Abstract
Gudden's tegmental nuclei provide major inputs to the rodent mammillary bodies, where they are thought to be important for learning and navigation. Comparable projections have yet to be described in the primate brain, where part of the problem has been in effectively delineating these nuclei. Immunohistochemical staining of tissue from a series of macaque monkeys (Macaca mulatta) showed that cells in the region of both the ventral and dorsal tegmental nuclei selectively stain for parvalbumin, thus helping to reveal these nuclei. These same tegmental nuclei were not selectively revealed when tissue was stained for SMI32, acetylcholinesterase, calbindin, or calretinin. In a parallel study, horseradish peroxidase was injected into the mammillary bodies of five cynomolgus monkeys (Macaca fascicularis). Retrogradely labeled neurons were consistently found in the three subdivisions of the ventral tegmental nucleus of Gudden, which are located immediately below, within, and above the medial longitudinal fasciculus. Further projections to the mammillary body region arose from cells in the anterior tegmental nucleus, which appears to be a rostral continuation of the infrafascicular part of the ventral tegmental nucleus. In the dorsal tegmental nucleus of Gudden, labeled cells were most evident when the tracer injection was more laterally placed in the mammillary bodies, consistent with a projection to the lateral mammillary nucleus. The present study not only demonstrates that the primate mammillary bodies receive parallel inputs from the dorsal and ventral tegmental nuclei of Gudden, but also helps to confirm the extent of these poorly distinguished nuclei in the monkey brain.
Indexing Terms: amnesia, head-direction, hypothalamus, memory, primate, raphe nucleus, tegmentum
Despite past uncertainty over the importance of the human mammillary bodies for memory (Victor et al., 1971; Kapur et al., 1998), there is now compelling evidence that these nuclei are vital for normal episodic memory (Dusoir et al., 1990; Van der Werf et al., 2000; Vann and Aggleton, 2004; Tsivilis et al., 2008; Vann et al., 2009). In order to understand how the mammillary bodies might contribute to memory, it is necessary to determine their anatomical connections. In nonprimate brains it has long been appreciated that the mammillary bodies receive two major sets of afferents – one from the subiculum (hippocampal formation) and the other from Gudden's tegmental nuclei (Valenstein and Nauta, 1959; Briggs and Kaelber, 1971; Meibach and Siegel, 1975; Swanson and Cowan, 1977; Hyakawa and Zyo, 1984; Allen and Hopkins, 1989). Although it has been established that there are dense subicular projections to the mammillary bodies in monkeys (Poletti and Cresswell, 1977; Rosene and Van Hoesen, 1977; Aggleton et al., 2005), it remains to be demonstrated whether the primate mammillary bodies also receive direct projections from Gudden's tegmental nuclei.
In most mammals, the tegmental nuclei of Gudden comprise a dorsal nucleus and a ventral nucleus (Petrovicky, 1971). In the rat these two nuclei have distinct, parallel connections (Fig. 1B). The dorsal tegmental nucleus projects to the lateral mammillary nucleus whereas the ventral tegmental nucleus projects to the medial mammillary nucleus (Hyakawa and Zyo, 1984; Allen and Hopkins, 1989; Hopkins, 2005). The search for corresponding projections from the tegmentum to the mammillary bodies in a primate brain is potentially problematic, as there has been uncertainty over the existence of some of these tegmental nuclei in the primate brain. Cytoarchitectonic studies have described only a reduced dorsal tegmental nucleus in the brains of monkeys and humans compared with other mammals (Petrovicky, 1971; Hayakawa and Zyo, 1983). The ventral tegmental nucleus of Gudden is regarded (Petrovicky, 1971) as being only very weakly developed in the rhesus monkey (Macacca mulatta; Fig. 1A). Indeed, some reports state that the ventral nucleus is completely absent in the human brain (Petrovicky, 1971; Hayakawa and Zyo, 1983), although other studies have located this nucleus in the human brain using a mixture of cytoarchitectonics and immunohistochemistry (Huang et al., 1992).
Figure 1.
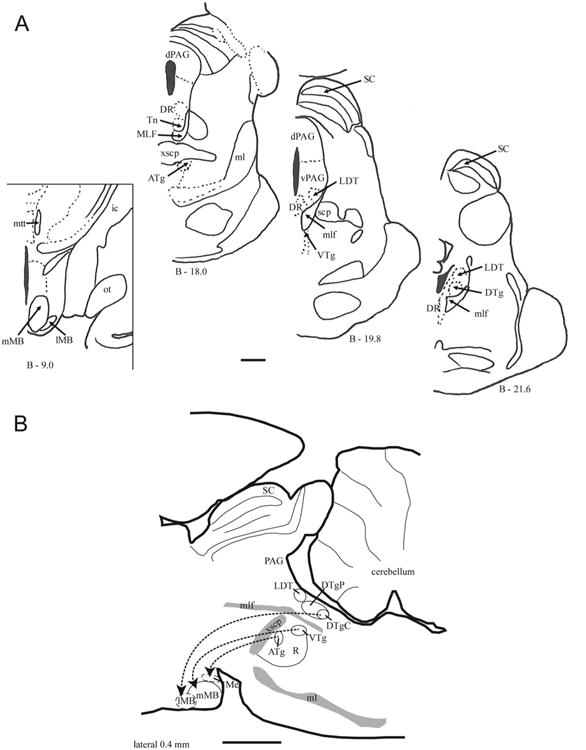
A: Series of coronal sections from the rhesus monkey showing the location of the Gudden's tegmental nuclei within the brain-stem. The three brainstem sections (on the right) are adapted from Paxinos et al. (2000), and their distance behind Bregma is indicated below each section. On the left is a coronal section through the midlevel of the mammillary bodies. B: Parasagittal section (laterality 0.4 mm) of the rat brain showing the relative positions of Gudden's tegmental nuclei and their topographic projections to different nuclei within the mammillary bodies. The lateral mammillary nucleus and pars medianus of the mammillary bodies are outlined with dashes as they are not located in the plane of the section. For abbreviations, see list. Scale bar = 2 mm.
The first goal of the present study was to examine potential markers (acetylcholinesterase [AChE), calbindin [CB], calretinin [CR], parvalbumin [PV], and SMI32) that might help to reveal the extent of Gudden's nuclei in the macaque monkey brain. AChE was selected, as both the dorsal and ventral tegmental nuclei show a strong affinity in the rat brain (Vann, 2009), although descriptions of the human tegmental nuclei (Huang et al., 1992) suggest that these nuclei need not contain especially high levels of AChE, i.e., there may be a species difference. CB, CR, and PV are all calcium binding proteins. PV also has a close affinity for γ-aminobutyric acid (GABA)ergic circuits. This property makes it of particular interest, as the projections from the ventral and dorsal tegmental nuclei in the rat to the mammillary nucleus are GABAergic (Allen and Hopkins, 1989; Wirtshafter and Stratford, 1993; Gonzalo-Ruiz et al., 1999). The antibody SMI32 recognizes a nonphosphorylated epitope of neurofilament protein. This antibody was selected as there is prior evidence that the suprafascicular portion of the ventral tegmental nuclei may stain positively for SMI32 (Paxinos et al., 2000).
The goal of delineating better Gudden's tegmental nuclei was closely linked with the second goal, namely, testing for direct projections from Gudden's tegmental nuclei to the mammillary bodies in an Old World monkey. For this second goal we examined five cynomolgus monkeys (Macaca fascicularis) in which the retrograde tracer horseradish peroxidase (HRP) had been injected into the mammillary body region. Not only should this information add to our understanding of mammillary body function but it could further assist in identifying better these two tegmental nuclear groups.
Materials and Methods
Animal care and housing
The findings were compiled from two different sources. The structural analyses regarding the distribution of AChE, CB, CR, PV, and SMI32 used tissue from adult rhesus monkeys prepared at the Laboratory of Neuropsychology, National Institute of Mental Health. The retrograde tracer experiments (HRP) were conducted on adult cynomolgus macaques at Oxford University. Both components of the study used archival tissue. All aspects of animal welfare, including housing, adhered strictly to the relevant Institute of Laboratory Animal Research Guide for the Care and Use of Laboratory Animals (structural analyses) and to the United Kingdom Home Office Licensing Laws (HRP injections). All animals were housed in pairs within a large room for holding macaque monkeys. They had free access to food and water.
Anatomical delineation of Gudden's tegmental nuclei
Serial coronal sections were stained for myelin following the process of Gallyas (1979) and for AChE, CB, PV, CR, and SMI32. For each marker it was possible to examine data from at least two different rhesus monkeys (Macaca mulatta). To limit animal usage, we did not prepare any additional cases with combined HRP injections and immunohistological analysis (as the separate information was already available). Four rhesus monkeys were perfused with 3 liters of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), and the brains were removed and cryoprotected through a series of glycerols in 0.1 M PBS (Rosene et al., 1986). The brains were blocked in the coronal plane and then quickly frozen in − 80°C isopentane. Sections were cut at 40 μm on a sliding microtome and then stored in PBS until processing.
AChE was visualized by using a modified version of a protocol by Tago et al. (1986). Initially, to quench endogenous peroxidase activity, sections were placed in 0.1% H2O2 for 30 minutes and rinsed in 0.1 M maleate buffer (pH 6.0). Sections were then incubated for 40 minutes in a solution of 15 mg acetylthiocholine iodide, 0.75 ml of 0.1 M sodium citrate, 1.5 ml of 30 mM cupric sulfate, and 1.5 ml of 5 mM potassium ferricyanide in 200 ml of maleate buffer (pH 6.0). Rinses in 30 mM Tris buffer (pH 7.6) preceded placement of the sections in a second incubation medium composed of 0.05 g of diaminobenzidine (DAB) and 3.75 g of nickel ammonium sulfate in 125 ml of 30 mM Tris buffer (solution pH 6.4). After 10 minutes of incubation, 12 drops of 0.1% H2O2 were added to this solution, and sections remained in solution for 12 minutes before a final rinsing in 3 mM Tris buffer. The tissue was mounted on gelatin-coated slides, air-dried, dehydrated in ethanol, and placed in xylenes before coverslipping.
