Abstract
Gait pattern classification may assist in clinical decision making and cluster analysis (CA) has been often adopted to this aim. The goal of this study was to identify, through CA, typical walking patterns in a group of 21 young subjects with CMT1A, a hereditary progressive neuropathy, and to study possible correlation with the disease’s clinical status. The protocol included kinematic/kinetic analysis of natural walking and more demanding locomotor tasks, i.e. toe- and heel-walking. Hierarchical cluster analysis was carried out on parameters related to primary signs (foot-drop and push-off deficit) and, separately, to compensatory mechanisms at proximal (pelvis, hip and knee) or distal (ankle) level.
CA on primary signs during natural walking identified three clusters: (1) pseudo-normal patients (PN), not significantly different from controls; (2) patients showing only foot-drop (FD); (3) patients with foot-drop and push-off deficit (FD&POD). Patients belonging to the PN subgroup showed distal abnormalities during heel-walking. The FD&POD subgroup was associated to a significantly worse clinical score (CMTES, p < 0.05). The main compensatory strategies, which occurred independently from primary clusterization, included augmented hip/knee flexion in swing (steppage) and early ankle plantarflexion at mid stance (vaulting). We concluded that, although a number of young CMT1A patients do not show typical primary deviations during natural walking, they do show significant abnormalities in more demanding locomotor tasks that should be therefore considered. It is also hypothesized that progression of this degenerative condition may be associated to the migration of patients to more severe clusters, with possible appearance of compensatory strategies.
Keywords: Gait analysis, Charcot–Marie–Tooth disease, Gait patterns, Cluster analysis, Toe walking, Heel walking
1. Introduction
Charcot–Marie–Tooth disease (CMT) is a heterogeneous group of hereditary neuropathies with an estimated prevalence of 17–40/100,000 [1]. It is characterized by length-dependent degeneration of the motor and sensory fibres with consequent weakness of distal limb muscles, distal sensory loss and frequent skeletal deformities [2]. The most common form (40–50% of all CMT cases) is CMT type 1A (CMT1A), associated with a duplication of chromosome 17p12 involving the peripheral myelin protein-22 (PMP22) gene. Motor and sensory dysfunction usually progress centripetally, starting from the intrinsic foot muscles and then spreading to the leg muscles, thus affecting locomotor function. Symptoms often start in childhood and the disease then follows a slowly progressive disabling course, although there is a large variability in severity.
The diversity of gait deviation stimulated the development of gait classification approaches, based on instrumented gait analysis (GA) data, to assist in diagnosis, clinical decision-making and communication [7]. Looking at GA literature, it appears that efforts have been mainly devoted to gait classification in cerebral palsy (CP) [7] and post-stroke hemiplegia [3].
Don et al. [4] already provided a gait classification of subjects with CMT based upon clinical examination: in their sample of 21 CMT patients, they found two distinctive gait patterns when considering two subgroups characterized by only foot-drop (FD) or foot-drop associated to plantarflexor muscles failure (FD&PFF). GA identified distinct abnormalities characterized by foot-drop during the swing phase of walking and a consequent compensatory increase of hip and knee flexion (“steppage pattern”) for the FD group. It also identified a marked decrease of plantar flexion at push-off with a compensatory increase of hip abduction and pelvic elevation on the swing side for FD&PFF group. However, a classification should allow the allocation of gait patterns into groups differentiated on the basis of a set of defined GA variables [7] and not through an “a priori” clinical classification. Moreover, in previous literature on GA applied to CMT, there was lack of homogeneity with respect to age and disease type [4,5] and the correlation of gait classification with clinical measures has not been addressed.
Several methods for quantitative gait data classification have been proposed [6]. A frequently used one is cluster analysis (CA), a global definition for a set of different mathematical algorithms which allow identification of homogeneous groups (clusters) in a given data-set. Review of the literature shows a very heterogeneous framework [7], with many CA approaches: Kinsella et al. [17] and Toro et al. [8] used hierarchical CA to identify gait patterns in stroke and CP patients; Giacomozzi et al. [9] and Rozumalski et al. [18] used k-means CA, applied to rheumatoid arthritis and CP patients; Fong-Chin et al. [10] studied subjects with ankle arthrodesis using fuzzy CA. Some authors used whole curves as CA data-set [9,10,18]; others selected specific kinematic and/or kinetic parameters [8,17]. It is still unclear which approach would offer best results, as Dobson [7] pointed out.
