10.1152/ajpregu.00406.2012.. [DOI] [PubMed] [Google Scholar]
- Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, et al. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol. 2011;110(6):1582–1591. doi: 10.1152/japplphysiol.00071.2011.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111(8):1201–1209. doi: 10.1172/JCI200314172.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, et al. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol. 2011;110(3):591–600. doi: 10.1152/japplphysiol.010702010.. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13(2):149–159. doi: 10.1016/j.cmet.2011.01.004.. [DOI] [PubMed] [Google Scholar]
- Martinez Cantarin MP, Ertel A, Deloach S, Fortina P, Scott K, Burns TL, Falkner B. Variants in genes involved in functional pathways associated with hypertension in African Americans. Clin Transl Sci. 2010;3(6):279–286. doi: 10.1111/j.1752-8062.2010.00242.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2013.5205. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GD, Marsh AP, Dove RW, Beavers D, Presley T, Helms C, et al. Plasma nitrate and nitrite are increased by a high-nitrate supplement but not by high-nitrate foods in older adults. Nutr Res. 2012;32(3):160–168. doi: 10.1016/j.nutres.2012.02.002.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahavandi M, Tavakkoli F, Millis RM, Wyche MQ, Habib MJ, Tavakoli N. Effects of hydroxyurea and L-arginine on the production of nitric oxide metabolites in cultures of normal and sickle erythrocytes. Hematology. 2006;11(4):291–294. doi: 10.1080/10245330600921998.. [DOI] [PubMed] [Google Scholar]
- Niu L, Wu J, Liao X, Chen F, Wang Z, Zhao G, Hu X. Physicochemical characteristics of orange juice samples from seven cultivars. Agricultural Sciences in China. 2008;7(1):41–47. doi: 10.1016/S1671-2927(08)60020-6. [DOI] [Google Scholar]
- Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y, Mortensen SP. Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol. 2012;590(21):5361–5370. doi: 10.1113/jphysiol.2012.239053.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini N, Serafini M, Colombi B, Del Rio D, Salvatore S, Bianchi M, Brighenti F. Total antioxidant activity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J Nutr. 2003;133(9):2812–2819. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- Shaarawy M, Nafei S, Abul-Nasr A, el-Sharkawy S, Younis A. Circulating nitric oxide levels in galactorrheic, hyperprolactinemic, amenorrheic women. Fertil Steril. 1997;68(3):454–459. doi: 10.1016/S0015-0282(97)00225-2.. [DOI] [PubMed] [Google Scholar]
- Smiechowska M. The content of nitrates V and VIII and vitamin C in juices obtained from organic and conventional raw materials. Pol J Food Nutr Sci. 2003;12/53(2):57–61. [Google Scholar]
- Tsuji S, Iharada A, Taniuchi S, Hasui M, Kaneko K. Increased production of nitric oxide by phagocytic stimulated neutrophils in patients with chronic granulomatous disease. J Pediatr Hematol Oncol. 2012;34(7):500–502. doi: 10.1097/MPH.0b013e3182668388.. [DOI] [PubMed] [Google Scholar]
- Umbrello M, Dyson A, Feelisch M, Singer M. The key role of nitric oxide in hypoxia: hypoxic vasodilation and energy supply-demand matching, Antioxid. Redox Signal. 2013;19(14):1690–1710. doi: 10.1089/ars.2012.4979.PMID:23311950. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, et al. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299(4):R1121–R1131. doi: 10.1152/ajpregu.00206.2010.. [DOI] [PubMed] [Google Scholar]
- Vincent SR. Nitric oxide neurons and neurotransmission. Prog Neurobiol. 2010;90(2):246–255. doi: 10.1016/j.pneurobio.2009.10.007.. [DOI] [PubMed] [Google Scholar]
- Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51(3):784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson-Berka JL, Rana I, Armani R, Agrotis A. Reactive oxygen species, Nox and angiotensin II in angiogenesis: implications for retinopathy. Clin Sci (Lond) 2013;124(10):597–615. doi: 10.1042/CS20120212.. [DOI] [PubMed] [Google Scholar]
- Zhu SG, Kukreja RC, Das A, Chen Q, Lesnefsky EJ, Xi L. Dietary nitrate supplementation protects against doxorubicin-induced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol. 2011;57(21):2181–2189. doi: 10.1016/j.jacc.2011.01.024.. [DOI] [PMC free article] [PubMed] [Google Scholar]