Abstract
The development and application of biomarkers to neurodegenerative diseases has become increasingly important in clinical practice and therapeutic trials. While substantial progress has been made at the basic science level in understanding the pathophysiology of HIV-Associated Neurocognitive Disorders (HAND), there are significant limitations in our current ability to predict the onset or trajectory of disease, and to accurately determine the effects of therapeutic interventions. Thus, the development of objective biomarkers is critical to further our understanding and treatment of HAND. In recent years, biomarker discovery efforts have largely been driven forward through the implementation of multiple “omics” approaches that include (but are not restricted to): Lipidomics, proteomics, metabolomics, genomics, transcriptomics, and advances in brain imaging approaches such as functional connectomics. In this paper we summarize our progress to date on lipidomic approaches to biomarker discovery, discuss how these data have influenced basic research on the neuropathology of HAND, and implications for the development of therapeutics that target metabolic pathways involved in lipid handling.
Overview
The introduction of combinational antiretroviral therapy (cART) in the early 1990s dramatically increased the expected lifespan of those infected the Human Immunodeficiency Virus (HIV)(Tozzi et al., 2007; Cardenas et al., 2009; Heaton et al., 2010). In addition to extending lifespan these therapeutics also decreased the incidence of cognitive impairment in HIV-infected patients. However, nearly two decades after the introduction of cART it is apparent that the prevalence of cognitive impairment is unchanged (and may be increasing), with approximately half of those infected with HIV likely to develop some form of cognitive impairment (Antinori et al., 2007; Chang et al., 2008; Valcour et al., 2008; Achim et al., 2009; Brew et al., 2009; Ances et al., 2010). Although cognitive impairments in cART treated patients tend to be less severe compared to untreated HIV-infected patients, they nonetheless can profoundly impact quality of life. Despite effective viral suppression with cART, brain volume loss, and evidence of age-influenced white matter damage are common in HIV-infected patients (Chang et al., 2008; McMurtray et al., 2008; Cardenas et al., 2009; Gongvatana et al., 2009). Examinations of brain tissues from cART treated patients shows evidence of metabolic disturbances, inflammation, synaptic and dendritic damage (McArthur et al.; Gelman, 2007; Pelle et al., 2008; Khanlou et al., 2009; Cohen et al., 2010; Nguyen et al., 2010; Kamat et al., 2012) These observations suggest that cART is not sufficient to prevent neural damage, and that an adjunctive neuroprotective therapy is required to protect the brain in HIV-infected patients. The development and validation of surrogate markers for brain damage are critical to facilitate therapeutic development, and could potentially identify HIV-infected patients at the earliest stages of neural damage, when a neuroprotective therapeutic would be most beneficial.
Cerebral Spinal Fluid Sphingolipid Content is a Surrogate Measure for Brain Sphingolipid Metabolism
In 2004 we first demonstrated that disruptions in brain sphingolipid metabolism are apparent in HIV infected patients. Accumulations of ceramide and sphingomyelin were notable in the frontal, parietal and temporal cortex of individuals infected with HIV, and there were anatomical differences in the particular species and sub-species of sphingolipid that accumulated. The degree of these metabolic disturbances in sphingolipid metabolism appeared to be related cognitive status (Haughey et al., 2004). In general, accumulations of ceramide and sphingomyelin were greater in patients with more severe forms of cognitive impairment. A significant discovery from this study was that the CSF sphingolipid content appeared to reflect a composite of the metabolic profile obtained from multiple brain regions. Thus, we reasoned that the sphingolipid composition of CSF could be a useful surrogate measure to estimate brain sphingolipid metabolism. As CSF can be collected from living patients, this approach allowed us to determine the cross sectional and temporal relationships of these lipid metabolites to changes in cognitive status. In a series of subsequent studies we measured CSF at one or more time points and quantified sphingolipid content. Data were then stratified according to longitudinal changes in cognitive functioning. The findings from these studies have begun to unravel complex temporal changes in sterol and sphingolipid metabolic profiles that are associated with HIV infection and temporal shifts in cognitive status. Recent evidence suggests that these metabolic disturbances in brains if HIV-infected patients may be a manifestation of underlying dysfunctions in endolysosomal systems (Bandaru et al., 2013).
Brain Sphingolipid Metabolism
Brain has one of the highest lipid contents of any organ in the human body. Our understanding of brain lipid metabolism, and in particular, roles that sphingolipids play in the regulation of neuronal and glial function is moving forward at a rapid pace. This current rate of discovery has been made possible due to a combination of technological advances in mass spectrometry, ongoing efforts to map pathways that regulate lipid metabolism (see lipidmaps.org), and an increased appreciation that dysregulations of sphingolipid metabolism play important roles in the pathogenesis of a number of different neurodegenerative conditions including AD, Parkinson’s disease, Multiple Sclerosis, and HIV Associated Neurocognitive Disorders (HAND)(Cutler et al., 2004; Haughey et al., 2004; Bandaru et al., 2009; Mielke et al., 2010; Fabelo et al., 2011).
Sphingolipids are a class of lipid derived from the alipathic amino alcohol sphingosine. The sphingosine backbone is O-linked to a charged head group such as ethanolamine, serine or choline, and amide-linked to an acyl group, such as a fatty acid. Ceramides are the simplest sphingolipid, consisting of a fatty acid chain attached by an amide linkage to sphingosine. Ceramide is a bioactive lipid that has been implicated in the regulation of numerous cellular processes including cell growth, survival, motility, protein traffic, neuronal plasticity, endolysosomal, autophagic and excitotoxic events, neurite outgrowth, synaptogenesis, neurotransmitter release, cytokine production, and the cellular response to inflammatory stimuli ((Jana et al., 2009; Zhang et al., 2009)). Such a wide variety of cellular functions are regulated by this family of lipids due to a complex network of interconnected metabolic pathways that are tissue, cellular, sub-cellular, and organelle specific. These metabolic pathways regulate the spatial and temporal patterns of ceramide production that determine the effects on these diverse cellular processes (reviewed in (Bikman and Summers, 2011; Worgall, 2011; Mencarelli and Martinez-Martinez, 2013). Ceramides can be produced de novo by the condensation of palmitate and serine to form 3-keto-dihydrosphingosine (by serine palmitoyl transferase). 3-keto-dihydrosphingosine is then reduced to dihydrosphingosine, which is acylated to produce dihydroceramide and finally converted to ceramide (by dihydroceramide desaturase). Ceramide can also be rapidly produced by reacylation of sphingosine (the salvage pathway) and by the hydrolysis of sphingomyelin (via a family of sphingomyelinases). Sphingomyelinases are categorized based on optimal pH for activity into acidic- (aSMase), alkaline- (alkSMase) and neutral (nSMase). Neutral sphingomyleinase-2 (nSMase2) is highly expressed in the CNS (Fensome et al., 2000; Hofmann et al., 2000; Clarke and Hannun, 2006), and is primarily located to the Golgi apparatus (Tomiuk et al., 1998; Hofmann et al., 2000), but can translocate to perinuclear regions in response to the antioxidant glutathione and to the plasma membrane in response to oxidative stress (Andrieu-Abadie and Levade, 2002; Levy et al., 2006; Castillo et al., 2007). nSMase2 activity is regulated by TNFα, IL-1, FasL, the HIV coat protein gp120 and oxidative stress (Liu and Hannun, 1997; Visnjic et al., 1999; Clarke and Hannun, 2006; Jana and Pahan, 2007; Wheeler et al., 2009). nSMase2 has been implicated in the neuropathogenesis associated with HAND and other inflammatory-associated conditions (France-Lanord et al., 1997; Cutler et al., 2002; Cutler et al., 2004; Haughey et al., 2004). Sphingomylein synthesis involves the transfer of choline from phosphatidyl choline to the head group of ceramide. This reaction is catalyzed by sphingomyelin synthases 1 and 2 that catalyze the conversion of ceramide and phosphatidylcholine to sphingomyelin and diacylglycerol. Human SMS1 is localized to the Golgi, while SMS2 resides primarily at the plasma membrane. Experimental evidence to date suggests that accumulations of ceramide and sphingomyelin in neurons may be related to the development and progression of HAND. The temporal order perturbations in sphingolipid metabolism provided clues that have fostered the development of therapeutics that target specific enzymes in these pathways.