The goal of the present work was therefore to study the classification of gait in young patients with CMT1A, through hierarchical CA, and to correlate this classification with the patients’ clinical status. Since the disease in unlikely to significantly affect gait in its early stages, we considered also more demanding tasks such as toe- and heel-walking [11].
2. Methods
2.1. Subjects
Twenty-one young subjects with CMT1A participated to the study (9 females, 12 males; mean ± SD: age 11.9 ± 2.8 years; body mass 44.5 ± 14.4 kg; height 152 ± 16 cm; CMTES1 4.4 ± 2.4; time from genetic diagnosis 3.1 ± 2.3 years; time from first clinical symptoms: 7.3 ± 3.9 years). Inclusion criteria were age < 18 years, diagnosis of CMT1A based on clinical and genetic criteria. Exclusion criteria were presence of other neurological diseases or unrelated clinical conditions affecting locomotor functions; inability to walk unaided barefoot; previous double or triple arthrodesis at the ankle limiting joint range of motion (ROM). Eighteen healthy age-matched subjects formed the control group (9 females, 9 males; mean ± SD: age 11.0 ± 3.3 years; body mass 41.4 ± 14.3 kg; height 146 ± 22 cm).
All subjects gave informed consent and the protocol was approved by the local Ethical Committee.
2.2. Instrumentation, protocol and data analysis
Kinematic data were collected using a 9-camera SMART-E motion capture system (BTS, Milano, Italy) sampling at 60 Hz; two consecutive force plates (Kistler, Winterthur, Switzerland), with 960 Hz sampling frequency, provided ground reaction forces (GRFs).
The total-body LAMB marker set was adopted, which included 29 retroreflective markers (12 mm diameter) positioned on the head, upper limbs, trunk, pelvis and lower limbs [20].
CMT1A subjects were asked to perform 5 trials at their natural speed (task NW); controls performed 15 trials at variable speed. Trials with speed not exceeding the global speed range of patients were selected for the analysis. Additional tasks included toe-walking (TW) and heel-walking (HW), performed at self-selected speed and with an effort to maximise the lift of the heel or toe from the ground during walking.
After acquisition, the markers’ coordinates were low-pass filtered at a cut-off frequency of 6 Hz. Anthropometric parameters of each subject were computed from markers’ positions recorded during the calibration trial, and used for estimation of internal joint centers. These, in turn, enabled calculation of trunk, pelvis and lower limb kinematics [16] during the locomotor tasks. Inverse dynamics was used to compute moments and powers at the ankle, knee and hip joints. Kinematic and kinetic data were computed in sagittal, frontal and horizontal anatomical planes; they were time-normalized as a percentage of the whole stride duration starting with a foot strike. Specific values were selected for each variable, according to their significance and relation to specific clinical signs. Each parameter was calculated as the average of five trials.
2.3. Cluster analysis: parameters
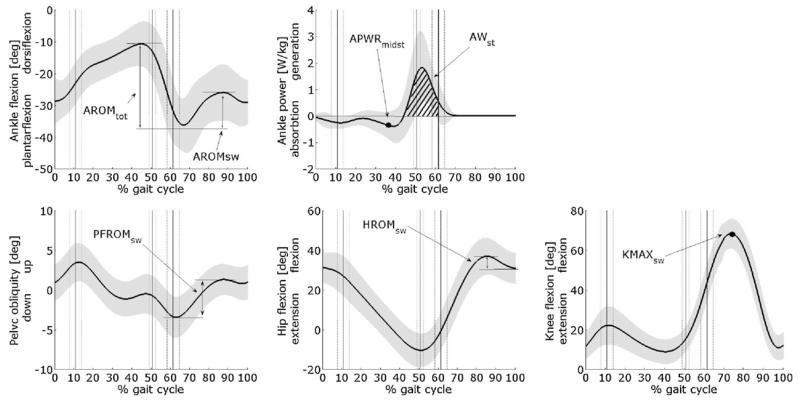
Cluster analysis on a target set of parameters extracted from the natural walking task was performed in order to determine homogeneous subgroups among patients. Two separate runs were carried out: a first one on parameters related to the disease-specific deficits (called “primary gait deviation”), and a second one on parameters connected to possible compensatory mechanisms. The first set of parameters included AROMratio and AWst (see Fig. 1). AROMratio, calculated as the ankle dorsiflexion ROM during swing phase divided by the ROM over the whole gait cycle, is related to foot-drop deficit and is expected to be larger in patients than in healthy subjects. AWst is the positive ankle work (calculated as integral of power curve) in stance phase, thus accounting for the push-off mechanism. The second set of parameters contains (Fig. 1) PFROMsw, calculated as the pelvic frontal ROM during swing, HROMsw, calculated as the peak hip flexion angle during swing minus hip angle at foot-strike, KMAXsw, calculated as the peak knee flexion angle in swing phase and APWRmidst, calculated as the value of ankle power curve at the time instant when controlateral toe passes the ipsilateral malleolus. These parameters, respectively, account for possible compensatory mechanisms at pelvis, hip, knee and ankle level to avoid foot drag: the last one, in particular, allows to detect an anticipated ankle plantarflexion to improve controlateral foot clearance (called “vaulting gait” in literature [12]). All these parameters showed good reliability in CMT patients [13].