Immunohistochemistry
Sections were placed in 0.6% H2O2 for 30 minutes to halt endogenous peroxidase activity and then rinsed in 0.1 M PBS (pH 7.4). Sections were subsequently agitated for 60 minutes in a blocking solution composed of 0.3% Triton X-100, 0.2% bovine serum albumin (BSA), and 3% normal horse serum in PBS before placement in the primary antibody incubation for 48 hours at 4°C (see Table 1 for dilution ratio). Several PBS rinses were carried out, and sections were then placed in secondary antibody solution (2% BSA, 3.75% normal serum, 0.3% Triton-X) for 60 minutes at room temperature for 60 minutes. After another series of PBS rinses, sections were incubated for 90 minutes in avidin-biotin-peroxidase complex (Vectastain Elite ABC kit, PK-6100, Vector, Burlingame, CA) before visualization by reaction using the DAB method for PV and the DAB-nickel method for CB, CR, and SMI32. The final reaction was stopped with rinses in 0.1 M PBS. Sections were then mounted on gelatin-coated slides and coverslipped. Control sections, stained by omitting the primary antibody, showed no specific staining. The resulting staining patterns for SMI32, PV, CB, and CR match previous descriptions (Saleem and Logothetis, 2007).
Table 1. Antibodies Used in This Study.
| Antibody | Supplier | Type | Host | Dilution | Immunogen |
|---|---|---|---|---|---|
| Anti-parvalbumin | Swant, #235, lot 10-11(F) | Monoclonal IgG1 | Mouse | 1:5,000 | Parvalbumin purified from carp muscle |
| Anti-SMI32 | Sternberger/Covance, #SMI32R | Monoclonal IgG1 | Mouse | 1:4,000 | Rat hypothalamus homogenate |
| Anti-calbindin D28k | Swant, #300, lot 07(F) | Monoclonal IgG1 | Mouse | 1:4,000 | Calbindin D28k purified from chicken gut |
| Anti-calretinin | Swant, #6B3, lot 010399 | Monoclonal | Mouse | 1:4,000 | Recombinant human calretinin-22k |
Antibody characterization
A list of the primary antibodies and their characteristics are presented in Table 1. Specificity of the antibodies was determined by the manufacturer (Swant, Bellinzona, Switzerland; Covance/Sternberger, Princeton, NJ). The antibodies were as follows:
PV: A mouse anti-PV monoclonal antibody was used to identify PV-positive fibers and cells. The antibody specifically stains the 45calcium binding spot of PV (MW 12,000, isoelectric focusing [IEF] 4.9) in a two-dimensional immunoblot from rat cerebellum. In a radioimunnoassay setup, the antibody measures PV with a sensitivity of 10 ng/assay with an affinity of 7.9 × 1012 L/M (Swant). The resulting staining patterns for PV match previous descriptions in the brainstem and cortex in a rhesus monkey (Saleem and Logothetis, 2007).
SMI 32: SMI 32 is a mouse monoclonal antibody to nonphosphorylated neurofilaments. On conventional immunoblots, SMI32 antibody recognizes two bands (200 and 180 kDa) that merge into a single nonphosphorylated neurofilament line on two-dimensional blots (Covance/Sternberger; Sternberger and Sternberger, 1983). This antibody has been shown to react with nonphosphorylated high-molecular-weight neurofilaments (200 kDa) of most mammalian species, including rats, cats, dogs, monkeys, and humans, and may also show some limited cross-reactivity with nonphosphorylated medium-molecular-weight neurofilaments (Covance/Sternberger). The resulting staining patterns for SMI32 match previous descriptions in the brainstem and cortex in a rhesus monkey (Saleem and Logothetis, 2007).
CB: A mouse anti-CB D-28k monoclonal antibody was used to identify CB-positive fibers and cells. This antibody reacts specifically with calbindin D-28K on immunoblots of extracts of brain tissue originating from monkey, human, guinea pig, rabbit, and rat. It does not cross-react with CR or other known calcium binding proteins. This antibody specifically stains the 45calcium binding spot of calbindin D-28k (MW 28,000, IEP 4.8) in a two-dimensional gel. In a radioimmunoassay it detects calbindin D-28k with a sensitivity of 10 ng/assay and an affinity of 1.6 × 1012 L/M (Swant). The resulting staining patterns for CB match previous descriptions in the hippocampus and prefrontal cortex of a rhesus monkey (Conde et al., 1994; Lavenex et al., 2009).
CR: A mouse anti-CR 22k monoclonal antibody was used to identify CR-positive fibers and cells. CA-22k is an alternative splice product of the CR gene and is identical with CR up to Arg178. After fusion, hybridoma cell were screened with human recombinant CR as the target, and the clone 6B3 was selected. This antibody reacts specifically with the CR in tissue originating in human and rat. The antibody specifically stains a single band of 29 kDa on immunoblots from human and rat brain extracts (Swant). The resulting staining patterns for CR match previous descriptions in the hippocampus and prefrontal cortex of a rhesus monkey (Conde et al., 1994; Lavenex et al., 2009).
Horseradish peroxidase injections
To test for direct inputs from Gudden's tegmental nuclei to the mammillary bodies, we examined the brains of five cynomolgus (Macaca fascicularis) monkeys that had previously received HRP injections in the mammillary bodies (Saunders, 1983; Aggleton et al., 2005). Comparisons with photomicrographs taken at the time of the original HRP reaction (Hanker-Yates technique) showed that the reaction product had not faded. Two of the five cynomolgus monkeys (MBfx4, MBfx5) had the fornix surgically transected up to 1 year prior to the HRP injection. Although fornix transection results in a decreased volume of the medial mammillary nucleus, this shrinkage is primarily due to the loss of neuropil with little or no reduction in cell numbers (Loftus et al., 2000). Consequently, these two cases should remain informative.
The nomenclature for the mammillary bodies follows that provided by Rose (1939) and, more recently, by Vann et al. (2007). The structure comprises two principal nuclear groups, the medial and lateral nuclei. The much larger, medial mammillary nucleus can readily be distinguished as it is surrounded by a dense fiber capsule. Lateral and ventral to the medial nucleus is the lateral mammillary nucleus. The lateral nucleus is distinguished by its relatively large cells, which stain intensely for Nissl substance.
Surgery: HRP injections in intact animals
The subjects were three male cynomolgus monkeys ranging in weight from 4.0 to 5.6 kg at the time of surgery (cases MB1–3). In two of the three cases, a 0.15-μl injection of 35% HRP (Boehringer, Mannheim, Germany) in 2% dimethyl-sulphoxide solution was directed at the left mammillary body; in the third case (MB1), the injection was aimed at the right mammillary body. Following the induction of anesthesia with intravenous Pentothal (Abbott, North Chicago, IL), the monkey was placed in a stereotaxic apparatus. A bone flap was made to the left of the dorsal midline, and a 1-μl Hamilton syringe (Bonaduz, Switzerland) was lowered into the mammillary region. The location of the mammillary bodies was determined from skull landmarks e.g., the posterior clinoid process, as revealed by X-ray (Aggleton and Passingham, 1981; Aggleton, 1985). Monkeys survived 48 hours and were then intravenously injected with pentothal and perfused intracardially with physiological saline followed by 1 liter of a solution of 2.5% paraformaldehyde and 1.5% glutaraldehyde in phosphate buffer (pH 7.2). The brains were blocked in the coronal plane and then cryoprotected in cold 30% sucrose in (0.1 M) phosphate buffer solution for 3 days. Frozen sections were cut at 50 μm, and a one-in-five series was collected in phosphate buffer.
Surgery: HRP injections in animals with fornix transections
Two male cynomolgus monkeys (MBfx 4 and 5), one weighing 4.0 kg and the other 5.6 kg at the time of surgery, received a complete transection of the fornix up to 1 year before the HRP injections. Both animals were first sedated with an intramuscular injection of Ketalar (JHP, Parsippany, NJ; 10 mg/kg) and anesthetized intravenously with Pentothal. A midline craniotomy and a unilateral dura flap exposed the midline. One hemisphere was gently retracted to access the corpus callosum. A small slit was made in the corpus callosum through which the anterior fornix was transected with a small metal sucker and cautery. The fornix section was made just caudal to the descending column. The details concerning the HRP injections are the same as those for the intact animals. The craniotomy for the HRP injections was made over the opposite (right) hemisphere to that previously used for the approach to the fornix. On completion of the surgery the dura and bone flap were replaced, and the skin was sutured. Bicillin was administered as a prophylactic measure.
HRP processing
Tissue was processed according to a modified Hanker-Yates technique (Hanker et al., 1977; Perry and Linden, 1982). For this reaction the brain sections were incubated in 700 ml of a 0.1 M sodium cacodylate buffer solution (pH 5.1) containing 2.4 g cobalt chloride, 1.6 g ammonium nickel sulfate, 700 mg catechol, and 350 mg p-phenylene-diamine for 15 minutes and then washed in phosphate buffer for 3–5 minutes. The sections were then transferred into a fresh solution of 700 mg catechol, 350 mg p-phenylenediaminem and 1 drop of hydrogen peroxide (H2O2), and incubated for 15 minutes. Sections were mounted on glass slides, counterstained with cresyl violet, and coverslipped.