Central Nervous System Inflammation is Associated with Ceramide Production
In multiple cohorts of HIV-infected patients we have consistently found that CSF accumulations of sphingomyelin and ceramide are associated with cognitive impairment (Haughey et al., 2004; Sacktor et al., 2004; Bandaru et al., 2007; Bandaru et al., 2013). However, deciphering the temporal relationship of these metabolites to cognitive status has proven to be more challenging.
Some of the earliest biochemical changes that may predict cognitive decline are consistent with the cellular response to an ongoing inflammatory process. Low-level neuroinflammation produced through the persistent activation of brain resident astrocytes and microglia is thought to be an important contributor to the neuropathogenesis of HAND. Brain and CSF levels of numerous inflammatory cytokines such as transforming growth factor-β (TGF-β), interleukins IL-1α , IL-1β, IL-6, tumor necrosis factor alpha (TNFα), and CD95 ligand have been repeatedly reported as elevated in HIV-infected patients in accordance with cognitive impairment (Grimaldi et al., 1991; Wahl et al., 1991; Tyor et al., 1992; Achim et al., 1993; Wesselingh et al., 1993; Saas et al., 1999; Avison et al., 2004). These effects were recapitulated by intact virions, or viral proteins in tissue culture, rodent, and primate models of HAND (Burdo et al., 2010; Roberts et al., 2010; El-Hage et al., 2011; Weed et al., 2012; Marker et al., 2013). TNFα and IL-1β are among the most frequently reported cytokines to be increased in brain and CSF of HIV-infected patients, and in pre-clinical models of HAND (Brabers and Nottet, 2006). These cytokines are known to be connected to ceramide associated stress signaling in neurons, and to cytokine/chemokine production in glia (Singh et al., 1998; Ayasolla et al., 2004; Davis et al., 2006; Wheeler et al., 2009; Martinez et al., 2012; Gu et al., 2013). TNFα and IL-1β receptors are linked to the generation of ceramide through sphingomyelin hydrolases (aSMase and nSMase as discussed above in the section on sphingolipid metabolism) that rapidly convert membrane sphingomyelin into ceramide. These actions occur through adaptor proteins and signaling intermediates that directly link TNF and IL-1 receptors to these hydrolytic enzymes (Adam-Klages et al., 1996; Schwandner et al., 1998). In addition to cytokines the HIV-coat protein gp120 has been shown to increase neuronal ceramide through actions that involved binding to CXCR4, increases of ER calcium, and a redox-sensitive translocation of nSMase2 to the plasma membrane (Haughey et al., 2004; Jana and Pahan, 2004b; David Wheeler, 2009). When localized to the plasma membrane neutral sphingomyelinase catalyzes the conversion of sphingomyelin to ceramide. As ceramide is a highly saturated lipid that packs tightly together, increases of membrane ceramide stabilize the structure of membrane microdomains (Haughey et al., 2004; Jana and Pahan, 2004b; David Wheeler, 2009). These microdomains (also called lipid rafts) are regions of the plasma membrane that exhibit decreased lateral mobility due to a focal enrichment of saturated lipids including cholesterol, sphingomyelin and ceramide. Membrane microdomains play important roles in signal transduction as delivery docks for protein insertion, and through the creation of transient signaling platforms that regulate protein scaffolding and downstream signaling.
NMDA Receptors Localize to Ceramide Enriched Membrane Microdomains
The insertion and removal of N-methyl D-aspartate (NMDA) receptors from the neuronal synapse is critical for the modulation of synaptic plasticity (Roche et al., 2001; Barria and Malinow, 2002; Nong et al., 2003; Lavezzari et al., 2004; Scott et al., 2004; Washbourne et al., 2004). Transmembrane receptors traffic within cells encased into intracellular vesicles. The insertion of receptors into the plasma membrane requires the fusion of receptor-laden vesicles with the plasma membrane. A specific, transient and focal reorganization of the plasma membrane is necessary for the insertion and surface expression of NMDA receptors (Wheeler et al., 2009). For example, applications of TNFα to neuronal cultures rapidly increased plasma membrane ceramide by mechanisms that involved a translocation and activation of the sphingomyelin hydrolase nSMase2. These increases of ceramide temporarily stabilized membrane microdomains, and facilitated the fusion of NMDA receptor containing vesicles with the plasma membrane. These newly inserted NMDA receptors were located to synapses in small clusters, where NMDA-evoked calcium transients were enhanced. NMDA-evoked excitatory post-synaptic currents, and long-term potentiation recorded from CA1 pyramidal cells in brain slice preparations were also enhanced following activation of nSMase2. Pharmacological, molecular and transgenic approaches each confirmed that nSMase2 was critical for NMDA receptor surface expression and functional changes in synaptic activity. Consistent with a role in plasticity, these increases of plasma membrane ceramide and NMDA receptor clustering were rapid and transient. Activation of nSMase2 increased ceramide and clustering of NMDA receptors within 2 min, and these effects returned to baseline within 10 min. Similar effects were found in vivo, in which nSMase2 plays an important role in memory formation. Pharmacological blockade of nSMase2 decreased the ceramide content in hippocampus and cortex, altered the number and molecular composition of NMDA and AMPA receptors, and impaired spatial and episodic-like memory in mice (Tabatadze et al., 2010). Together these studies identified nSMase2-generated ceramide as an important regulator of membrane microdomain stability, NMDA receptor trafficking, with associated effects that regulate cellular and behavioral manifestations of memory.
In the setting of HIV-infection, the virus in combination with inflammatory cytokines may over activate nSMase2 resulting in a chronic elevation of ceramide with prolonged stabilization of NMDA receptors to membrane microdomains. In gp120 treated neurons, clusters of NMDA receptors were trapped into stabilized membrane microdomains, where the receptors were unable to laterally disperse or internalize, even following strong agonist induction (Xu et al., 2011). Similar effects were observed in gp120 transgenic mice where accumulations of ceramide and over activation of nSMase2 were accompanied by modifications in the subunit composition of NMDA receptors, and hyper phosphorylation of NR1 subunits on serine 896 (indicative of increased surface expression). Isolation of lipid raft fractions from the cortex of these animals showed that NMDA receptors were preferentially located to flotilin-1 positive lipid rafts fractions. Daily administrations of a nSMase2 inhibitor to gp120 mice blunted nSMase2 activity, normalized brain ceramide content, and reduced NR1 serine 896 phosphorylation (Chen, 2012). Thus, prolonged increases of ceramide that are apparent in the brains and CSF of HIV-infected individuals may perturb cognitive function through a stabilization of NMDA receptors into membrane microdomains where excessive calcium signaling may evoke cellular stress pathways (Figure 2A).