Fig. 1.
Graphical description of the parameters selected for cluster analysis. Parameters related to primary signs: AROMratio = AROMsw/AROMtot, AWst; parameters related to possible compensatory mechanisms: PFROMsw, HROMsw, KMAXsw, and APWRmidst.
Statistical analysis included (i) a T-test to compare the whole CMT1A group vs controls and (ii) an ANOVA and Tukey HSD post hoc for the comparison between clusters and controls. The significance level was set at 0.05.
2.4. Cluster analysis: technique
Ahierarchical cluster analysis technique was applied to the CMT1A group: Euclidean distance was chosen as metric, and the Ward linkage method was adopted [17]. The parameters were standardized by subtracting the means and dividing by standard deviations. The optimal number of clusters was quantitatively determined by calculation of the agglomeration coefficient while increasing the number of clusters from 1 to 21 and adopting the “stopping rule” [17] (a large percentage decrease in the coefficient followed by a plateau) [21]. This choice was then confirmed by visually inspecting the dendrogram. Thus, no a priori hypotheses were formulated on the number of possible gait patterns. The gait patterns obtained by the clustering algorithm were interpreted by the clinical perspective, considering the healthy controls’ pattern as reference.
3. Results
Young subjects with CMT1A showed, overall, significant foot-drop, reduced push-off, increased ROM at hip joint and increased peak knee flexion in swing. However, by inspecting individual kinematic and kinetic patterns, some variability between subjects was observed.
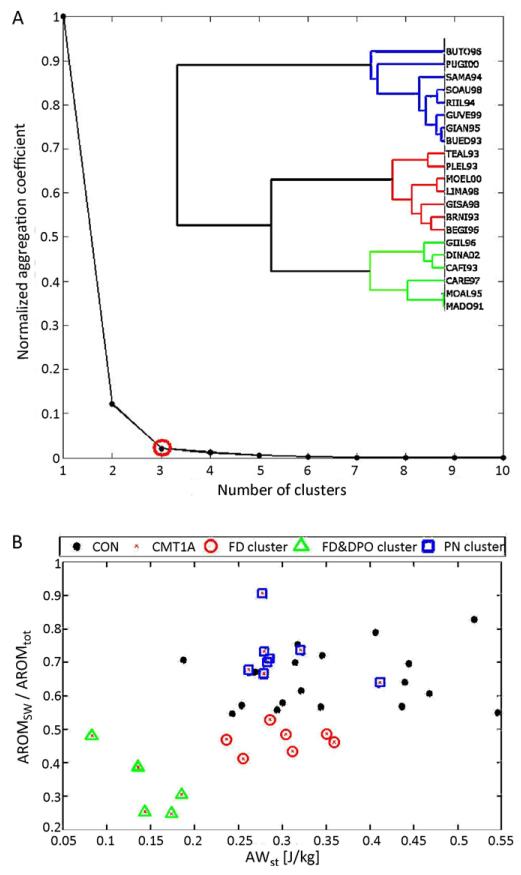
The optimal number of clusters for CA on parameters related to primary gait deviations during natural walking was 3 (see Fig. 2A). Cluster 1 included 8 CMT1A patients without primary gait deviations that we called pseudo-normal (PN) group, since they were not significantly different from the group of healthy controls. Cluster 2 included 7 patients with foot-drop (called FD group) and cluster 3 included the remaining 6 CMT1A patients who presented both a foot-drop and a push-off deficit (named FD&POD group).Fig. 2B shows, the distribution of these clusters superimposed to the group of healthy controls in the plane of primary parameters’ values (AROMratio, AWst). In Table 1 the mean (SD) values of some gait parameters for the control group, the whole CMT1A group and the three clusters are reported, together with statistically significant differences.