Data collection
Sections were examined by using a Zeiss Imager Z.1 microscope, and images were captured by using a Zeiss Axiocam HRC camera. The brightness, contrast, and evenness of illumination was modified to match, as far as possible, sections from the different monkeys at the time of the image acquisition by using the Axiovision software (version 4.8.2). Images were then imported to Adobe (San Jose, CA) Photoshop for sizing for inclusion into the figures.
Results
Nomenclature, cytoarchitecture, and chemoarchitecture
Locating the dorsal and ventral tegmental nuclei of Gudden in the macaque brain is problematic, as descriptions of these nuclei in the primate brain differ markedly in the extent, terminology, and even the presence of these nuclei. In addition, there may be substantial inter-species differences (Petrovicky, 1971; Hayakawa and Zyo, 1983; Huang et al., 1992). For these reasons the Results section begins with a brief historical overview for each of the two major subdivisions within Gudden's nuclei that explains both current knowledge and the terminology to be adopted.
The primate dorsal tegmental nucleus of Gudden: historical perspective
The dorsal tegmental nucleus of Gudden (DTg) in the monkey (Figs. 1A, 2C) and human brain is the easier of the two tegmental nuclei to identify. This nucleus lies within the central gray matter just above the dorsomedial edge of the medial longitudinal fasciculus (mlf) and medial to the lateral dorsal tegmental nucleus (Petrovicky, 1971; Hayakawa and Zyo, 1983; Huang et al., 1992). The DTg of the rhesus monkey was originally subdivided into two major portions, pars ventralis and pars dorsalis (Petrovicky, 1971), with a third part (pars caudalis) being a separate, caudal continuation of pars dorsalis. Subsequent descriptions of the human tegmentum (Huang et al., 1992) have also divided the DTg into two main parts: 1) a dense central part (pars centralis or DTgC), equivalent to the pars ventralis of Petrovicky (1971), and 2) a pericentral part (DTgP) equivalent to the pars dorsalis, as well as a third caudal part, the posterodorsal tegmental nucleus (PDTg), considered a homologue of the pars caudalis (Petrovicky, 1971). In their atlas of the rhesus monkey brain, Paxinos et al. (2000) were able to delineate the dorsal tegmental nucleus using the same nomenclature as that coined by Huang et al. (1992) for the human brain.
Figure 2.
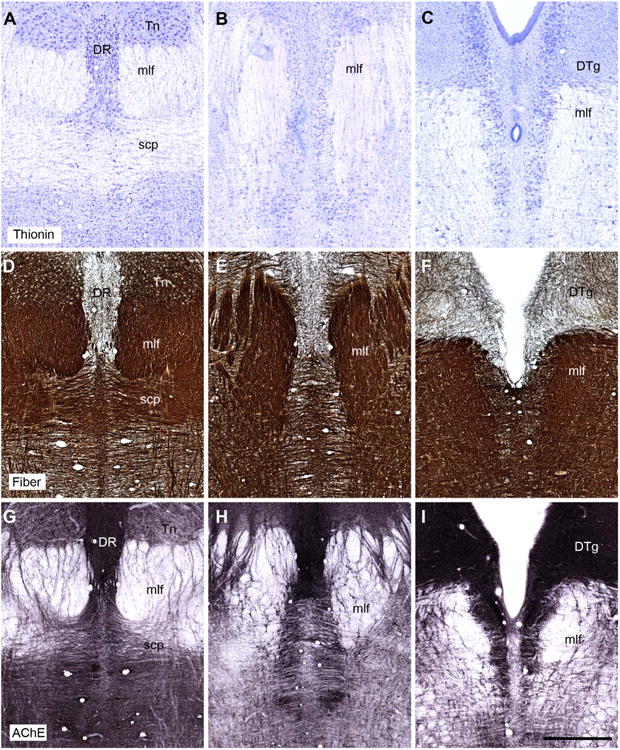
A–I: Coronal sections showing the appearance of the tegmental and raphe regions in the rhesus monkey (Macaca mulatta). The top row shows sections stained for Nissl substance (thionin stain). The middle row shows fiber-stained sections (Gallyas). The bottom row shows sections stained for acetylcholinesterase. Note how the dorsal tegmental nucleus of Gudden (DTg) can be seen as a discrete entity, whereas the ventral tegmental nucleus of Gudden fails to emerge as a distinct nuclear aggregation using these three stains. The sections showing the stains are taken from three different animals, but are at comparable anterior-posterior (AP) levels. For abbreviations, see list. Scale bar = 1 mm in I (applies to A–I).
The present study adheres to the terminology of Paxinos et al. (2000). The most superior part of the dorsal tegmental nucleus is the globular pericentral part (DTgP), the pars dorsalis of Petrovicky (1971). The DTgP consists of only lightly Nissl-stained cells that are fairly uniform in size and smaller than the surrounding cells in the central gray. At the rostral, ventral edge of this subnucleus is a group of somewhat larger cells (the pars centralis or DTgC) that stain for Nissl more darkly and lie close to the dorsal margin of the medial longitudinal fasciculus (Petrovicky, 1971). At some levels the combined pair of sub-nuclei is encapsulated by white matter (Petrovicky, 1971). It should be noted that these two subnuclei (DTgP and DTgC) appear very similar and are difficult to distinguish in Nissl-stained sections (Petrovicky, 1971; Huang et al., 1992). Indeed, in the green monkey (Cercopithecus aetiops), they have been described as inseparable (Hayakawa and Zyo, 1983). The caudal subdivision, the PDTg, is found at the level of the locus coeruleus, where it is sited just medial to the laterodorsal tegmental nucleus (Paxinos et al., 2000). The PDTg is composed of small, lightly Nissl-stained cells that contrast with the much larger, dense staining cells in locus coeruleus.
Dorsal tegmental nucleus: chemoarchitecture
The findings for PV are described first, as it was the only marker to show a clear, positive affinity for both the dorsal and ventral tegmental nuclei. Cells and fibers positive for PV appeared at the rostral limit of the dorsal tegmental nucleus (Fig. 3C) and continued caudally along the length of this nucleus to reach the PDTg, where this staining then became appreciably lighter. The dorsal tegmental nucleus stood out from both the lateral dorsal tegmental nucleus and the raphe nucleus, as neither area contained cells that typically stained for PV. The PV-positive cells in the dorsal tegmental nucleus appeared continuous with those in the suprafascicular part of the ventral tegmental nucleus.
Figure 3.
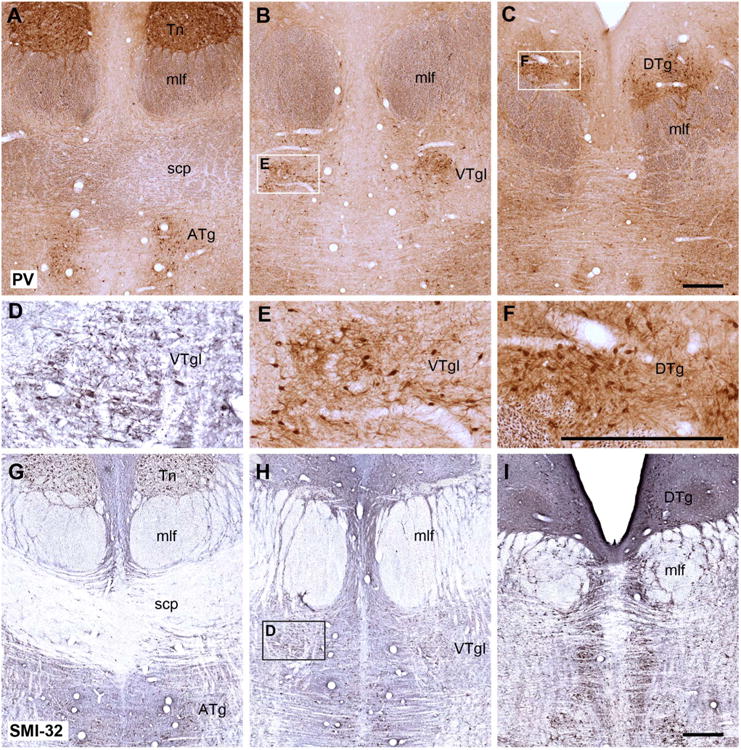
Coronal sections from a rhesus monkey brain showing the appearance of the dorsal and ventral tegmentum at three different anterior-posterior levels stained immunohistochemically for parvalbumin (A–C,E,F) and for SMI32 (D,G–I). The parvalbumin staining is of particular interest as it appears to show an affinity for Gudden's tegmental nuclei but not the adjacent raphe nucleus. Insets outlined in B, C, and H are shown at a higher magnification, such that E and F show parvalbumin-positive cells taken from B and C, respectively, whereas D shows SMI32-positive cells in the ventral tegmental nucleus taken from H. For abbreviations, see list. Scale bar = 0.5 mm in C (applies to A–C) F (applies to D–F), and I (applies to G–I).
None of the other staining procedures revealed a selective, positive affinity for the dorsal tegmental nucleus. The fiber stain (Gallyas) confirmed that at some levels the dorsal tegmental nucleus can appear encapsulated (Fig. 2F), with the nucleus being more lightly stained than most adjacent parts of the central gray, e.g., the lateral dorsal tegmental nucleus. This difference in fiber staining was, however, never sufficient to define precisely the extent of this nucleus. A similar situation was found for AChE (Fig. 2I): many stained fibers ran across the dorsal tegmental region, but the lateral dorsal tegmental nucleus contained more staining for both fibers and cells. Likewise, the marker SMI32 revealed the dorsal tegmental nucleus (Fig. 3) but only because adjacent areas showed higher immunopositivity. At some caudal levels a ring of SMI32-immunopositive cells and fibers formed a border around the edges of the dorsal tegmental nucleus as the immediately adjacent dorsal raphe and, to a lesser extent, the lateral dorsal tegmental nucleus contained stained cells.