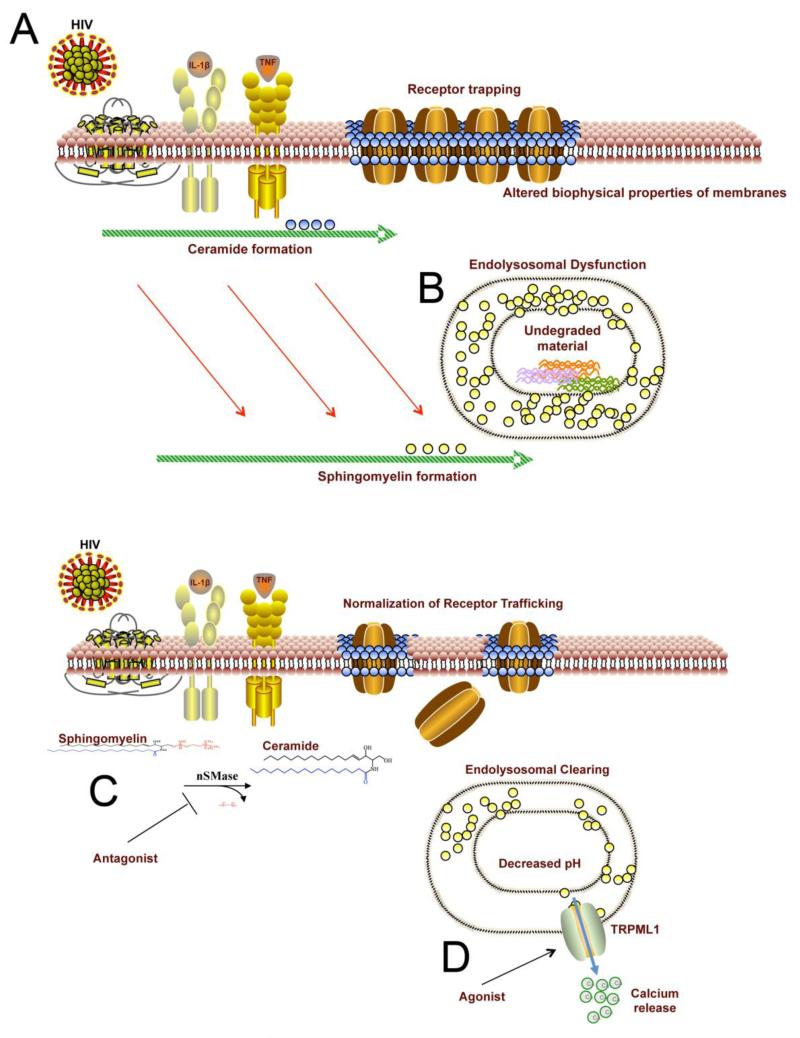
Figure 2. Accumulation of Ceramide and Sphingomyelin Perturb Protein Trafficking and Endolysosomal Function.
A) HIV, TNFα and IL-1β increase the size and stabilize the structure of membrane microdomains through increased ceramide formation. NMDA receptor clusters are trapped into stabilized membrane microdomains. B) Ceramide is the direct precursor to sphingomyelin. When sphingomyelin is overproduced, it becomes sequestered into lysosomes. This sequestration of sphingomyelin in lysosomes impairs the derivative capacity of endolysosomes and undegraded proteins accumulate. C) Inhibition of nSMase2 can prevent IL1β, TNFα and HIV from inducing ceramide formation. Reducing ceramide formation in this setting normalizes the lipid content of the plasma membrane and restores normal receptor trafficking. D) Agonists of the lysosomal TRPML1 channel induce the release of calcium. The release of calcium from lysosomes reduces the ionic gradient and hydrogen to be pumped into the lumen. This lowers the luminal pH and helps to clear debris through restoration of the lysosomal derivative capacity (lysosomal hydrolases have a low pH optima).
Accumulations of Sphingomyelin may Provoke Endolysosomal Dysfunction
We have just recently completed a study that describes interactions between CSF lipidoses and temporal shifts in the cognitive status in a demographically diverse multicenter collection of HIV-infected subjects. These findings provided evidence that the onset and progression of HAND may involve a progressive disturbance in ceramide, sphingomyelin, and cholesterol metabolism that is reminiscent of the biochemical manifestations associated with a lysosomal storage disorder (Bandaru et al., 2013). Increases in a single ceramide species and reduced esterification of cholesterol were associated with prodromal stages of cognitive decline, while progressive cognitive impairments were characterized by accumulations in multiple sphingomyelin species. The implications of these findings are that perturbed endolysosomal function may contribute to cognitive decline in HIV-infected subjects, and is consistent with reports of enlarged endolysosomal systems and defects of autophagy in neuronal cultures, brain tissues of HIV-infected subjects, and in primates infected with SIV (Gelman et al., 2005; Alirezaei et al., 2008b; Alirezaei et al., 2008a; Chen et al., 2013). These pathological observations are consistent with our biochemical findings of perturbed sphingolipid balance, since the molecular response to an overabundance of sphingolipids is to sequester these products into lysosomes. These findings suggest that HIV-associated perturbations in sphingolipid metabolism may eventually lead to dysfunctions in endolysosomal systems (Figure 2A).
The Endolysosomal System
Organization of the endolysosomal pathway is complex and there are a various models explaining how organelles within the system relate to one another. One of the most accepted is the maturation model which suggests each organelle is a transient but distinct entity that matures along a defined pathway (Luzio et al., 2007). In this model, endocytosed material is first compartmentalized into early endosomes, which then mature into a transient late endosomes that continue to mature into lysosomes (a terminal organelle). Early endosomes are the major sorting station in the endolysosomal system that allows for the recycling of membrane receptors and other materials back to the membrane surface. From the early endosomes internalized material designated for degradation is separated into clumps of vesicles called multivesicular bodies that mature into late endosomes, and eventually to lysosomes (Sachse et al., 2002). Substrates can enter the endolysosomal system through endocytosis, autophagy, and phagocytosis. Endocytosis allows the digestion of exogenous proteins through clathrin-mediated or clathrin independent actions. Clathrin-mediated endocytosis depends on surface membrane receptors that bind to substrates before invagination to form a vesicle. Although the majority of endocytosis occurs via the clathrin route, some substrates enter through clathrin-independent routes such as the CLIC/GEEC and flotillin-dependent pathways. Autophagy is responsible for the removal of endogenous proteins and is critical for metabolic and homeostatic function (Kroemer and Jaattela, 2005). Four autophagic mechanisms have been described that include macro-autophagy, micro-autophagy, crinophagy and chaperone-mediated autophagy. Macro-autophagy is the primary route of the autophagic pathway and occurs when large regions of the cytoplasm are sequestered into a double membrane autophagosome destined for degradation. The autophagosome travels through the cytoplasm and fuses with the lysosomal membrane where proteolysis of the cargo can occur. Proteolysis occurs primarily in the late endosomes and lysosomes as both contain lysosomal hydrolases (digestive enzymes). However there are there are distinct differences in morphology and proteolytic capacity of the two structures. Late endosomes have a complex morphology that is organized by microtububles while lysosomes are simpler, electron-dense organelles. Late endosomes contain only ~20% of the total hydrolase pool, yet are the primary site of proteolysis in the endolysosomal pathway. Lysosomes on the other hand contain the bulk of the total hydrolase pool but only contribute approximately 20% of total proteolytic capacity. Proper functioning of the endolysosomal system is critical for normal cellular metabolism, and perturbations that cause lipids to accumulate in these compartments disrupts endolysosomal function.