Fig. 2.
Results of cluster analysis based on primary gait deviations. (A) Dendogram and normalized aggregation coefficient vs number of clusters. (B) Representation of CMT1A patients’ clusters in the primary parameters’ plane: PN, pseudo-normal CMT1A patients (squares); FD, CMT1A patients with foot-drop (circles); FD&POD, CMT1A patients with foot-drop and push-off deficit (triangles). Healthy controls are reported as reference (filled circle).
Table 1.
Mean (SD) of some clinical data and kinematic and kinetic parameters related to natural walking for control subjects and different CMT1A primary clusters. Shaded cells mark the parameters used for CA on primary gait deviations. The last three rows report the number of CMT1A subjects in each cluster related to compensatory mechanisms (NoC: no compensatory mechanisms; HKC: hip and/or knee hyperflexion in swing–steppage gait; VG: early ankle plantarflexion in mid stance–vaulting gait).
| Parameter | Controls | CMT1A (all) | PN-CMT1A | FD-CMT1A | FD&DPO-CMT1A |
|---|---|---|---|---|---|
| N of subjects | 18 | 21 | 8 | 7 | 6 |
| Age [years] | 11.0 (3.3) | 12.0 (2.9) | 11.6 (2.6) | 12.0 (2.9) | 12.3 (3.5) |
| CMTES | - | 4.4 (2.4) | 3.1 (1.4) | 4.0 (2.4) | 6.7 (2.0)b |
| Tdiagnosis [years] | - | 3.1 (2.3) | 3.3 (1.9) | 2.7 (3.0) | 3.2 (2.2) |
| Tsymptoms [years] | - | 7.3 (3.9) | 5.3 (3.7) | 8.4 (3.9) | 8.1 (3.9) |
| Gait speed/BH [%BHs−1] | 77 (7) | 76(11) | 77 (13) | 79 (9) | 69 (9)a |
| Cadence [stepsmin−1] | 116(11) | 116 (9) | 113 (13) | 117 (6) | 113 (9) |
| Stride length/BH [%] | 79.8 (4.1) | 78.2 (7.4) | 80.4 (6.4) | 81.0 (5.2) | 73.5 (8.0)a |
| AROMsw/AROMtot | 0.65 (0.09) | 0.53 (0.18)* | 0.72 (0.08) | 0.47 (0.04)a,b | 0.34 (0.9)a,b |
| AWst [J/kg] | 0.36 (0.10) | 0.26 (0.08)* | 0.30 (0.05) | 0.30 (0.05) | 0.14 (0.04)a |
| HROMtot [deg] | 42.1 (3.7) | 48.9 (6.3)* | 48.1 (6.3) | 47.4 (5.7) | 51.8 (7.0)a |
| KROMtot [deg] | 59.7 (5.0) | 67.8 (6.4) | 64.7 (5.7) | 63.3 (7.0) | 59.7 (6.5) |
| AROMtot [deg] | 32.5 (6.7) | 25.8 (5.7)* | 28.0 (7.8) | 26.3 (3.8) | 22.4 (2.1)a |
| N of CMT1A subjects in NoC group | - | 12 | 5 | 3 | 4 |
| N of CMT1A subjects in HKC group | - | 6 | 1 | 3 | 2 |
| N of CMT1A subjects in VG group | - | 3 | 2 | 1 | 0 |
Significant differences (p < 0.05 ANOVA and Tukey HSD post hoc) between CMT1A clusters and controls.
Significant differences (p < 0.05 ANOVA and Tukey HSD post hoc) between FD or FD&DPO cluster and PN cluster.
Significant differences between CMT1A and controls (p < 0.05 T-test).