The dorsal tegmental nucleus contained appreciable numbers of CR-positive cells that were concentrated in the medial and ventral portions of the nucleus (Fig. 4A,C). The most ventral cells appeared continuous with CR-positive cells in the region occupied by the suprafascicular part of the ventral tegmental nucleus. Even though the lateral dorsal tegmental nucleus appeared to contain numbers of CR-immunopositive cells similar to those in the dorsal tegmentum, the dorsal tegmental nucleus became more visible after staining for CR because this nucleus still contained fewer positive-stained fibers than the adjoining areas, e.g., the dorsal raphe nucleus (Fig. 4A). Finally, the dorsal tegmental nucleus failed to stain for CB (Fig. 4B,D), so that in some sections the region again stood out by virtue of its lack of immunoreactivity.
Figure 4.
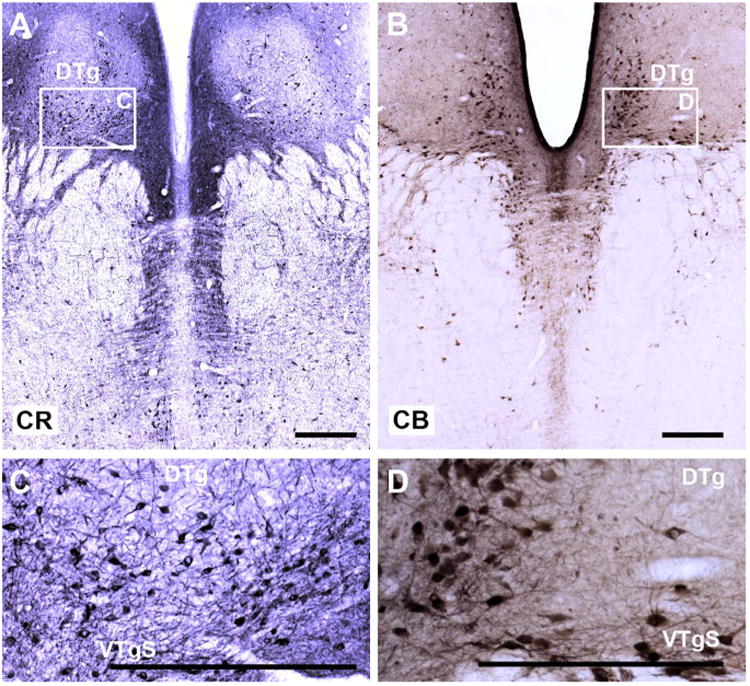
A–D: Coronal sections from a rhesus monkey brain showing the distribution of calretinin-positive cells (A,C) and calbindin-positive cells (B,D) in the tegmentum. As inset C shows, cells in both the dorsal tegmental and ventral tegmental nucleus are positive for calretinin, but this staining is not selective, as numerous raphe cells are also positive. Whereas cells in the ventral tegmentum also show a strong affinity for calbindin (B,D) this was not the case for the dorsal tegmentum. Again, adjacent cells in the dorsal raphe were immunopositive for calbindin. For abbreviations, see list. Scale bar = 0.5 mm in A–D.
The primate ventral tegmental nucleus of Gudden: historical perspective
The ventral tegmental nucleus of Gudden (VTg) is typically described as consisting of much larger cells than the dorsal tegmental nucleus and is placed pairwise beside the raphe nucleus. In many species, including some primates, the VTg is split immediately above (pars suprafascicularis) and immediately below (pars infrafascicularis) the medial longitudinal fasciculus (Petrovicky, 1971; Hayakawa and Zyo, 1983), with some intercalated cells squeezed among the fibers of the fasciculus. Despite being divided into these two parts, the cell types appear the same above and below the medial longitudinal fasciculus and are thought to reflect essentially one nucleus (Petrovicky, 1971). It has also been stated that in the rhesus monkey the pars suprafascicularis is only weakly developed and no pars infrafascicularis is present (Petrovicky, 1971), although others have reported a more extensive ventral tegmental nucleus in the rhesus monkey (Crosby and Woodburne, 1943). Subsequent descriptions of the human ventral tegmental nucleus of Gudden (Huang et al., 1992) have detailed three subdivisions within the VTg, two of which match the terminology of Petrovicky (1971). These are a small suprafascicular subdivision (VTgS) that is present above the medial longitudinal fasciculus, and an infrafascicular subdivision (VTgI) just below the same tract. In addition, the “principal part” of the ventral tegmental nucleus of Gudden (VTgP) is intercalated among the fibers of the medial longitudinal fasciculus. These same three subdivisions (Huang et al., 1992), which have been adopted in one rhesus monkey brain atlas (Paxinos et al., 2000), form the basis of the current terminology.
In nonprimates, a further tegmental nucleus has been described that has been regarded as part of the ventral tegmental group. The nucleus centralis superioris pars compacta lies rostral and ventral to the pars infrascicularis of the VTg (Petrovicky, 1971; Hayakawa and Zyo, 1983). This same nucleus has been shown to project directly to the pars medianus of the medial mammillary nucleus in species such as rats (Hayakawa and Zyo, 1984; Allen and Hopkins, 1989; Hopkins, 2005), although in some of these studies this rostral tegmental region is called the anterior ventral tegmental nucleus (Allen and Hopkins, 1989; Hopkins, 2005). In rats this nucleus is placed under the caudal part of the decussation of the superior cerebellar peduncle (Hopkins, 2005). A corresponding, anterior tegmental nucleus has been indicated in the rhesus monkey (Paxinos et al., 2000), where it is also primarily located underneath the decussation of the superior cerebellar peduncle but extends caudally to reach the VTgI, so that these cell groups join just behind the decussation.
Ventral tegmental nucleus: chemoarchitecture
A number of staining procedures appeared to react with cells in the region occupied by the ventral tegmental nucleus, but they also reacted with cells in the raphe nucleus, making it very difficult to detect any borders. The only exception was PV, which stained positively for the ventral tegmental nucleus but not for the median raphe nucleus (Fig. 3A–C).
Cells along the length of the ventral tegmental nucleus appeared immunopositive for PV. This affinity started in the anterior tegmental nucleus, causing cells in this region under the decussation of the superior cerebellar peduncle to stand out from the background (Fig. 3A). This stained group of cells could be followed caudally behind the decussation, where they continued in the infrafascicular part of the ventral tegmental nucleus, i.e., below the medial longitudinal fasciculus (Fig. 3B). These immunopositive cells were also found within and immediately above the medial longitudinal fasciculus, corresponding with the principal and suprafascicular parts of the ventral tegmental nucleus, respectively (Fig. 3C).
Neither Nissl staining (Figs. 1A, 2B) nor a white matter stain (Gallyas) helped to reveal the ventral tegmental nucleus, as so many fibers criss-cross this region (Fig. 2D–F). Staining for AChE also failed to highlight the anterior tegmental nucleus or the infrascicular portion of the ventral tegmental nucleus (Fig. 2G–I). Although some intercalated cells both within and immediately above the medial longitudinal fasciculus stained for AChE, so did cells in the raphe nuclei, making it impossible to detect a clear border. The same situation was found for CB, CR, and SMI32, as immunopositive cells were found in the supra-fascicular region and the principal parts of the ventral tegmental nucleus, i.e., immediately above and intercalated within the medial longitudinal fasciculus (SMI32; Figs. 3E, 4). In all of these cases, however, laterally placed cells in the median raphe nucleus also stained positively for CB, CR, and SMI32 and so the ventral tegmental nucleus could not be distinguished by these markers alone (Figs. 3, 4). In the case of CR, the stained cells in the suprafascicular part appeared to extend dorsally into the region of the dorsal tegmental nucleus (Fig. 4A,C).
Tegmental projections to the mammillary body region
Location of the HRP injection sites within the mammillary bodies
Every animal received a single injection centered in the medial mammillary nucleus (mMB) that was close to the rostrocaudal center of this nucleus (Fig. 5). The placement of the HRP injections did, however, vary in the medial to lateral plane. In cases MB3 and MBfx4, the injection tract was in the medial half of the mMB, whereas in MB1 it was placed in the center of the mMB. In both MB2 and MBfx5 the injection tract was in the lateral half of mMB.
Figure 5.