Typical and “Atypical” Disorders of Lysosomal Storage
Though rare, mutations in genes that code for lysosomal enzymes produce a series of diseases that are collectively known as lysosomal storage disorders. To date nearly 70 genetically distinct lysosomal storage disorders have been described, and many are associated with dysfunctions in macromolecular storage that result in physical disruption of the endolysosomal system. Although there is a great deal of variability in the severity of lysosomal storage disorders, those that involve sphingolipidoses are frequently associated with serious neurological and cognitive symptoms. Gaucher disease, Tay-Sachs disease, Sandhoff disease, and Niemann-Pick C disease are typical lysosomal storage disorders that result from genetic mutations in enzymes involved with lipid metabolism. In each of these disorders, sphingolipid metabolism is impaired and one or more lipid products accumulate in endolysomal compartments. Neural degeneration is a universal feature of these disorders involving atrophy, dendritic, axonal, and white matter degeneration (Sun et al.; Nixon et al., 2008; Bellettato and Scarpa, 2010)
Neurodegenerative pathology caused by altered lipid metabolism and dysfunctions in the endolysosomal system are not restricted to these typical lysosomal storage disorders. A growing body of evidence supports the notion that accumulations in certain lipid metabolites play a role in the neuropathology of a number of neurodegenerative diseases in addition to HAND (as discussed above). For instance Alzheimer’s disease (AD) is a progressive and age dependent neurodegenerative disorder that is characterized by dementia and memory loss. The hallmarks of AD include widespread neuronal death associated with the accumulation of extracellular deposits of amyloid β-peptide (Aβ) into plaques and intraneuronal aggregates of the microtubule associated protein tau into neurofibrillary tangles. While the cause of sporadic AD remains elusive, there is evidence that altered sphingolipid and sterol metabolism may create conditions that are favorable for the formation and aggregation of Aβ (van Echten-Deckert and Walter, 2012). One of the first studies to show that ceramides are elevated early in the pathogenesis of AD found nearly a three-fold elevation of ceramides in white matter of AD patients that peaked at the stage of very mild dementia (Han et al., 2002). A subsequent study showed that long-chain ceramides, especially ceramide C24, accumulates in brains of AD patients (Cutler et al., 2004). Ceramide has been shown to be important for Aβ formation by increasing the half-life of β-secretase. The addition of C6 ceramide to CHO cells stably transfected with APP (CHO-APP) increased Aβ by increasing β- but not γ-secretase cleavage of the amyloid precursor protein (Kalvodova et al., 2005). It has also been demonstrated that β-amyloid can increase ceramide levels by mechanisms that involve nSMase, lipid peroxidation and oxidative stress (Ayasolla et al., 2004; Jana and Pahan, 2004a; Ju et al., 2005; Satoi et al., 2005; Malaplate-Armand et al., 2006). The addition of nSMase to CHO-APP cells increased ceramide and the formation of β-amyloid while the inhibition of ceramide synthesis with fumonisin B1 decreased ceramide and β-amyloid (Puglielli et al., 2003). Likewise, β-amyloid peptides induced the NADPH oxidase-mediated production of superoxide radicals in neurons that was involved in the activation of nSMase, but not aSMase, via hydrogen peroxide. (Jana and Pahan, 2004a). Thus, ceramide can promote β-amyloid formation through induction and stabilization of β-secretase, and β-amyloid can induce ceramide by redox-sensitive up regulation of nSMase. In addition to HIV and AD, disorders of lipid metabolism have also been linked to Parkinson’s disease (Brugg et al., 1996; France-Lanord et al., 1997; Lwin et al., 2004; Cheng et al., 2011; Lee et al., 2011), and Multiple Scelrosis (Kim et al.; van Doorn et al.; Glabinski, 1993; Chen et al., 2005; Hait et al., 2006; Narayanan et al., 2006; Wheeler et al., 2008). If indeed sphingolipidoses are common to many neurodegenerative conditions (Haughey, 2010), this suggests that accumulations of sphingolipids in lysosomes with consequent disruption of endolysosomal systems may be a common neurodegenerative phenotype.
Surrogate Marker Guided Therapeutic Development
Reducing neuroinflammation has proven to be a difficult therapeutic target. Moreover, not all inflammation is detrimental or should be inhibited. Inflammation alerts the body to infection or damage. It is when the immunological response becomes non-specific or prolonged that inflammation can itself damage multiple organ systems including brain. However, blocking inflammatory pathways can have unanticipated consequences that damage neural, vascular and cardiac systems, depending on the drug, target, duration of dosing, and timing of treatment (Karplus and Saag, 1998; Sugaya et al., 2000; Hoozemans et al., 2008; Jaturapatporn et al., 2012). Alternate therapeutic approached to blocking inflammation have been to target noxious downstream effectors of inflammatory signaling (Nixon, 2009; Marker et al., 2013). Lipidomic approaches discussed here have begun to identify several possible targets for therapeutic intervention that are downstream of inflammatory cytokine signaling. For example, nSMase2 is activated by inflammatory stimuli such as IL-1β, TNFα, and the HIV-coat protein gp120. Inhibiting nSMase2 protects neurons and mitochondria by reducing increases of ceramide and normalizing NMDA receptor trafficking (Haughey et al., 2004; Tsakiri et al., 2008; Novgorodov and Gudz, 2011; Gu et al., 2013)(Figure 2B). Attempts to manufacture therapeutically useful inhibitors of nSMase2 have been difficult. As this enzyme regulates the hydrolysis of sphingomyelin to ceramide, small molecule inhibitors of the enzymes catalytic site are hydrophobic (ie they look like a lipid). Hence, solubility can be an issue with these compounds, and oral bioavailability is often poor. On the plus side, once in circulation, these compounds have good brain penetrance. Alternative methods of biodelivery such as intranasal and nanoformulations to increase absorption may be required to improve delivery of these more hydrophilic compounds. Thus, although nSMase2 is an attractive therapeutic target, its ‘drugability” has yet to be determined.
A second potential therapeutic target that is downstream of inflammatory signaling is lysosomal located calcium channels. Inflammatory associated increases of ceramide can be converted to sphingomyelin. Accumulations of sphingomyelin in endolysosomal compartments results in intraluminal accumulations of calcium that are associated with reductions in cellular energetics. Overly abundant calcium in lysosomes increases positive charge in the lumen, and prevents acidification of the compartment by impairing the hydrogen pump (the increased ionic gradient prevents pumping of positive charge into the lumen). Agonists that induce calcium efflux from these stores through activation of TRPM1 may help to dissipate this transmembrane potential and restore the derivative capacity of lysosomes (Figure 2B). In pre-clinical models of lysosomal storage disease TRPML1 agonists have been shown to restore intraluminal pH, and clear sphingolipids from lysosomes (Shen et al., 2012). These results suggest that small molecule therapeutics designed to restore lysosomal function may have alternative uses as therapeutics for neurodegenerative disease.
Challenges for Biomarker Discovery in cART treated HIV-Infected Patients
Declines in cognitive status prior to the introduction of cART were frequent in patients with advanced HIV-infection, commonly involved an encephalitic process, were typically progressive, and a predicted death (Porwit et al., 1989; Wigdahl and Kunsch, 1989; Griffin et al., 1990). Biomarkers for cognitive impairment pre-cART were tied to infectious markers such as viral load, CD4 counts, immunological and inflammatory indicators (Brew et al., 1990; Griffin et al., 1990; McArthur et al., 1992; Tyor et al., 1992; Wiley et al., 1992; Portegies et al., 1993; Haughey et al., 2004). The introduction of cART shifted the neurological manifestations of HIV-infection so that the majority of cognitive impairments were mild (asymptomatic neurocognitive impairment), with bidirectional transitions in severity. The association of virological, immunological and inflammatory biomarkers to cognitive status in cART treated patients is not entirely clear in the ear of cART therapy. For instance, although viral load seems to no longer be a useful surrogate for cognitive status (i.e. neurocognitive impairments can occur in patients with little or no detectable viral load), CSF inflammatory markers such as MCP-1, TNFα, ceramide, and immunological markers such as neopterin, may continue to be useful surrogate measures (Sevigny et al., 2004; Hagberg et al., 2010; Bandaru et al., 2013; Yilmaz et al., 2013). However, there is not currently a consensus on what markers (biological and/or imaging) are the most useful surrogates measures of cognitive status in HIV-infected patients. There are currently a number of complex technical, social and economic challenges that that will need to be resolved as the field refines its search for a consensus group of HAND biomarkers.