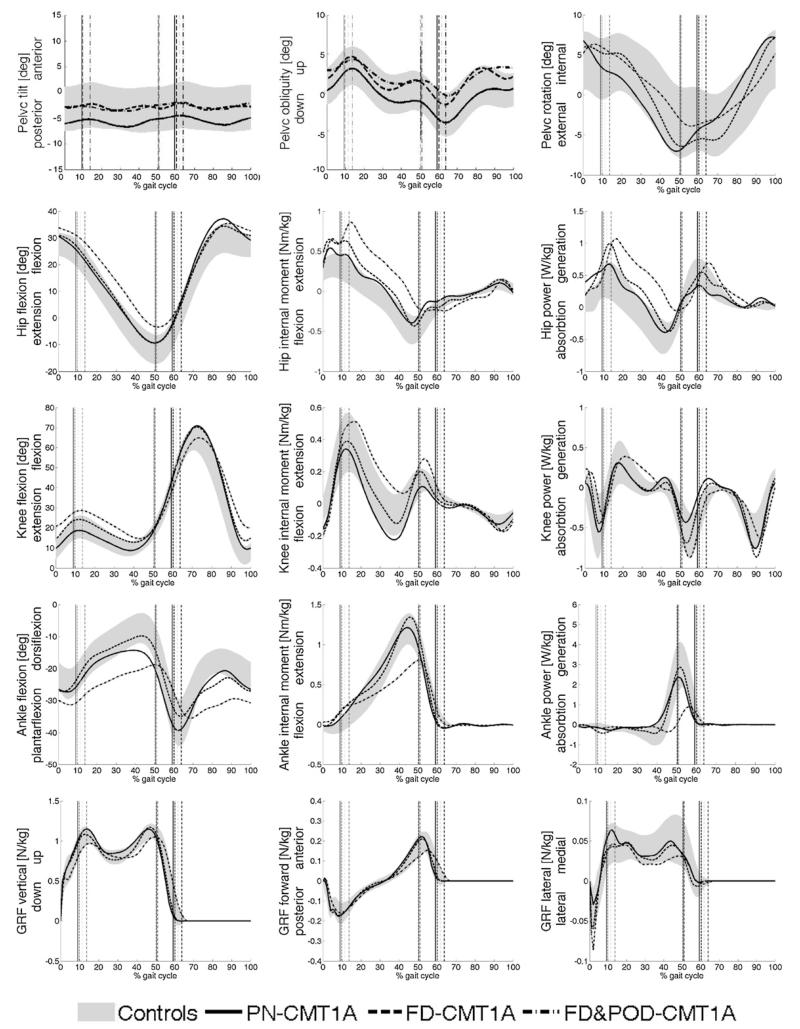
In Fig. 3 the time course of the main kinematic and dynamic variables of natural walking are reported for the three CMT1A subgroups, superimposed to the range (±1SD) of controls. It is to note that FD&POD patients, in addition to primary gait deviations, showed also a considerable increase of hip extensor moment and power production during stance, attributable to a greater activation of hip extensor muscles to counterbalance the ankle push-off deficit.
Fig. 3.
Kinematics and kinetics of the control group (grey area, mean ± 1SD) and three CMT1A clusters based on primary gait deviations. PN: pseudo-normal; FD: foot-drop; FD&POD: foot-drop and push-off deficit.
The optimal number of clusters for CA on parameters related to compensatory mechanisms was 6 and those clusters could be grouped, a posteriori, into 3 main clinically significant clusters: the first (including 12 subjects) did not show any important compensatory mechanisms (NoC group), the second (6 subjects) presented an increase of hip and/or knee peak flexion during swing (HKC group), the third (3 subjects) presented an early ankle plantarflexion (VG group), associated to an increase of plantar-flexion internal moment and ankle power production at mid stance (see electronic addendum). The latter two clusters can be associated, respectively, to the “steppage gait” and “vaulting gait” described in literature to improve foot clearance. Interestingly, the compensation clusters resulted independent from the primary clusterization, as shown by the stochastic distribution of number of subjects with different secondary signs in each primary cluster reported in the last rows of Table 1 (χ2 test on observed vs expected frequencies, p = 0.39).