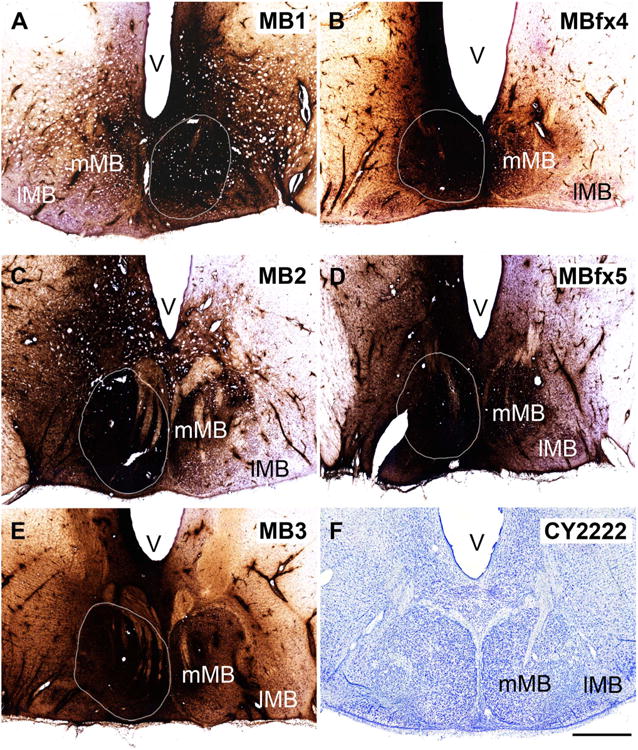
A–F: Photomicrographs of the single injection sites within the mammillary bodies from the five cynomolgus macaques described in this study. The extent of the medial mammillary nucleus (mMB) is shown by a white outline. In all cases the injection was centered in the mMB, with variable spread into the lateral mammillary nucleus (lMB). The dark reaction product extends beyond the boundaries of the mammillary bodies, although the total extent of this product is appreciably greater than the active area of HRP uptake. F shows a coronal section through the lateral and medial mammillary bodies of a cynomolgus monkey (Nissl stain). V, ventricle. Scale bar = 1 mm in F (applies to a–F).
In all five cases the HRP injection appeared extensive (Fig. 5), and so defining the active extent of each injection is important. The active injection site was assumed to correspond to that region with a continuous, uniform dense spread of reaction product. This area was appreciably smaller than the total region that appears dark in Figure 5, i.e., it did not include many adjacent hypothalamic sites where only cell bodies appeared to be associated with the reaction product. Evidence that the area of active uptake was appreciably smaller than the extent of all reaction product came from the three cases in which the fornix was intact. Here, it was possible to compare the amounts of retrograde label in the subiculum across the two hemispheres. In two cases (MB2, MB3), numerous subicular labeled cells were found ipsilateral to the injection site, but none were found contralateral, i.e., although considerable spread of HRP reaction product could be seen across the midline (especially in MB3; Fig. 5) this was not sufficient for retrograde transport to the subiculum in that hemisphere. In the remaining case (MB1) only a tiny proportion of labeled cells was seen in the contralateral subiculum.
Applying the above criteria, it is most likely that in MB2 and MB3 the ipsilateral mMB was largely filled, with little or no active uptake in the contralateral side. Furthermore, in case MB3 the injection did not appear to reach the lateral mammillary nucleus (lMB) in either hemisphere. In case MB1, there was evidence of spread across to the contralateral mMB, but the involvement of the lMB appeared to be solely ipsilateral. Inspection of the two cases with fornix transections MBfx4 and MBfx5 also suggested limited spread into the contralateral mMB. In case MBfx5 there was clear involvement of the ipsilateral lMB, but in MBfx4 the lMB appeared to contain little of the HRP injection. These observed patterns of HRP product were consistent with the conclusion from the tract placements that the injection sites went from medial to lateral in the order MB3, MBfx4, MB1, MB2, and MBfx5. Consequently the injections in MB2 and MBfx5 were those most likely to have involved the lMB as well as the mMB. Finally, due to the vertical approach, there was some additional HRP in the posterior hypothalamic area (dorsal to the mammillary bodies) in all cases.
Retrograde label in the dorsal tegmental nucleus of Gudden
Across the five cases, there was more variation in the amount of retrograde label in the DTg than in the VTg. The animal with the most medial injection site (MB3) stood out, as not a single labeled cell could be located within the DTg. In a second case in which the injection was centered in the middle of the mMB (MB1), very few HRP-labeled cells were present, and these cells almost all comprised a highly restricted group at the junction of the DTgC with the DTgP in the ipsilateral hemisphere. Likewise, MBfx4 had only a few labeled DTg cells and these were mainly found close to the borders with the raphe and the lateral dorsal tegmental nucleus. In contrast, the two cases with most lateral injections (MBfx5, MB2) contained a clear clump of HRP-labeled cells at the rostral, ventral margin of the DTg just above the medial longitudinal fasciculus (Figs. 6F, 7F). This label filled much, if not all, of the DTgC at this level. This HRP label continued in a much more scattered form both dorsally and caudally, so that the DTgP was also labeled, although not at the same concentration as the DTgC. It should, however, be added that distinguishing a clear border between the DTgC and DTgP was often not possible. In three cases (MB2, MBfx4, MBfx5) some corresponding label was also present in the DTgC and DTgP in the contralateral hemisphere, although always at a very low density. The amount of ipsilateral (and contralateral) label then decreased the more caudal the section.
Figure 6.
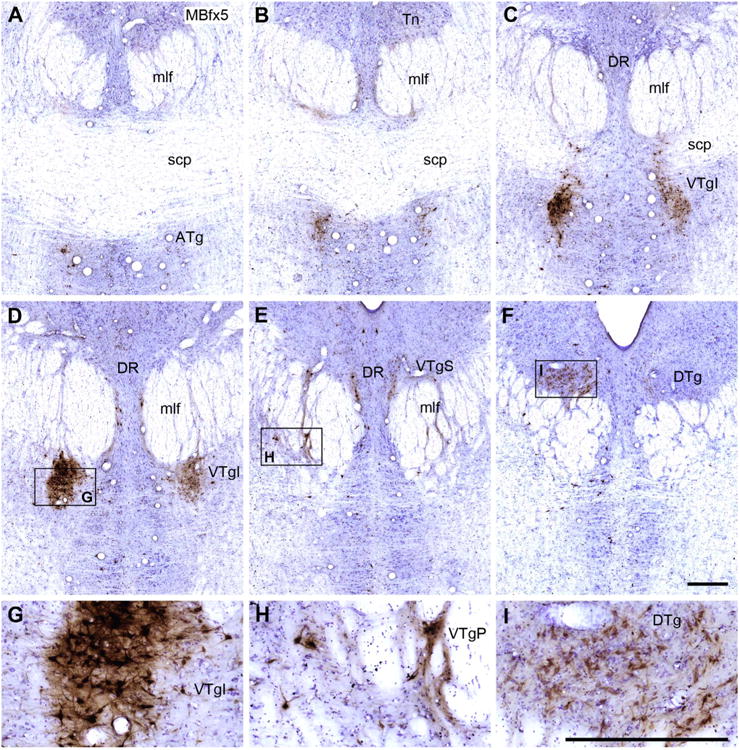
Six coronal Nissl-stained sections from case MBfx5 taken at different anterior (A) to posterior (F) levels through the tegmental area. The injection is presumed to have involved much of the medial and lateral mammillary nuclei. The HRP-positive cells are stained brown and form dense aggregates, especially in the ventral tegmental nucleus of Gudden. A–F: Four tegmental areas containing HRP-positive cells: ATg, VTgI, VTgP, and DTg. G,H: HRP-positive cells in the ventral tegmental nucleus. Note that the label “VTgI” in Figure 6C, D, and G is placed adjacent to the actual site of the nucleus. I: HRP-positive cells primarily located in the dorsal tegmental nucleus of Gudden, pars centralis. For abbreviations, see list. Scale bar = 0.5 mm in F (applies to A–F) and I (applies to G–I).
Figure 7.
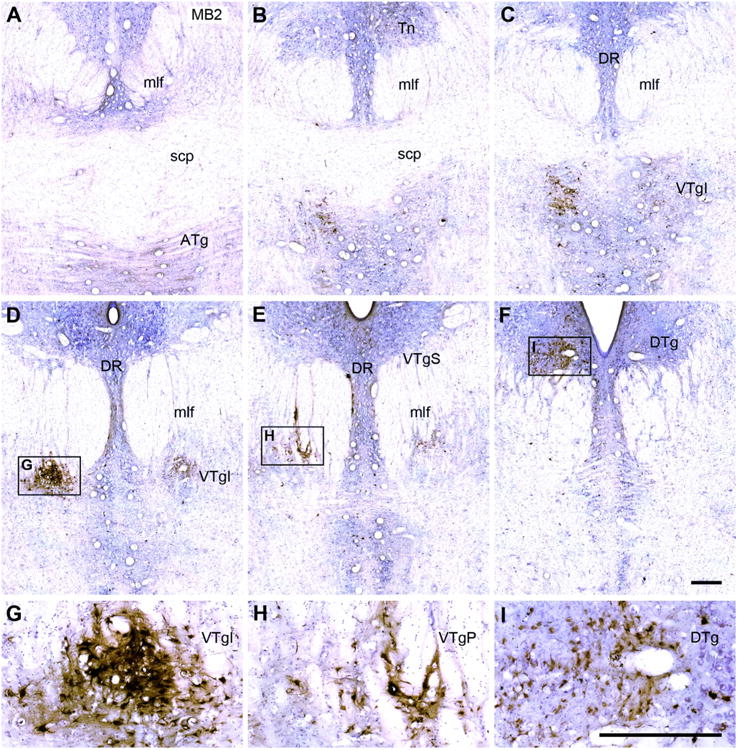
A–F: Six coronal Nissl-stained sections from case MB2 taken at different anterior (A) to posterior (F) levels through the tegmental area. The injection is presumed to have involved much of the medial and lateral mammillary nuclei. The HRP-positive cells are stained brown and form dense aggregates, especially in the ventral tegmental nucleus of Gudden. Note that the label “VTgI” in Figure 7C, D, and G is placed adjacent to the actual site of the nucleus. G,H,I: The HRP label has been magnified in these insets. Inset I shows HRP-positive cells primarily located in the dorsal tegmental nucleus of Gudden, pars centralis. For abbreviations, see list. Scale bar = 0.5 mm in I (applies to A–I).