There has been a noticeable shift in biomarker discovery from cross sectional approaches to longitudinal designs. Early biomarker studies often grouped HIV-infected patients based on cognitive status at a single time point. A temporal course of events was sometimes inferred from differences in biomarkers obtained from a single time point that were based on statistical associations with cognitive status at that time point. Before cART it was a reasonable assumption that cognitive decline typically followed a forward progression. The temporal course of cognitive status is more complicated in cART treated patients. Hence, the findings from cross sectional studies are more limited, and should not be used to infer a temporal course of events. Longitudinal approaches are now more commonly used and can include samples collected a single time point with longitudinal clinical and cognitive testing data, to multiple time points that include sample collections, clinical and cognitive testing data. While these longitudinal approaches are considerably more informative, they too have limitations. The vast majority of cognitive impairments in cART treated patients fall into the categories of asymptomatic neurocognitive impairments (abnormalities in two or more cognitive abilities), and minor cognitive disorder (cognitive impairment with mild functional impairment). These mild to moderate forms of impairment in the spectrum of HAND can be influenced by psychiatric conditions, drug and alcohol use, nutrition, sleep disturbances, aging, alcohol drug use (including tobacco) and socioeconomic status. Similarly, some of the biomarkers isolated from CSF or blood can be influenced by these same risk factors. In addition, antiretroviral drugs can alter some biomarkers through effects on insulin sensitivity, lipid handling, and cellular stress responses. The effects of these variables on biomarker measures can be more or less prominent depending on the particular biomarker in question, characteristics of the cohort, population size, and the length of time that the study encompasses. Although many of these variables can be controlled for in statistical analyses, it is difficult to control for all possible variables in any given population.
While there is no easy answer to address these potential confounds in biomarker studies, examinations of larger populations with mixed demographic and risk factors may aid in the discovery of biomarkers that are less influenced by these variables. The future success of biomarkers in the HIV field will require a great deal of co-operation between centers that have banked and prospective sample collections (including brain imaging), laboratories who study biomarkers, federal and private support to harness these considerable resources into meaningful and directed approaches for the discovery, consolidation and validation of biomarkers specifically related to cognitive status.
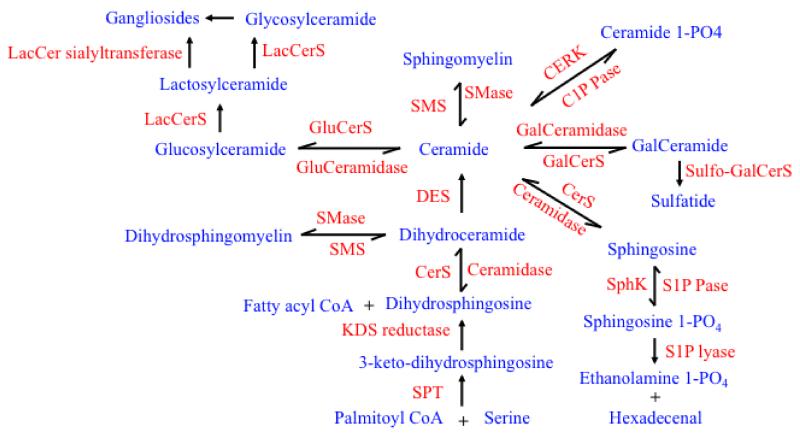
Figure 1. Metabolic pathways for sphingolipid metabolism.
Sphingolipid products are shown in blue, and enzymes that catalyze these reactions are shown in red. SMase (Sphingomyelinase), SMS (Sphingomylen synthase), DES (Dihydroceramide desaturase) CERK (Ceramide kinase), CerS (Ceramide synthase), KDS (reductase= 3-keto-dihydrosphingosine reductase), SPT (Serine palmitoyl transferase), S1P Pase (Sphingosine 1-phosphate phosphatase), S1P lyase (Sphingosine 1-phosphate lyase), SphK (Sphingosine kinase), Cer1P Pase (Ceramide 1-phosphate phosphatase), GluCerS (Glucosylceramide synthase), GluCeramidase (Glucosyl ceramidase), GalCerS (Galactosylceramide synthase), LacCerS (Lactosylceramide synthase), GlyCerS, (Glycosylceramide synthase), Sulfo-GalCerS (Sulfo galactosylceramide synthase), Palmitoyl COA (Palmitoyl coenzyme A hydrolase).
Acknowledgments
This work was supported by National Institutes of Health grants MH077542, MH075673, AG034849, and MH096636
Footnotes
Conflict of interest
The authors have no conflicts of interest related to this work to report.
References
- Achim CL, Heyes MP, Wiley CA. Quantitation of human immunodeficiency virus, immune activation factors, and quinolinic acid in AIDS brains. J Clin Invest. 1993;91:2769–2775. doi: 10.1172/JCI116518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achim CL, Adame A, Dumaop W, Everall IP, Masliah E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam-Klages S, Adam D, Wiegmann K, Struve S, Kolanus W, Schneider-Mergener J, Kronke M. FAN, a novel WD-repeat protein, couples the p55 TNF-receptor to neutral sphingomyelinase. Cell. 1996;86:937–947. doi: 10.1016/s0092-8674(00)80169-5. [DOI] [PubMed] [Google Scholar]
- Alirezaei M, Kiosses WB, Fox HS. Decreased neuronal autophagy in HIV dementia: a mechanism of indirect neurotoxicity. Autophagy. 2008a;4:963–966. doi: 10.4161/auto.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS One. 2008b;3:e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, McCutchan JA, Ellis RJ. HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis. 2010;201:336–340. doi: 10.1086/649899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieu-Abadie N, Levade T. Sphingomyelin hydrolysis during apoptosis. Biochim Biophys Acta. 2002;1585:126–134. doi: 10.1016/s1388-1981(02)00332-3. [DOI] [PubMed] [Google Scholar]
- Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avison MJ, Nath A, Greene-Avison R, Schmitt FA, Bales RA, Ethisham A, Greenberg RN, Berger JR. Inflammatory changes and breakdown of microvascular integrity in early human immunodeficiency virus dementia. J Neurovirol. 2004;10:223–232. doi: 10.1080/13550280490463532. [DOI] [PubMed] [Google Scholar]
- Ayasolla K, Khan M, Singh AK, Singh I. Inflammatory mediator and beta-amyloid (25-35)-induced ceramide generation and iNOS expression are inhibited by vitamin E. Free Radic Biol Med. 2004;37:325–338. doi: 10.1016/j.freeradbiomed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Bandaru VV, McArthur JC, Sacktor N, Cutler RG, Knapp EL, Mattson MP, Haughey NJ. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68:1481–1487. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, Troncoso J, Wheeler D, Pletnikova O, Wang J, Conant K, Haughey NJ. ApoE4 disrupts sterol and sphingolipid metabolism in Alzheimer’s but not normal brain. Neurobiol Aging. 2009;30:591–599. doi: 10.1016/j.neurobiolaging.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, Mielke MM, Sacktor N, McArthur JC, Grant I, Letendre S, Chang L, Wojna V, Pardo C, Calabresi P, Munsaka S, Haughey NJ. A lipid storage-like disorder contributes to cognitive decline in HIV-infected subjects. Neurology. 2013 doi: 10.1212/WNL.0b013e3182a9565e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Bellettato CM, Scarpa M. Pathophysiology of neuropathic lysosomal storage disorders. J Inherit Metab Dis. 2010;33:347–362. doi: 10.1007/s10545-010-9075-9. [DOI] [PubMed] [Google Scholar]
- Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest. 2011;121:4222–4230. doi: 10.1172/JCI57144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur J Clin Invest. 2006;36:447–458. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G. Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol. 2009;4:163–174. doi: 10.1007/s11481-008-9143-1. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Bhalla RB, Paul M, Gallardo H, McArthur JC, Schwartz MK, Price RW. Cerebrospinal fluid neopterin in human immunodeficiency virus type 1 infection. Ann Neurol. 1990;28:556–560. doi: 10.1002/ana.410280413. [DOI] [PubMed] [Google Scholar]
- Brugg B, Michel PP, Agid Y, Ruberg M. Ceramide induces apoptosis in cultured mesencephalic neurons. Journal of neurochemistry. 1996;66:733–739. doi: 10.1046/j.1471-4159.1996.66020733.x. [DOI] [PubMed] [Google Scholar]
- Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas V, Meyerhoff D, Studholme C, Kornak J, Rothlind J, Lampiris H, Neuhaus J, Grant R, Chao L, Truran D, Weiner M. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol. 2009:1–10. doi: 10.1080/13550280902973960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo SS, Levy M, Thaikoottathil JV, Goldkorn T. Reactive nitrogen and oxygen species activate different sphingomyelinases to induce apoptosis in airway epithelial cells. Exp Cell Res. 2007;313:2680–2686. doi: 10.1016/j.yexcr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Chang L, Wong V, Nakama H, Watters M, Ramones D, Miller EN, Cloak C, Ernst T. Greater than age-related changes in brain diffusion of HIV patients after 1 year. J Neuroimmune Pharmacol. 2008;3:265–274. doi: 10.1007/s11481-008-9120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JT, Collins DL, Freedman MS, Atkins HL, Arnold DL. Local magnetization transfer ratio signal inhomogeneity is related to subsequent change in MTR in lesions and normal-appearing white-matter of multiple sclerosis patients. Neuroimage. 2005;25:1272–1278. doi: 10.1016/j.neuroimage.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Chen X, Hui L, Geiger NH, Haughey NJ, Geiger JD. Endolysosome involvement in HIV-1 transactivator protein-induced neuronal amyloid beta production. Neurobiol Aging. 2013;34:2370–2378. doi: 10.1016/j.neurobiolaging.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Bandaru VVR, Geiger JD, Haughey NJ. Inhibition of neutral sphingomyelinase protects neurons from excitotoxic damage by normalizing brain ceramide and NMDA receptor phosphorylation. In Process. 2012 [Google Scholar]
- Cheng D, Jenner AM, Shui G, Cheong WF, Mitchell TW, Nealon JR, Kim WS, McCann H, Wenk MR, Halliday GM, Garner B. Lipid pathway alterations in Parkinson’s disease primary visual cortex. PLoS One. 2011;6:e17299. doi: 10.1371/journal.pone.0017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CJ, Hannun YA. Neutral sphingomyelinases and nSMase2: bridging the gaps. Biochim Biophys Acta. 2006;1758:1893–1901. doi: 10.1016/j.bbamem.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Gongvatana A, Buchthal S, Schifitto G, Clark U, Paul R, Taylor M, Thompson P, Tate D, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B. Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. J Neurovirol. 2010 doi: 10.3109/13550284.2010.520817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Pedersen WA, Camandola S, Rothstein JD, Mattson MP. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 2002;52:448–457. doi: 10.1002/ana.10312. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David Wheeler EK, Bandaru Veera V.R, Wang Yue, Knorr David, Poirier Christophe, Mattson Mark P., Geiger Jonathan D., Haughey Norman J. TNFα-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. Journal of Neurochemistry. 2009 doi: 10.1111/j.1471-4159.2009.06038.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CN, Tabarean I, Gaidarova S, Behrens MM, Bartfai T. IL-1beta induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J Neurochem. 2006;98:1379–1389. doi: 10.1111/j.1471-4159.2006.03951.x. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Dever SM, Fitting S, Ahmed T, Hauser KF. HIV-1 coinfection and morphine coexposure severely dysregulate hepatitis C virus-induced hepatic proinflammatory cytokine release and free radical production: increased pathogenesis coincides with uncoordinated host defenses. J Virol. 2011;85:11601–11614. doi: 10.1128/JVI.05239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabelo N, Martin V, Santpere G, Marin R, Torrent L, Ferrer I, Diaz M. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol Med. 2011;17:1107–1118. doi: 10.2119/molmed.2011.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensome AC, Rodrigues-Lima F, Josephs M, Paterson HF, Katan M. A neutral magnesium-dependent sphingomyelinase isoform associated with intracellular membranes and reversibly inhibited by reactive oxygen species. J Biol Chem. 2000;275:1128–1136. doi: 10.1074/jbc.275.2.1128. [DOI] [PubMed] [Google Scholar]
- France-Lanord V, Brugg B, Michel PP, Agid Y, Ruberg M. Mitochondrial free radical signal in ceramide-dependent apoptosis: a putative mechanism for neuronal death in Parkinson’s disease. J Neurochem. 1997;69:1612–1621. doi: 10.1046/j.1471-4159.1997.69041612.x. [DOI] [PubMed] [Google Scholar]
- Gelman BB. The neuropathology of HIV. Handb Clin Neurol. 2007;85:301–317. doi: 10.1016/S0072-9752(07)85018-4. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Soukup VM, Holzer CE, 3rd, Fabian RH, Schuenke KW, Keherly MJ, Richey FJ, Lahart CJ. Potential role for white matter lysosome expansion in HIV-associated dementia. J Acquir Immune Defic Syndr. 2005;39:422–425. doi: 10.1097/01.qai.0000164250.41475.f2. [DOI] [PubMed] [Google Scholar]
- Glabinski A. [Cytotoxic cytokines in multiple sclerosis] Neurologia i neurochirurgia polska. 1993;27:869–875. [PubMed] [Google Scholar]
- Gongvatana A, Schweinsburg BC, Taylor MJ, Theilmann RJ, Letendre SL, Alhassoon OM, Jacobus J, Woods SP, Jernigan TL, Ellis RJ, Frank LR, Grant I. White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol. 2009;15:187–195. doi: 10.1080/13550280902769756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE, McArthur JC, Cornblath DR. Soluble interleukin-2 receptor and soluble CD8 in serum and cerebrospinal fluid during human immunodeficiency virus-associated neurologic disease. J Neuroimmunol. 1990;28:97–109. doi: 10.1016/0165-5728(90)90024-h. [DOI] [PubMed] [Google Scholar]
- Grimaldi LM, Martino GV, Franciotta DM, Brustia R, Castagna A, Pristera R, Lazzarin A. Elevated alpha-tumor necrosis factor levels in spinal fluid from HIV-1-infected patients with central nervous system involvement. Ann Neurol. 1991;29:21–25. doi: 10.1002/ana.410290106. [DOI] [PubMed] [Google Scholar]
- Gu L, Huang B, Shen W, Gao L, Ding Z, Wu H, Guo J. Early activation of nSMase2/ceramide pathway in astrocytes is involved in ischemia-associated neuronal damage via inflammation in rat hippocampi. J Neuroinflammation. 2013;10:109. doi: 10.1186/1742-2094-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L, Cinque P, Gisslen M, Brew BJ, Spudich S, Bestetti A, Price RW, Fuchs D. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Han X, D MH, McKeel DW, Jr., Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis. J Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- Haughey NJ. Sphingolipids in neurodegeneration. Neuromolecular Med. 2010;12:301–305. doi: 10.1007/s12017-010-8135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- Heaton RK, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K, Tomiuk S, Wolff G, Stoffel W. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc Natl Acad Sci U S A. 2000;97:5895–5900. doi: 10.1073/pnas.97.11.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoozemans JJ, Rozemuller JM, van Haastert ES, Veerhuis R, Eikelenboom P. Cyclooxygenase-1 and -2 in the different stages of Alzheimer’s disease pathology. Curr Pharm Des. 2008;14:1419–1427. doi: 10.2174/138161208784480171. [DOI] [PubMed] [Google Scholar]