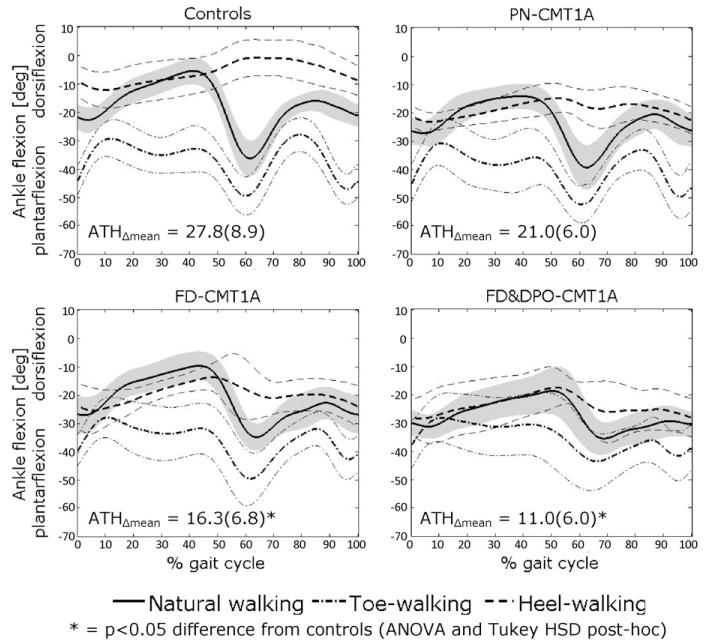
Concerning toe- and heel-walking trials, most CMT1A patients presented abnormal patterns, even those belonging to the pseudo-normal cluster. As an example, Fig. 4 shows the time course of ankle flexion-extension angle during normal walking, heel-walking and toe-walking for the healthy control group, PN, DP and DP&POD clusters of CMT1A. It is apparent that, although the ankle pattern of PN patients during natural walking was very similar to that of controls, during heel-walking it was considerably less dorsiflexed. Abnormalities in demanding tasks were more marked in FD and FD&POD clusters, which showed also a less plantarflexed toe-walking than controls and, therefore, a reduced offset between ankle patterns in toe- and heel-walking. The above behavior is numerically described by the difference between the mean ankle angle of toe- and heel-walking (ATHΔmean), which was smaller in all CMT1A clusters than in controls, although statistical significance at Tukey HSD post hoc was achieved only for FD and FD&POD clusters (see values reported in Fig. 4).
Fig. 4.
Pattern of ankle flexion-extension angle during natural walking, heel-walking and toe-walking for the healthy control group (CON) and three CMT1A clusters based on primary gait deviations. Mean ± 1SD band is shown.
4. Discussion
Instrumented gait analysis provides reliable data for gait pattern classification. This may assist in clinical decision making and in following up patient functional status. Cluster analysis is an objective and quantitative approach for pattern classification of gait data [8-10,17,18].
Although some papers have been published on CMT gait analysis [4,5,14,15], a classification of gait patterns based on cluster analysis, has not been previously performed. Don et al. [4], in particular, could not detect a reliable gait pattern when the whole group of 21 patients with different types of CMT was considered. They identified two different patterns for different combinations of – clinically defined – foot drop and plantar flexion failure. Along the same line, in the present paper, focused on young subjects with CMT1A, we considered two different gait classifications, based, respectively, on disease-specific primary gait deviations and possible compensatory mechanisms at pelvis, hip, knee and ankle level.
We chose to use hierarchical CA since it can both provide a graphic representation (dendrogram) and a quantitative index (agglomeration coefficient) about the data aggregation, useful in the critic point of determining the optimal number of clusters. We chose two sets of parameters for CA, one related to disease-specific primary deficits, the other to compensatory mechanisms: the choice was based on clinical appropriateness (i.e. the parameters should have a clear clinical meaning) and on good reliability.
Results showed that our group of young CMT1A patients, overall, walked at similar speed, cadence and stride length as the control group (Table 1). However, they showed, in accordance with Newman et al. [5], significant differences both at distal (ankle) and proximal (knee, hip) level, with a quite large intersubject variability. Considering the subgroups obtained by the cluster analysis based on primary gait deviations, we found significant abnormalities in natural walking, compared to controls, only in clusters 2 and 3 (which showed, respectively, foot-drop alone and foot-drop associated to push-off deficit). Conversely, cluster 1 did not show any significant difference to the controls, and was defined as a pseudo-normal subgroup. The presence of a significant number of “asymptomatic walkers” (38% of the whole CMT1A group) has not been previously described. This may be attributed to the young age of our subjects and their consequent mild to moderate disease severity. In this subgroup, however, reduced dorsiflexion was observed during heel-walking, compared to healthy controls. This task is therefore appropriate to provide sensitive indexes to evaluate patients at an early stage.