Caudal to the DTgP, scattered HRP-labeled cells were present in the periventricular region, but we could not identify a particular patch of label that corresponded to the location of the PDTg. Instead, there were occasional HRP-positive cells across a region that included the lateral dorsal tegmental nucleus, the dorsal raphe, and the PDTg. In some cases there was an increase of label in locus coeruleus so that it formed a final, caudal grouping of HRP-positive cells. This label in the locus coeruleus was most evident in cases MB2, MB1, MBfx4, and in two of these cases (MB1 and MBfx4) there was appreciable label on the side contralateral to the injection site, in addition to that found ipsilateral.
Retrograde label in the ventral tegmental nucleus of Gudden
The location of label in VTg was very similar in all five cases; the principal difference concerned the numbers of retrogradely filled cells. A series of photomicrographs from two cases (MBfx5 and MB2) helps to show the distribution of these retrogradely labeled cells (Figs. 6, 7). A continuous band of labeled cells, which started from the anterior tegmental nucleus (ATg; below the superior cerebellar peduncle), extended caudally and then dorsally through the medial part of the medial longitudinal fasciculus, so that the label was found in the VTgI, VTgP, and, finally, VTgS (the most caudal of the three nuclei). This label was always predominantly ipsilateral to the injection site, and for all three subdivisions (VTgI, VTgP, and VTgS) the labeled cells were interrupted by numerous white matter tracts. The label in the VTgI, VTgP, and VTgS mainly appeared to be in large stellate cells, but because of all of the white matter tracts the various subdivisions were indistinct when a Nissl stain alone was used (Fig. 2). Whereas many of the labeled cells in VTgP were intercalated within the medial longitudinal fasciculus, others were found immediately adjacent to the medial border of this tract, i.e., just lateral to the pontine raphe nucleus. These more medial cells tended to be slightly smaller than those in the VTgI or VTgS, and sometimes appeared fusiform. One of the few differences across cases was that the animal with the most medial injection site (MB3) did not appear to contain any labeled cells in the suprafascicular subregion (VTgS).
Although the HRP label in the anterior tegmental nucleus appeared somewhat different from that in the other VTg subdivisions as the cellular group was more discrete, there was little else to suggest that this nucleus was not part of the VTg. More caudal, at the posterior limit of the superior cerebellar peduncle, the label in the anterior tegmental nucleus (ATg) became almost continuous with that in the VTgI (Figs. 6A–C, 7A–C) where the label was found a little more dorsal (immediately under the medial longitudinal fasciculus). Finally, in all cases only a few labeled cells were also present in the raphe nucleus, just medial to the VTg. These raphe cells were most numerous in cases MB2 and MBfx5
Discussion
The present study had two inter-related goals. The first was to look for a marker for Gudden's tegmental nuclei, especially the ventral tegmental nuclei, given the difficulties in identifying these cell groups from Nissl-stained sections in the primate brain (Petrovicky, 1971). The second was to determine whether Gudden's tegmental nuclei project to the primate mammillary bodies, as is known to be the case in the rodent.
There have been persistent problems in identifying Gudden's tegmental nuclei. This situation can be traced back to their first description (Gudden, 1884), in which Gudden claimed (as reported by Petrovicky, 1971) that these nuclei were only very weakly developed in the monkey and could not be identified in the human brain. Later studies of the midbrain of the rhesus monkey located fibers that seemed to run between the mammillary bodies and the tegmentum, and so tentatively determined the location and appearance of the ventral and dorsal tegmental nuclei (Crosby and Woodburne, 1943, 1951). Even so, Petrovicky (1971) regarded the ventral tegmental nucleus of the rhesus monkey as only very weakly developed with only a suprafascicular part, i.e., lacking the two more ventral subdivisions. This concept of a rudimentary ventral tegmental nucleus accorded with a subsequent description of the green monkey (Cercopithecus aetiops) brain that failed to find any aspect of the ventral tegmental nucleus of Gudden (Hayakawa and Zyo, 1983). Problems in distinguishing the ventral tegmental nucleus of Gudden in Nissl-stained sections have meant that it is often not specifically identified in brain atlases of macaque monkeys (e.g., Snider and Lee, 1961; Shantha et al., 1968; Saleem and Logothetis, 2007).
Inspection of our Nissl-stained sections showed yet again that the ventral tegmental nucleus is especially difficult to delineate (Fig. 2). For this reason, we combined the present HRP findings with our immunohistochemical findings to help better define the extent of these nuclei. The validity of this approach depends on whether all parts of Gudden's tegmental nuclei project to the mammillary bodies in the macaque monkey brain. Support comes from: 1) descriptions of efferents from the macaque mammillary bodies that terminate in the same tegmental areas (Veazey et al., 1982), and 2) the long-established presence of dense projections from throughout both the dorsal and ventral tegmental nuclei of Gudden to the mammillary bodies in nonprimate brains (Hyakawa and Zyo, 1984; Allen and Hopkins, 1989; Hopkins, 2005).
In collaboration with the HRP results, a number of potential markers were examined to determine whether they might help to reveal Gudden's tegmental nuclei in the macaque brain. In the rat, both the dorsal and ventral tegmental nuclei contain appreciable levels of acetylcholinesterase (Gonzalo-Ruiz et al., 1999; Vann, 2009). In the macaque monkey, these same nuclei showed variable staining for acetylcholinesterase, which did not result in the nuclei becoming especially visible. A similar situation is found in the human brain, with the exception of the PDTg, which is revealed by its high acetylcholinsterase levels (Huang et al., 1992). Marked species differences regarding the levels of acetylcholinesterase in these tegmental nuclei have, however, already been noted (Leichnetz et al., 1989). Other markers (calbindin and calretinin) showed positive reactivity with cells in regions that corresponded to parts of the ventral tegmental nucleus (infrafascicular and suprafascicular), but the adjacent raphe nucleus also showed positive staining. As a consequence, it was not possible to highlight just the target nuclei. For these same markers, the dorsal tegmental nucleus often showed poor immunoreactivity, which sometimes enabled the region to become more visible. Likewise, SMI32 appeared to label cells and fibers within the ventral tegmental nucleus (pars suprafascicularis and principalis) but did not provide a selective marker for these nuclei (see also Paxinos et al., 2000).
Parvalbumin was an exception to this pattern. Parvalbumin appeared to have a strong, selective affinity for cells in both the ventral and dorsal tegmental nuclei. The ventral staining included the anterior tegmental nucleus, so creating an almost continuous body of immunolabeled cells from below the superior cerebellar peduncle, at its rostral limit, to the posterior dorsal tegmental nucleus, at its caudal limit. The relative lack of label in adjacent sites, such as the raphe nucleus, ensured the prominence of these tegmental nuclei. Parvalbumin staining is often associated with GABAergic sites (Celio, 1986). It is, therefore, relevant that the projections from the tegmental nuclei in the rat to the mammillary nucleus are GABAergic and are thought to create an inhibitory loop that regulates hippocampal interactions with the mammillary bodies (Allen and Hopkins, 1989; Wirtshafter and Stratford, 1993; Gonzalo-Ruiz et al., 1999). It must, however, be noted that the parvalbumin data and the HRP findings came from separate cohorts of monkeys. The HRP injections were made some decades ago, and so it was not possible to combine subsequently the tracer experiments with the immunohistochemical analyses in order to be definitive as to whether the parvalbumin-positive cells themselves project to the mammillary bodies.
Despite this limitation, it was possible to use the HRP findings as an initial guide, along with the parvalbumin results, to identify those cells that form all three divisions of the ventral tegmental nucleus (VTgI, VTgP, and VTgS) of the macaque monkey (Figs. 3, 6, 7). It immediately became clear that the more ventral nuclei (especially the VTgI) are broken up by numerous white matter tracts passing in several different planes, thus explaining the difficulty that previous studies have found in identifying discrete sites such as the infrafascicular portion (VTgI). The finding of additional HRP-positive cells in a separate nucleus just anterior and ventral to the VTgI, the anterior tegmental nucleus (Paxinos et al., 2000), suggests an affinity between this nucleus and the three ventral tegmental nuclei. Indeed, studies of the rat brain have sometimes treated this rostral area as part of the ventral tegmental nucleus, and have also reported that it has a specific input to the pars medianus of the medial mammillary nucleus (Hayakawa and Zyo, 1984; Allen and Hopkins, 1989; Hopkins, 2005). This anterior tegmental nucleus corresponds to the nucleus centralis superioris pars compacta (Petrovicky, 1971; Hayakawa and Zyo, 1983).
Our tracer studies showed that every monkey with an HRP injection centered in the mammillary bodies contained groups of retrogradely labeled cells in the ventral tegmental nucleus of Gudden. Retrograde label was also found in the dorsal tegmental nucleus, but this label was most evident in those cases with the most laterally placed HRP injection in the mammillary bodies. The only part of Gudden's tegmental nuclei not to contain HRP-positive cells was the caudal division of the dorsal nucleus (PDTg). The location of much of the tegmental retrograde label closely matches those sites thought to receive mammillary body projections in the cynomolgus monkey (Veazey et al., 1982), strongly indicating that these connections are reciprocal, as is known to be the case in the rat (Allen and Hopkins, 1989; Hopkins, 2005).