- Jana A, Pahan K. Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase. Implications for Alzheimer’s disease. J Biol Chem. 2004a;279:51451–51459. doi: 10.1074/jbc.M404635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K. Human immunodeficiency virus type 1 gp120 induces apoptosis in human primary neurons through redox-regulated activation of neutral sphingomyelinase. J Neurosci. 2004b;24:9531–9540. doi: 10.1523/JNEUROSCI.3085-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K. Oxidative stress kills human primary oligodendrocytes via neutral sphingomyelinase: implications for multiple sclerosis. J Neuroimmune Pharmacol. 2007;2:184–193. doi: 10.1007/s11481-007-9066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Hogan EL, Pahan K. Ceramide and neurodegeneration: susceptibility of neurons and oligodendrocytes to cell damage and death. J Neurol Sci. 2009;278:5–15. doi: 10.1016/j.jns.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaturapatporn D, Isaac MG, McCleery J, Tabet N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. The Cochrane database of systematic reviews. 2012;2:CD006378. doi: 10.1002/14651858.CD006378.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju TC, Chen SD, Liu CC, Yang DI. Protective effects of S-nitrosoglutathione against amyloid beta-peptide neurotoxicity. Free Radic Biol Med. 2005;38:938–949. doi: 10.1016/j.freeradbiomed.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60:234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus TM, Saag KG. Nonsteroidal anti-inflammatory drugs and cognitive function: do they have a beneficial or deleterious effect? Drug safety: an international journal of medical toxicology and drug experience. 1998;19:427–433. doi: 10.2165/00002018-199819060-00001. [DOI] [PubMed] [Google Scholar]
- Khanlou N, Moore DJ, Chana G, Cherner M, Lazzaretto D, Dawes S, Grant I, Masliah E, Everall IP. Increased frequency of alpha-synuclein in the substantia nigra in human immunodeficiency virus infection. J Neurovirol. 2009;15:131–138. doi: 10.1080/13550280802578075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Steelman AJ, Zhang Y, Kinney HC, Li J. Aberrant upregulation of astroglial ceramide potentiates oligodendrocyte injury. Brain Pathol. 22:41–57. doi: 10.1111/j.1750-3639.2011.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat Rev Cancer. 2005;5:886–897. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Wang S, Slone SR, Yacoubian TA, Witt SN. Defects in very long chain fatty acid synthesis enhance alpha-synuclein toxicity in a yeast model of Parkinson’s disease. PLoS One. 2011;6:e15946. doi: 10.1371/journal.pone.0015946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Castillo SS, Goldkorn T. nSMase2 activation and trafficking are modulated by oxidative stress to induce apoptosis. Biochem Biophys Res Commun. 2006;344:900–905. doi: 10.1016/j.bbrc.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hannun YA. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J Biol Chem. 1997;272:16281–16287. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- Lwin A, Orvisky E, Goker-Alpan O, LaMarca ME, Sidransky E. Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab. 2004;81:70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Malaplate-Armand C, Florent-Bechard S, Youssef I, Koziel V, Sponne I, Kriem B, Leininger-Muller B, Olivier JL, Oster T, Pillot T. Soluble oligomers of amyloid-beta peptide induce neuronal apoptosis by activating a cPLA2-dependent sphingomyelinase-ceramide pathway. Neurobiol Dis. 2006;23:178–189. doi: 10.1016/j.nbd.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Marker DF, Tremblay ME, Puccini JM, Barbieri J, Gantz Marker MA, Loweth CJ, Muly EC, Lu SM, Goodfellow VS, Dewhurst S, Gelbard HA. The new small-molecule mixed-lineage kinase 3 inhibitor URMC-099 is neuroprotective and anti-inflammatory in models of human immunodeficiency virus-associated neurocognitive disorders. J Neurosci. 2013;33:9998–10010. doi: 10.1523/JNEUROSCI.0598-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez TN, Chen X, Bandyopadhyay S, Merrill AH, Tansey MG. Ceramide sphingolipid signaling mediates Tumor Necrosis Factor (TNF)-dependent toxicity via caspase signaling in dopaminergic neurons. Mol Neurodegener. 2012;7:45. doi: 10.1186/1750-1326-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Nance-Sproson TE, Griffin DE, Hoover D, Selnes OA, Miller EN, Margolick JB, Cohen BA, Farzadegan H, Saah A. The diagnostic utility of elevation in cerebrospinal fluid beta 2-microglobulin in HIV-1 dementia. Multicenter AIDS Cohort Study. Neurology. 1992;42:1707–1712. doi: 10.1212/wnl.42.9.1707. [DOI] [PubMed] [Google Scholar]
- McMurtray A, Nakamoto B, Shikuma C, Valcour V. Cortical atrophy and white matter hyperintensities in HIV: the Hawaii Aging with HIV Cohort Study. J Stroke Cerebrovasc Dis. 2008;17:212–217. doi: 10.1016/j.jstrokecerebrovasdis.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencarelli C, Martinez-Martinez P. Ceramide function in the brain: when a slight tilt is enough. Cell Mol Life Sci. 2013;70:181–203. doi: 10.1007/s00018-012-1038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Haughey NJ, Ratnam Bandaru VV, Schech S, Carrick R, Carlson MC, Mori S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG. Plasma ceramides are altered in mild cognitive impairment and predict cognitive decline and hippocampal volume loss. Alzheimers Dement. 2010;6:378–385. doi: 10.1016/j.jalz.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S, Francis SJ, Sled JG, Santos AC, Antel S, Levesque I, Brass S, Lapierre Y, Sappey-Marinier D, Pike GB, Arnold DL. Axonal injury in the cerebral normal-appearing white matter of patients with multiple sclerosis is related to concurrent demyelination in lesions but not to concurrent demyelination in normal-appearing white matter. Neuroimage. 2006;29:637–642. doi: 10.1016/j.neuroimage.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Nguyen TP, Soukup VM, Gelman BB. Persistent hijacking of brain proteasomes in HIV-associated dementia. Am J Pathol. 2010;176:893–902. doi: 10.2353/ajpath.2010.090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon GF. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol. 2009;158:982–993. doi: 10.1111/j.1476-5381.2009.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, Yang DS, Lee JH. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy. 2008;4:590–599. doi: 10.4161/auto.6259. [DOI] [PubMed] [Google Scholar]
- Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Nature. 2003;422:302–307. doi: 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- Novgorodov SA, Gudz TI. Ceramide and mitochondria in ischemic brain injury. International journal of biochemistry and molecular biology. 2011;2:347–361. [PMC free article] [PubMed] [Google Scholar]
- Pelle MT, Bazille C, Gray F. Neuropathology and HIV dementia. Handb Clin Neurol. 2008;89:807–818. doi: 10.1016/S0072-9752(07)01270-5. [DOI] [PubMed] [Google Scholar]
- Portegies P, Godfried MH, Hintzen RQ, Stam J, van der Poll T, Bakker M, van Deventer SJ, van Lier RA, Goudsmit J. Low levels of specific T cell activation marker CD27 accompanied by elevated levels of markers for non-specific immune activation in the cerebrospinal fluid of patients with AIDS dementia complex. J Neuroimmunol. 1993;48:241–247. doi: 10.1016/0165-5728(93)90198-8. [DOI] [PubMed] [Google Scholar]
- Porwit A, Parravicini C, Petren AL, Barkhem T, Costanzi G, Josephs S, Biberfeld P. Cell association of HIV in AIDS-related encephalopathy and dementia. APMIS. 1989;97:79–90. doi: 10.1111/j.1699-0463.1989.tb00759.x. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J Biol Chem. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- Roberts TK, Eugenin EA, Morgello S, Clements JE, Zink MC, Berman JW. PrPC, the cellular isoform of the human prion protein, is a novel biomarker of HIV-associated neurocognitive impairment and mediates neuroinflammation. Am J Pathol. 2010;177:1848–1860. doi: 10.2353/ajpath.2010.091006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Saas P, Boucraut J, Quiquerez AL, Schnuriger V, Perrin G, Desplat-Jego S, Bernard D, Walker PR, Dietrich PY. CD95 (Fas/Apo-1) as a receptor governing astrocyte apoptotic or inflammatory responses: a key role in brain inflammation? J Immunol. 1999;162:2326–2333. [PubMed] [Google Scholar]
