Abstract
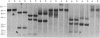
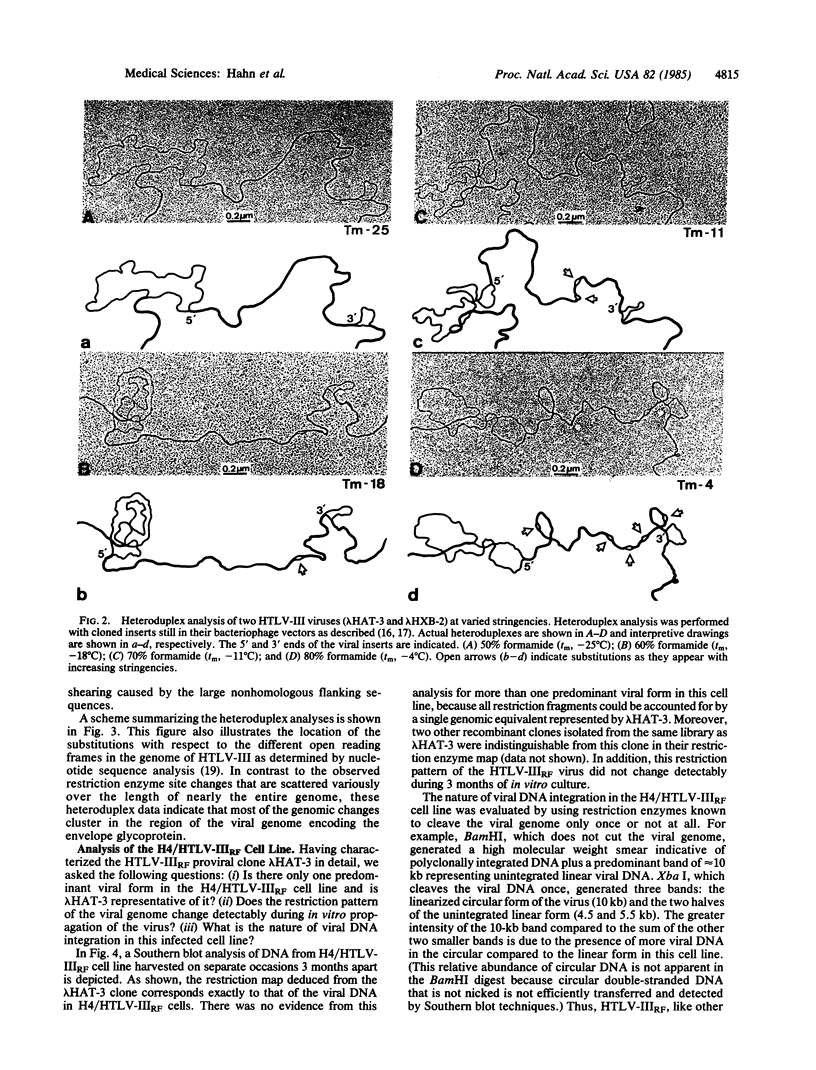
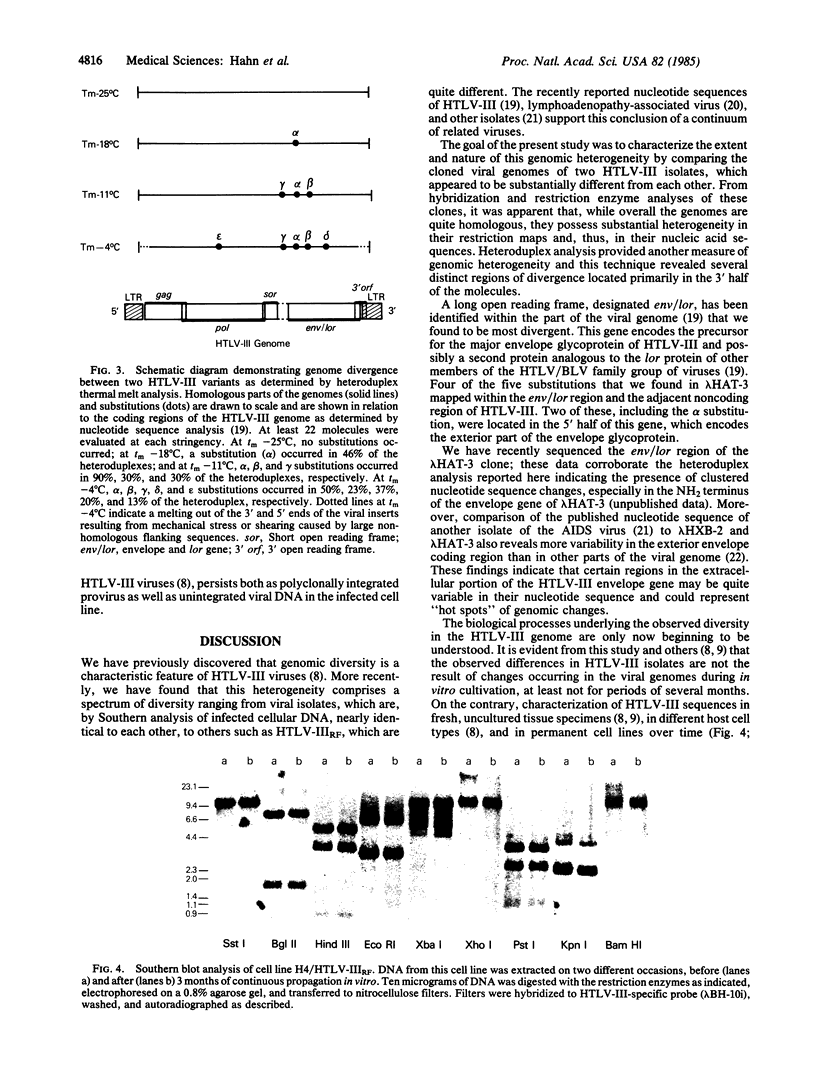
Converging lines of research have linked human T-cell lymphotropic virus type III (HTLV-III) to the pathogenesis of the acquired immune deficiency syndrome. A characteristic feature of this virus is its genomic heterogeneity, which occurs to varying degrees in different viral isolates. To define further the nature and extent of these genomic changes, we compared the molecularly cloned genomes of two variant HTLV-III isolates by extensive restriction enzyme mapping and heteroduplex thermal melt analysis. Both viral isolates were found to be highly related to each other throughout their entire genomic complement, yet they differed markedly in their restriction enzyme maps. Electron microscopic heteroduplex analysis revealed several distinct regions of divergence located almost exclusively in the part of the genome that encodes the viral envelope gene. In vitro culture of one of these viruses over a period of 3 months did not result in any genomic changes as determined by restriction analysis of viral DNA. These results, as well as the recently published nucleotide sequences of other HTLV-III isolates, indicate that the most substantial variation among HTLV-III isolates is located in the envelope. These findings raise the possibility that viral isolates from different individuals could have important biological differences in their envelope antigens, a consideration relevant to ongoing attempts to develop a vaccine against HTLV-III.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Narayan O. A physical map of the linear unintegrated DNA of Visna virus. Virology. 1981 Aug;113(1):412–415. doi: 10.1016/0042-6822(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Pedersen F. S., Narayan O., Haseltine W. A. Genomic changes associated with antigenic variation of visna virus durig persistent infection. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4454–4458. doi: 10.1073/pnas.77.8.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Hyman R. W. A study in evolution: the DNA base sequence homology between coliphages T7 and T3. J Mol Biol. 1971 Dec 14;62(2):287–301. doi: 10.1016/0022-2836(71)90428-1. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. The tandem direct repeats within the long terminal repeat of murine leukemia viruses are the primary determinant of their leukemogenic potential. J Virol. 1984 Dec;52(3):945–952. doi: 10.1128/jvi.52.3.945-952.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Wong-Staal F., Gallo R. C., Clements J. E., Narayan O., Gilden R. V. Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science. 1985 Jan 11;227(4683):173–177. doi: 10.1126/science.2981428. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Arya S. K., Popovic M., Gallo R. C., Wong-Staal F. Molecular cloning and characterization of the HTLV-III virus associated with AIDS. Nature. 1984 Nov 8;312(5990):166–169. doi: 10.1038/312166a0. [DOI] [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Montelaro R. C., Parekh B., Orrego A., Issel C. J. Antigenic variation during persistent infection by equine infectious anemia virus, a retrovirus. J Biol Chem. 1984 Aug 25;259(16):10539–10544. [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Ratner L., Gallo R. C., Wong-Staal F. HTLV-III, LAV, ARV are variants of same AIDS virus. Nature. 1985 Feb 21;313(6004):636–637. doi: 10.1038/313636c0. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P. A. Visna of sheep; a slow, demyelinating infection. Br J Exp Pathol. 1958 Oct;39(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Schüpbach J., Sarngadharan M. G., Gallo R. C. Antigens on HTLV-infected cells recognized by leukemia and AIDS sera are related to HTLV viral glycoprotein. Science. 1984 May 11;224(4649):607–610. doi: 10.1126/science.6324349. [DOI] [PubMed] [Google Scholar]
- Seligmann M., Chess L., Fahey J. L., Fauci A. S., Lachmann P. J., L'Age-Stehr J., Ngu J., Pinching A. J., Rosen F. S., Spira T. J. AIDS--an immunologic reevaluation. N Engl J Med. 1984 Nov 15;311(20):1286–1292. doi: 10.1056/NEJM198411153112005. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Gonda M. A., Flickinger G. H., Hahn B. H., Gallo R. C., Wong-Staal F. Genomes of evolutionarily divergent members of the human T-cell leukemia virus family (HTLV-I and HTLV-II) are highly conserved, especially in pX. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4544–4548. doi: 10.1073/pnas.81.14.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G. M., Hahn B. H., Arya S. K., Groopman J. E., Gallo R. C., Wong-Staal F. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science. 1984 Dec 7;226(4679):1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Harper M. E., Hahn B. H., Epstein L. G., Gajdusek D. C., Price R. W., Navia B. A., Petito C. K., O'Hara C. J., Groopman J. E. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985 Jan 11;227(4683):177–182. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- Young H. A., Gonda M. A., De Feo D., Ellis R. W., Nagashima K., Scolnick E. M. Heteroduplex analysis of cloned rat endogenous replication-defective (30 S) retrovirus and Harvey murine sarcoma virus. Virology. 1980 Nov;107(1):89–99. doi: 10.1016/0042-6822(80)90275-5. [DOI] [PubMed] [Google Scholar]