Age, gender, time from genetic diagnosis and time from first clinical symptoms were not significantly different among clusters (although Tsymptoms was slightly reduced in PN patients) (see Table 1). Contrarily, cluster 3 was associated to a significantly more advanced stage of the disease, according to CMTES score, than cluster 1. Cluster 2 presented with an intermediate level of severity. This result suggests that, along with disease progression, CMT subjects could progressively migrate from cluster 1 to cluster 2. Subsequently, when the calf muscles are also involved, the push-off deficit appears too and patients would shift to cluster 3. Proximal compensatory mechanisms would also alter during the progression from cluster 1 to 3, as discussed above.
Results from more demanding tasks support this view: a progressive reduction of the offset between the ankle patterns in toe- and heel-walking is shown from PN to FD to FD&POD clusters (see Fig. 4), with an involvement of dorsiflexors first (reduced dorsiflexion in heel-walking of PN patients) followed by plantar flexors (reduced plantarflexion in toe-walking of FD and especially of FD&POD patients). To confirm this hypothesis, a longitudinal natural history study should be performed on a cohort of subjects with CMT, to follow changes in their gait pattern over time.
With regard to cluster analysis on compensatory mechanisms, the results indicated that the largest cluster was that of patients without significant secondary gait deviations. The rest of the patients presented mainly excessive hip and/or knee flexion or vaulting gait. The young age of our patients may again explain the large number of subjects not showing such compensatory mechanisms. Only 2 subjects presented the pelvic elevation described by Don et al. [4] in about half of their sample. This might be due to their different approach, based on a clinical “a priori” patient classification into two clusters, or to the older age, and thus different clinical status, of their patients.
It is interesting to note that three patients from the PN group, presented compensatory mechanisms, i.e. vaulting or steppage gait. This does not necessarily contradict our earlier discussion. Although they did not show primary gait deviations during natural walking, they did so during more demanding tasks. Compensatory actions have indeed been documented at an early stage of other diseases affecting the locomotor system, when gait alterations are not yet visually detectable [16].
A large sample is generally considered a critical factor for the application of cluster analysis. Despite the relatively small number of patients included in the study, it was possible to identify clinically significant clusters that could be used to monitor the functional progression of the disease. Nevertheless, to strengthen the findings reported here and to extend their applicability to a broader range of patients, it would be desirable to consider a larger sample of CMT subjects, including adults and more severely affected patients, followed-up in a longitudinal study.
Supplementary Material
Acknowledgements
The financial support of the Italian Ministry of Health (Ricerca Finalizzata RF – FCG – 2007-666049) and of Telethon-Italy (GUP10010) is gratefully acknowledged.
The authors would like to thank Dr.Eng. Serena Frittoli for her help in processing part of the gait analysis data.
Footnotes
CMTES (CMT Examination Score) is the clinical component of the CMT Neuropathy Score (CMTNS), a validated composite scale for CMT [19], and has a score ranging between 0 (normal) and 28 (worst).
Conflict of interest statement
None.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.gaitpost.2011.08.023.
References
- [1].Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62(April (4)):310–8. doi: 10.1136/jnnp.62.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pareyson D, Marchesi C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol. 2009;8(July (7)):654–67. doi: 10.1016/S1474-4422(09)70110-3. [DOI] [PubMed] [Google Scholar]
- [3].Kaczmarczyk K, Wit A, Krawczyk M, Zaborski J. Gait classification in post-stroke patients using artificial neural networks. Gait Posture. 2009;30(August (2)):207–10. doi: 10.1016/j.gaitpost.2009.04.010. [DOI] [PubMed] [Google Scholar]