- Sachse M, Ramm G, Strous G, Klumperman J. Endosomes: multipurpose designs for integrating housekeeping and specialized tasks. Histochem Cell Biol. 2002;117:91–104. doi: 10.1007/s00418-001-0348-0. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Haughey N, Cutler R, Tamara A, Turchan J, Pardo C, Vargas D, Nath A. Novel markers of oxidative stress in actively progressive HIV dementia. J Neuroimmunol. 2004;157:176–184. doi: 10.1016/j.jneuroim.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Satoi H, Tomimoto H, Ohtani R, Kitano T, Kondo T, Watanabe M, Oka N, Akiguchi I, Furuya S, Hirabayashi Y, Okazaki T. Astroglial expression of ceramide in Alzheimer’s disease brains: a role during neuronal apoptosis. Neuroscience. 2005;130:657–666. doi: 10.1016/j.neuroscience.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Schwandner R, Wiegmann K, Bernardo K, Kreder D, Kronke M. TNF receptor death domain-associated proteins TRADD and FADD signal activation of acid sphingomyelinase. J Biol Chem. 1998;273:5916–5922. doi: 10.1074/jbc.273.10.5916. [DOI] [PubMed] [Google Scholar]
- Scott DB, Michailidis I, Mu Y, Logothetis D, Ehlers MD. Endocytosis and degradative sorting of NMDA receptors by conserved membrane-proximal signals. J Neurosci. 2004;24:7096–7109. doi: 10.1523/JNEUROSCI.0780-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, Schifitto G, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Cohen B, Epstein LG, Marder K. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–2090. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- Shen D, Wang X, Li X, Zhang X, Yao Z, Dibble S, Dong XP, Yu T, Lieberman AP, Showalter HD, Xu H. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I, Pahan K, Khan M, Singh AK. Cytokine-mediated induction of ceramide production is redox-sensitive. Implications to proinflammatory cytokine-mediated apoptosis in demyelinating diseases. J Biol Chem. 1998;273:20354–20362. doi: 10.1074/jbc.273.32.20354. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Uz T, Kumar V, Manev H. New anti-inflammatory treatment strategy in Alzheimer’s disease. Japanese journal of pharmacology. 2000;82:85–94. doi: 10.1254/jjp.82.85. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liou B, Ran H, Skelton MR, Williams MT, Vorhees CV, Kitatani K, Hannun YA, Witte DP, Xu YH, Grabowski GA. Neuronopathic Gaucher disease in the mouse: viable combined selective saposin C deficiency and mutant glucocerebrosidase (V394L) mice with glucosylsphingosine and glucosylceramide accumulation and progressive neurological deficits. Hum Mol Genet. 19:1088–1097. doi: 10.1093/hmg/ddp580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Savonenko A, Song H, Bandaru VV, Chu M, Haughey NJ. Inhibition of neutral sphingomyelinase-2 perturbs brain sphingolipid balance and spatial memory in mice. J Neurosci Res. 2010;88:2940–2951. doi: 10.1002/jnr.22438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiuk S, Hofmann K, Nix M, Zumbansen M, Stoffel W. Cloned mammalian neutral sphingomyelinase: functions in sphingolipid signaling? Proc Natl Acad Sci U S A. 1998;95:3638–3643. doi: 10.1073/pnas.95.7.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- Tsakiri N, Kimber I, Rothwell NJ, Pinteaux E. Interleukin-1-induced interleukin-6 synthesis is mediated by the neutral sphingomyelinase/Src kinase pathway in neurones. Br J Pharmacol. 2008;153:775–783. doi: 10.1038/sj.bjp.0707610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Valcour V, Watters MR, Williams AE, Sacktor N, McMurtray A, Shikuma C. Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. J Neurovirol. 2008;14:362–367. doi: 10.1080/13550280802216494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn R, Nijland PG, Dekker N, Witte ME, Lopes-Pinheiro MA, van het Hof B, Kooij G, Reijerkerk A, Dijkstra C, van van der Valk P, van Horssen J, de Vries HE. Fingolimod attenuates ceramide-induced blood-brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes. Acta Neuropathol. 124:397–410. doi: 10.1007/s00401-012-1014-4. [DOI] [PubMed] [Google Scholar]
- van Echten-Deckert G, Walter J. Sphingolipids: critical players in Alzheimer’s disease. Prog Lipid Res. 2012;51:378–393. doi: 10.1016/j.plipres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Visnjic D, Batinic D, Banfic H. Different roles of protein kinase C alpha and delta isoforms in the regulation of neutral sphingomyelinase activity in HL-60 cells. Biochem J. 1999;344(Pt 3):921–928. doi: 10.1042/0264-6021:3440921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl SM, Allen JB, McCartney-Francis N, Morganti-Kossmann MC, Kossmann T, Ellingsworth L, Mai UE, Mergenhagen SE, Orenstein JM. Macrophage- and astrocyte-derived transforming growth factor beta as a mediator of central nervous system dysfunction in acquired immune deficiency syndrome. J Exp Med. 1991;173:981–991. doi: 10.1084/jem.173.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Liu XB, Jones EG, McAllister AK. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J Neurosci. 2004;24:8253–8264. doi: 10.1523/JNEUROSCI.2555-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed M, Adams RJ, Hienz RD, Meulendyke KA, Linde ME, Clements JE, Mankowski JL, Zink MC. SIV/macaque model of HIV infection in cocaine users: minimal effects of cocaine on behavior, virus replication, and CNS inflammation. J Neuroimmune Pharmacol. 2012;7:401–411. doi: 10.1007/s11481-011-9281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur JC, Farber JM, Griffin JW, Griffin DE. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993;33:576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- Wheeler D, Bandaru VV, Calabresi PA, Nath A, Haughey NJ. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 2008;131:3092–3102. doi: 10.1093/brain/awn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D, Knapp E, Bandaru VV, Wang Y, Knorr D, Poirier C, Mattson MP, Geiger JD, Haughey NJ. Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J Neurochem. 2009;109:1237–1249. doi: 10.1111/j.1471-4159.2009.06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigdahl B, Kunsch C. Role of HIV in human nervous system dysfunction. AIDS Res Hum Retroviruses. 1989;5:369–374. doi: 10.1089/aid.1989.5.369. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Achim CL, Schrier RD, Heyes MP, McCutchan JA, Grant I. Relationship of cerebrospinal fluid immune activation associated factors to HIV encephalitis. Aids. 1992;6:1299–1307. doi: 10.1097/00002030-199211000-00010. [DOI] [PubMed] [Google Scholar]
- Worgall TS. Sphingolipid synthetic pathways are major regulators of lipid homeostasis. Adv Exp Med Biol. 2011;721:139–148. doi: 10.1007/978-1-4614-0650-1_9. [DOI] [PubMed] [Google Scholar]
- Xu H, Bae M, Tovar YRLB, Patel N, Bandaru VV, Pomerantz D, Steiner JP, Haughey NJ. The Human Immunodeficiency Virus Coat Protein gp120 Promotes Forward Trafficking and Surface Clustering of NMDA Receptors in Membrane Microdomains. J Neurosci. 2011;31:17074–17090. doi: 10.1523/JNEUROSCI.4072-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz A, Yiannoutsos CT, Fuchs D, Price RW, Crozier K, Hagberg L, Spudich S, Gisslen M. Cerebrospinal fluid neopterin decay characteristics after initiation of antiretroviral therapy. J Neuroinflammation. 2013;10:62. doi: 10.1186/1742-2094-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li X, Becker KA, Gulbins E. Ceramide-enriched membrane domains--structure and function. Biochim Biophys Acta. 2009;1788:178–183. doi: 10.1016/j.bbamem.2008.07.030. [DOI] [PubMed] [Google Scholar]