In all five monkeys with HRP injections, the tegmental projections were most numerous on the side ipsilateral to the injection site, although there were always some labeled cells in the corresponding regions in the contralateral hemisphere. This bilateral label raises the question of whether there was spread of HRP across the midline. Defining the effective extent of an HRP injection is difficult (Mesulam, 1982), and at first glance the injection sites seemed large, with reaction product extending into adjacent hypothalamic nuclei and across the midline. Indeed, the presence of occasional labeled cells in the ipsilateral medial nucleus of the amygdala (all cases except MB3) suggests some limited spread into adjacent, ipsilateral hypothalamic nuclei (Amaral et al., 1992) that could potentially add to some of the tegmental label (Leichnetz et al., 1989; Petrovicky, 1973; Hayakawa et al., 1993). Further inspection did, however, show that in the three cases with an intact fornix the transported label in the subiculum was either entirely ipsilateral (MB2, MB1) or was very infrequent in the contralateral hemisphere (MB3). The implications are that: 1) relatively little, if any, active HRP uptake took place across the midline in the contralateral medial mammillary bodies in cases MB2 and MB1 as otherwise labeled cells would have been found in the contralateral subiculum, given that the subiculum projects ipsilaterally to all fields of the medial mammillary nucleus (Aggleton et al., 2005); and 2) the area of active uptake is, therefore, considerably smaller than the total area of reaction product around the injection site (as seen in Fig. 5).
Consequently, the present findings suggest that there is a population of crossed tegmental–mammillary body projections. This evidence is most convincing for the projections from the dorsal tegmental nucleus, as contralateral label was found in those cases with the most lateral HRP injections (e.g., MBfx5, MB2), i.e., those injections furthest from the midline. Even so, confirmation would require more discrete injections. The potential presence of crossed-connections remains noteworthy as investigations into nonprimate brains have found that both the dorsal tegmental projections (to the lateral mammillary nucleus) and the ventral tegmental projections (to the medial mammillary nucleus) remain ipsilateral (Hyakawa and Zyo, 1984; Allen and Hopkins, 1989; Hopkins, 2005).
Consistent with other species (Hyakawa and Zyo, 1984; Allen and Hopkins, 1989; Hopkins, 2005), there was evidence that the projections from the dorsal tegmental nucleus terminate in the lateral mammillary nucleus of the macaque. Furthermore, studies in the rat indicate that this projection originates principally from the central (ventral) portion of the dorsal tegmental nucleus (Hopkins, 2005). The same relationship was found in the present study, i.e., the dorsal tegmental label was concentrated in pars centralis (DTgC). Evidence that the monkey DTg preferentially projects to the lateral mammillary nucleus comes from the lack of dorsal tegmental label in the case with the most medial mammillary body injection (MB3) and the greatest amount of label in the two cases (MB2, MBfx5) with the most lateral injections. Confirmation of this arrangement would, however, require more discrete mammillary body injections. Likewise, it could not be determined whether the dorsal tegmental projection solely terminates in the lateral mammillary nucleus, as is the case in rats (Hopkins, 2005). Nevertheless, some evidence comes from the reciprocal projections in the cynomolgus monkey, where the lateral mammillary nucleus is thought to project exclusively to the dorsal tegmental nucleus (Veazey et al., 1982).
The present study found evidence that a number of sites adjacent to Gudden's tegmental nuclei might project to the mammillary bodies. Scattered HRP-positive cells were found in the dorsal raphe, locus coeruleus, and lateral dorsal tegmental nucleus. Inputs to the mammillary bodies arising from these same nuclei have been reported in other species (Hyakawa and Zyo, 1984; Allen and Hopkins, 1989). Of these additional connections, those with the dorsal raphe and the locus coeruleus may prove to be reciprocal (Veazey et al., 1982).
This first demonstration in a primate brain of projections from Gudden's tegmental nuclei to the mammillary bodies raises the issue of their possible functional role. Both electrophysiological and anatomical studies of the rat brain indicate that the dorsal and ventral tegmental nuclei have very different properties, and so should be regarded as separate components in two parallel, functional pathways that connect with the mammillary bodies (Vann and Aggleton, 2004). The dorsal tegmental nucleus is thought to code for angular head velocity and is regarded as a vital element of the rodent head-direction system via its connections with the lateral mammillary nucleus (Blair et al., 1998; Bassett and Taube, 2001; Bassett et al., 2007). As a consequence the dorsal tegmental nucleus is assumed to be important for spatial navigation. The rat ventral tegmental nucleus sends inhibitory projections to the medial mammillary nucleus (Allen and Hopkins, 1989) and is thought to have a role in moderating, or even generating theta rhythm (Bassant and Poindessous-Jazat, 2001; Kocsis et al., 2001; Vertes et al., 2004). A recent study on the impact of selective lesions in the ventral tegmental nucleus of Gudden (Vann, 2009) observed striking learning deficits in rats on an array of spatial tasks that depend on hippocampal function. Such results strengthen the notion that the tegmental–mammillary body connections support aspects of memory (Vann, 2010). Further evidence comes from the report of a patient with persistent amnesia attributed to pathology in the area of the ventral tegmental nucleus (Goldberg et al., 1981), although this study lacks additional MRI confirmation.
In summary, the present findings help to establish the existence of the various tegmental nuclei of Gudden in the macaque monkey and show, in conjunction with studies of mammillary body efferents (Veazey et al., 1982), that these tegmental nuclei are reciprocally connected with the mammillary bodies. The discovery of these connections prompts further questions about the potential role of these tegmental connections in learning and memory (Vann, 2010).
Acknowledgments
The authors are grateful to M. Mishkin, M. Malloy, and L. Woods for their support and technical assistance.
Grant sponsors: The Royal Society, the Wolfson Trust, National Institute of Mental Health Intramural Research Program.
Abbreviations
- AChE
Acetylcholinesterase
- ATg
Anterior tegmental nucleus
- B
Bregma
- CB
Calbindin
- CR
Calretinin
- dPAG
Dorsal periaqueductal gray
- DR
Dorsal raphe nucleus, DTg, Dorsal tegmental nucleus of Gudden
- DTg
Dorsal tegmental nucleus of Gudden
- DTgC
Dorsal tegmental nucleus of Gudden, central part
- DTgP
Dorsal tegmental nucleus of Gudden, pericentral part
- HRP
Horseradish peroxidase
- ic
Internal capsule
- LDT
Lateral dorsal tegmental nucleus
- lMB
Lateral mammillary nucleus
- Me
Mammillary bodies, pars medianus
- mlf
Medial longitudinal fasciculus
- ml
Medial lemniscus
- mMB
Medial mammillary nucleus
- MTT
Mammillothalamic tract
- ot
Optic tract
- PAG
Periaqueductal gray
- PDTg
Posterodorsal tegmental nucleus
- PV
Parvalbumin
- R
Raphe nucleus
- SC
Superior colliculus
- scp
Superior cerebellar peduncle
- Tn
Trochlear nucleus
- V
Ventricle
- vPAG
Ventral periaqueductal gray
- VTg
Ventral tegmental nucleus of Gudden
- VTgI
Ventral tegmental nucleus of Gudden, infrafascicular subdivision
- VTgP
Ventral tegmental nucleus of Gudden principal subdivision
- VTgS
Ventral tegmental nucleus of Gudden, suprafascicular subdivision
- xscp
Decussation of superior cerebellar peduncle
Literature Cited
- Aggleton JP. X-ray localization of limbic structures in the cynomolgus monkey (Macaca fascicularis) J Neurosci Methods. 1985;14:101–108. doi: 10.1016/0165-0270(85)90121-9. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Passingham RE. Stereotaxic surgery under X-ray guidance in the rhesus monkey, with special reference to the amygdala. Exp Brain Res. 1981;44:271–276. doi: 10.1007/BF00236564. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Vann SD, Saunders RC. Projections from the hippocampal region to the mammillary bodies in macaque monkeys. Eur J Neurosci. 2005;22:2519–2530. doi: 10.1111/j.1460-9568.2005.04450.x. [DOI] [PubMed] [Google Scholar]
- Allen GV, Hopkins DA. Mamillary body in the rat: topography and synaptology of projections from the subicular complex, prefrontal cortex, and midbrain tegmentum. J Comp Neurol. 1989;286:311–336. doi: 10.1002/cne.902860303. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley Periodicals; 1992. pp. 1–66. [Google Scholar]
- Bassant MH, Poindessous-Jazat F. Ventral tegmental nucleus of Gudden: a pontine hippocampal theta generator? Hippocampus. 2001;11:809–813. doi: 10.1002/hipo.1096. [DOI] [PubMed] [Google Scholar]
- Bassett JP, Taube JS. Neural correlates for angular head velocity in the rat dorsal tegmental nucleus. J Neurosci. 2001;21:5740–5751. doi: 10.1523/JNEUROSCI.21-15-05740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JP, Tullman ML, Taube JS. Lesions of the tegmentomammillary circuit in the head direction system disrupt the head direction signal in the anterior thalamus. J Neurosci. 2007;27:7564–7577. doi: 10.1523/JNEUROSCI.0268-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Cho J, Sharp PE. Role of the lateral mammillary nucleus in the rat head-direction circuit: a combined single unit recording and lesion study. Neuron. 1998;21:1387–1397. doi: 10.1016/s0896-6273(00)80657-1. [DOI] [PubMed] [Google Scholar]