- [4].Don R, Serrao M, Vinci P, Ranavolo A, Cacchio A, Ioppolo F, et al. Foot drop and plantar flexion failure determine different gait strategies in Charcot-Marie-Tooth patients. Clin Biomech (Bristol Avon) 2007;22:905–16. doi: 10.1016/j.clinbiomech.2007.06.002. [DOI] [PubMed] [Google Scholar]
- [5].Newman CJ, Walsh M, O’Sullivan R, Jenkinson A, Bennett D, Lynch B, et al. The characteristics of gait in Charcot-Marie-Tooth disease types I and II. Gait Posture. 2007;26:120–7. doi: 10.1016/j.gaitpost.2006.08.006. [DOI] [PubMed] [Google Scholar]
- [6].Chau T. A review of analytical techniques for gait data. Part 1. Fuzzy, statistical and fractal methods. Gait Posture. 2001;13(February (1)):49–66. doi: 10.1016/s0966-6362(00)00094-1. [DOI] [PubMed] [Google Scholar]
- [7].Dobson F, Morris ME, Baker R, Graham HK. Gait classification in children with cerebral palsy: a systematic review. Gait Posture. 2007;25(January (1)):140–52. doi: 10.1016/j.gaitpost.2006.01.003. [DOI] [PubMed] [Google Scholar]
- [8].Toro B, Nester CJ, Farren PC. Cluster analysis for the extraction of sagittal gait patterns in children with cerebral palsy. Gait Posture. 2007;25:157–65. doi: 10.1016/j.gaitpost.2006.02.004. [DOI] [PubMed] [Google Scholar]
- [9].Giacomozzi C, Martelli F, Nagel A, Schmiegel A, Rosenbaum D. Cluster analysis to classify gait alterations in rheumatoid arthritis using peak pressure curves. Gait Posture. 2009;29:220–4. doi: 10.1016/j.gaitpost.2008.08.004. [DOI] [PubMed] [Google Scholar]
- [10].Fong-Chin S, Wen-Lan W, Yuh-Min C, You-Li C. Fuzzy clustering of gait patterns of patients after ankle arthrodesis based on kinematic parameters. Med Eng Phys. 2001:23–83. doi: 10.1016/s1350-4533(01)00020-0. [DOI] [PubMed] [Google Scholar]
- [11].Bovi G, Rabuffetti M, Mazzoleni P, Ferrarin M. A multiple-task gait analysis approach: kinematic, kinetic and EMG reference data for healthy young and adult subjects. Gait Posture. 2011;33(Jan (1)):6–13. doi: 10.1016/j.gaitpost.2010.08.009. [DOI] [PubMed] [Google Scholar]
- [12].Chantraine F, Detrembleur C, Lejeune TM. Effect of the rectus femoris motor branch block on post-stroke stiff-legged gait. Acta Neurol Belg. 2005;105(Sep-tember (3)):171–7. [PubMed] [Google Scholar]
- [13].Ferrarin M, Bovi G, Rabuffetti M, Mazzoleni P, Montesano A, Moroni I, Pagliano E, Marchi A, Marchesi C, Beghi E, Pareyson D. Reliability of instrumented movement analysis as outcome measure in Charcot-Marie-Tooth disease: results from a multitask locomotor protocol. Gait Posture. 2011;34(May (1)):36–43. doi: 10.1016/j.gaitpost.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kuruvilla A, Costa JL, Wright RB, Yoder DM, Andriacchi TP. Characterization of gait parameters in patients with Charcot-Marie-Tooth disease. Neurol India. 2000;48(March (1)):49–55. [PubMed] [Google Scholar]
- [15].Vinci P, Paoloni M, Ioppolo F, Gargiulo P, Santilli V. Gait analysis in a patient with severe Charcot-Marie-Tooth disease: a case study with a new orthotic device for footdrop. Eur J Phys Rehabil Med. 2010;46(September (3)):355–61. [PubMed] [Google Scholar]
- [16].Watelain E, Dujardin F, Babier F, Dubois D, Allard P. Pelvic and lower limb compensatory actions of subjects in an early stage of hip osteoarthritis. Arch Phys Med Rehabil. 2001;82(December (12))):1705–11. doi: 10.1053/apmr.2001.26812. [DOI] [PubMed] [Google Scholar]
- [17].Kinsella S, Moran K. Gait pattern categorization of stroke participants with equines deformity of the foot. Gait Posture. 2008;27:144–51. doi: 10.1016/j.gaitpost.2007.03.008. [DOI] [PubMed] [Google Scholar]
- [18].Rozumalski A, Schwartz M. Crouch gait patterns defined using k-means cluster analysis are related to underlying clinical pathology. Gait Posture. 2009;30:155–60. doi: 10.1016/j.gaitpost.2009.05.010. [DOI] [PubMed] [Google Scholar]
- [19].Shy ME, Blake J, Krajewski K, Fuerst DR, Laura M, Hahn AF, et al. Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology. 2005;64(April (7))):1209–14. doi: 10.1212/01.WNL.0000156517.00615.A3. [DOI] [PubMed] [Google Scholar]
- [20].Rabuffetti M, Crenna P. A modular protocol for the analysis of movement in children. Gait Posture. 2004;20:S77–8. [Google Scholar]
- [21].Hair JF, Anderson RE, Tatham RL, Black WC. Multivariate data analysis. New Jersey Prentice-Hall; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.