- Briggs TL, Kaelber W. Efferent fiber connections of the dorsal and deep tegmental nuclei of Gudden. An experimental study in the cat. Brain Res. 1971;29:17–29. doi: 10.1016/0006-8993(71)90414-8. [DOI] [PubMed] [Google Scholar]
- Celio MR. Parvalbumin in most γ-aminobutyric acid containing neurons of the rat cerebral cortex. Science. 1986;231:995–997. doi: 10.1126/science.3945815. [DOI] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Crosby EC, Woodburne RT. The nuclear pattern of the non-tectal portions of the midbrain and isthmus in primates. J Comp Neurol. 1943;78:441–482. [Google Scholar]
- Crosby EC, Woodburne RT. The mammalian midbrain and isthmus regions. Part II. The fiber connections. J Comp Neurol. 1951;94:1–32. doi: 10.1002/cne.900940102. [DOI] [PubMed] [Google Scholar]
- Dusoir H, Kapur N, Brynes DP, McKinstry S, Hoare RD. The role of diencephalic pathology in human memory disorder. Brain. 1990;113:1695–1706. doi: 10.1093/brain/113.6.1695. [DOI] [PubMed] [Google Scholar]
- Gallyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1:203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- Goldberg E, Antin SP, Bilder RM, Jr, Gerstman LJ, Hughes JE, Mattis S. Retrograde amnesia: possible role of mesencephalic reticular activation in long-term memory. Science. 1981;213:1392–1394. doi: 10.1126/science.7268442. [DOI] [PubMed] [Google Scholar]
- Gonzalo-Ruiz A, Romera JC, Sanz JM, Morte L. Localization of amino acids, neuropeptides and cholinergic neurotransmitter markers in identified projections from the mesencephalic tegmentum to the mammillary nuclei of the rat. J Chem Neuroanat. 1999;16:117–133. doi: 10.1016/s0891-0618(98)00063-5. [DOI] [PubMed] [Google Scholar]
- von GuddenB. Vers dtsch Natforsch. Magdeburg: Tagungsblatt; 1884. Uber das Corpus mammillare und die sogenannten Schenkel des Fornix. 57. [Google Scholar]
- Hanker JS, Yates PE, Metz CB, Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horeseradish-peroxidase. J Histochem. 1977;9:789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Ito H, Zyo K. Neuroanatomical study of the afferent projections to the supramammillary nucleus in the rat. Anat Embryol. 1993;188:139–148. doi: 10.1007/BF00186247. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Zyo K. Comparative cyoarchitectonic study of Gudden's tegmental nuclei in some mammals. J Comp Neurol. 1983;216:233–244. doi: 10.1002/cne.902160302. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Zyo K. Comparative anatomical study of the tegmentomammillary projections in some mammals: a horseradish peroxidise study. Brain Res. 1984;300:335–349. doi: 10.1016/0006-8993(84)90844-8. [DOI] [PubMed] [Google Scholar]
- Hopkins DA. Neuroanatomy of head direction cell circuits. In: Wiener SI, Taube JS, editors. Head direction cells and the neural mechanisms of spatial orientation. Cambridge, MA: MIT Press; 2005. pp. 17–44. [Google Scholar]
- Huang XF, Tork I, Halliday GM, Paxinos G. The dorsal, posterodorsal, and ventral tegmental nuclei: a cyto- and chemoarchitectonic study in the human. J Comp Neurol. 1992;318:117–137. doi: 10.1002/cne.903180202. [DOI] [PubMed] [Google Scholar]
- Kapur N, Crewes H, Wise R, Abbott P, Carter M, Millar J, Lang D. Mammillary body damage results in memory impairment but not amnesia. Neurocase. 1998;4:509–517. [Google Scholar]
- Kocsis B, Vertes RP. Characterization of neurons of the supramammillary nucleus and mammillary body that discharge rhythmically with hippocampal theta in the rat. J Neurosci. 1994;14:7040–7052. doi: 10.1523/JNEUROSCI.14-11-07040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Lavenex PB, Bennett JL, Amaral DG. Postmortem changes in the neuroanatomical characteristics of the primate brain: hippocampal formation. J Comp Neurol. 2009;512:27–51. doi: 10.1002/cne.21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichnetz GR, Carleton SM, Katayama Y, Gonzalo-Ruiz A, Holstege G, DeSalles AAF, Hayes RL. Afferent and efferent connections of the cholinoceptive medial pontine reticular formation (region of the ventral tegmental nucleus) in the cat. Brain Res Bull. 1989;22:665–688. doi: 10.1016/0361-9230(89)90087-7. [DOI] [PubMed] [Google Scholar]
- Loftus M, Knight RT, Amaral DG. An analysis of atrophy in the medial mammillary nucleus following hippocampal and fornix lesions in humans and nonhuman primates. Exp Neurol. 2000;163:180–190. doi: 10.1006/exnr.2000.7361. [DOI] [PubMed] [Google Scholar]
- Meibach RC, Siegel A. The origin of the fornix fibers which project to the mammillary bodies in the rat: a horseradish peroxidase study. Brain Res. 1975;88:508–512. doi: 10.1016/0006-8993(75)90662-9. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Tracing neural connections with horseradish peroxidase. New York: Wiley; 1982. [Google Scholar]
- Paxinos G, Huang XF, Petrides M, Toga AW. The rhesus monkey brain in stereotaxic coordinates. San Diego, CA: Academic Press; 2000. [Google Scholar]
- Perry VH, Linden R. Evidence for dendritic competition in the developing retina. Nature. 1982;297:683–685. doi: 10.1038/297683a0. [DOI] [PubMed] [Google Scholar]
- Petrovicky P. Structure and incidence of Gudden's tegmental nuclei in some mammals. Acta Anat (Basel) 1971;80:272–286. doi: 10.1159/000143694. [DOI] [PubMed] [Google Scholar]
- Petrovicky P. Note on the connections of Gudden's tegmental nuclei. I. Efferent ascending connections in the mammillary peduncle. Acta Anat. 1973;86:165–190. [PubMed] [Google Scholar]
- Poletti CE, Cresswell G. Fornix system efferent projections in the squirrel monkey: an experimental degeneration study. J Comp Neurol. 1977;175:101–128. doi: 10.1002/cne.901750107. [DOI] [PubMed] [Google Scholar]
- Rose J. The cell structure of the mamillary body in the mammals and in man. J Anat. 1939;74:91–115. [PMC free article] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Roy NJ, Davis BJ. A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J Histochem Cytochem. 1986;34:1301–1315. doi: 10.1177/34.10.3745909. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. London: Academic Press; 2007. [Google Scholar]
- Saunders RC. PhD thesis. Oxford University; 1983. Some experiments on memory involving the fornix-mammillary system. [Google Scholar]
- Shantha TR, Manocha SL, Bourne GH. A stereotaxic atlas of the Java monkey brain (Macaca irus) Baltimore, MD: Williams & Wilkins; 1968. [Google Scholar]
- Snider RS, Lee JC. A stereotaxic atlas of the monkey brain (Macaca mulatta) Chicago: University of Chicago Press; 1961. [Google Scholar]
- Sternberger A, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and non-phosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Tago H, Kimura H, Maeda T. Visualization of detailed acetycholinesterase fiber and neuron staining in the rate brain by a sensitive histochemical procedure. J Histochem Cytochem. 1986;34:1431–1438. doi: 10.1177/34.11.2430009. [DOI] [PubMed] [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 2008;11:834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Nauta WJH. A comparison of the distribution of the fornix system in the rat, guinea pig, cat, and monkey. J Comp Neurol. 1959;113:337–362. doi: 10.1002/cne.901130302. [DOI] [PubMed] [Google Scholar]
- Van der Werf Y, Witter MP, Uylings HBM, Jolles J. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia. 2000;38:613–627. doi: 10.1016/s0028-3932(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Vann SD, Saunders RC, Aggleton JP. Distinct, parallel pathways link the medial mammillary bodies to the anterior thalamus in macaque monkeys. Eur J Neurosci. 2007;26:1575–1586. doi: 10.1111/j.1460-9568.2007.05773.x. [DOI] [PubMed] [Google Scholar]
- Vann SD. Gudden's ventral tegmental nucleus is vital for memory: re-evaluating diencephalic inputs for amnesia. Brain. 2009;132:2372–2384. doi: 10.1093/brain/awp175. [DOI] [PubMed] [Google Scholar]
- Vann SD. Re-evaluating the role of the mammillary bodies in memory. Neuropsychologia. 2010;48:2316–2327. doi: 10.1016/j.neuropsychologia.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. The mammillary bodies—two memory systems in one? Nat Rev Neurosci. 2004;5:35–44. doi: 10.1038/nrn1299. [DOI] [PubMed] [Google Scholar]
- Vann SD, Saunders RC, Aggleton JP. Distinct, parallel pathways link the medial mammillary bodies to the anterior thalamus in macaque monkeys. Eur J Neurosci. 2007;26:1575–1586. doi: 10.1111/j.1460-9568.2007.05773.x. [DOI] [PubMed] [Google Scholar]
- Vann SD, Tsivilis D, Denby CE, Quamme J, Yonelinas AP, Aggleton JP, Montaldi M, Mayes AR. Impaired recollection but spared familiarity in patients with extended hippocampal system damage: convergence across three methods. Proc Natl Acad Sci U S A. 2009;106:5442–5447. doi: 10.1073/pnas.0812097106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RB, Amaral DG, Cowan WM. The morphology and connections of the posterior hypothalamus in the cynomolgus monkey (Macaca fascicularis). II Efferent connections. J Comp Neurol. 1982;207:135–156. doi: 10.1002/cne.902070204. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Viana Di Prisco G. Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cogn Neurosci Rev. 2004;3:173–200. doi: 10.1177/1534582304273594. [DOI] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsak-off syndrome. Philadelphia: Davis: 1971. [Google Scholar]
- Wirtshafter D, Stratford TR. Evidence for GABAergic projections from the tegmental nuclei of Gudden to the mammillary body in the rat. Brain Res. 1993;630:188–194. doi: 10.1016/0006-8993(93)90656-8. [DOI] [PubMed] [Google Scholar]


