Abstract
Objectives
To determine gender or race differences in associations between adiposity and leptin, and whether adiponectin moderates these relationships.
Methods
Subjects were 441 adolescents, 14–18 years old (44% black; 50% female). Percent body fat (%BF) from dual-energy x-ray absorptiometry. Leptin and adiponectin were measured using immunoassays.
Results
Among the four groups (white boys, white girls, black boys and black girls), white girls had the highest adiponectin (p=0.0017) and black girls had the highest leptin (p=0.0164). Percent BF and leptin were positively correlated (p=0.0164). The %BF-leptin relationship was stronger in boys than girls (p<0.0001). Those with lower adiponectin had a stronger %BF-leptin relationship than those with high adiponectin in the entire sample (p=0.0220). Statistical models were adjusted for age, race, gender and the interaction between race and gender.
Conclusion
Our data suggest a protective metabolic interaction for adiponectin and lend additional support for obesity prevention strategies in adolescents.
Keywords: adolescents, percent body fat, leptin, adiponectin
Introduction
In addition to functioning as a storage repository for triglycerides, adipose tissue is an active endocrine organ with the ability to secrete a variety of modulators in response to metabolic signals. Leptin is one such protein, believed to increase with adiposity and promote obesity-related metabolic disease in adults1. A second is adiponectin, for which low levels are correlated with higher adiposity and an increased susceptibility to metabolic disease2–4. The high prevalence of adolescent obesity5–8 calls for close investigation into the precise relationships between adiposity and adipokines in this population. However, adolescent population studies are currently underrepresented in the literature, particularly in blacks.
The relationship between adiposity and leptin has been investigated in both children and adults1, 9, 10. Data from a study of 166 black adolescents showed all adiposity variables to be significantly correlated with leptin in both males and females, but trunk fat contributed the most to circulating leptin only in girls11. Another, including a total of 74 black and white subjects reported that gender differences in serum leptin could not be fully explained by relative body composition alone12. A larger study of 1,021 subjects aged 13–21 years showed leptin levels to be strongly associated with percent body fat (%BF), a relationship significantly stronger in males than in females13. However, this study was conducted in a population of rural Chinese adolescents. Therefore, gender and race variations for the adiposity and leptin relationship in black and white American adolescents remain unclear.
As adiponectin circulates in very high levels compared to other adipokines and has numerous effector functions, it is reasonable to consider a role for adiponectin to initiate cross-talk and modify adipocyte leptin release and leptin receptor activation14. In animal studies, peripheral expression of transgene adiponectin reduces food intake and body weight15, and injection of recombinant adiponectin has been shown to activate the hypothalamic leptin signaling pathway16. While these two adipokines remain central to the process of energy homeostasis, their precise interaction has not been well established. Additional investigations are needed to explore the ability of adiponectin to moderate circulating leptin and potential gender and race differences. This cross-sectional study of healthy, black and white adolescents thus aimed to 1) characterize the circulating levels of leptin and adiponectin concentrations according to race and gender; 2) to explore the relationship of adiposity with leptin in this population and investigate potential gender and race differences; and 3) to explore adiponectin as a potential modifier of these relationships.
Methods
Experimental Subjects
Subjects were 14–18 year old adolescents recruited via flyers distributed in local high schools. We sought a broad sample that included blacks and whites of both genders, from urban and suburban schools. Subjects were apparently healthy, had no contraindications to any study procedures, and were taking no medications that might affect the measured parameters. Interested youths and parents signed written informed assent and consent forms, respectively. All procedures were approved by the Human Assurance Committee at the Medical College of Georgia. Data collection took place between January 2001 and June 2005. Analyses were conducted on 441 subjects for whom %BF, leptin and adiponectin values were available.
Protocol
To reduce the discomfort of the blood draw, subjects were instructed to apply Emla cream (5 g tube) 1 h prior to reporting to the lab at 8 am. Subjects were also instructed to be in a fasting state, (i.e. having had nothing to eat or drink besides water from 8 pm to testing). Approximately 45 mL of blood were drawn for various measures, including leptin and adiponectin. After the blood draw, subjects were fed a standardized low nitrate/nitrite breakfast (e.g., cereal bars, 100% fruit juice), and then underwent a series of measurements including body composition.
Body composition
Weight was assessed with a balance scale and height was assessed with a stadiometer. BMI was calculated from these measurements. Body composition was measured with dual-energy x-ray absorptiometry (DXA) using the Hologic QDR-4500W (Waltham, MA). DXA provides measurement of %BF17. For some subjects, DXA values were not available from the Hologic QDR-4500W model, but were available only from the Hologic QDR-1000W model. For these individuals, prediction equations were derived using linear regression, with race, sex and QDR-1000W measurement as the predictor variables. The multiple R-square value for %BF was 0.99.
Pubertal Status
To assess pubertal development, subjects were placed in a private room alone. They were asked to read a prepared script and view a series of pictures showing different stages of pubertal development for their gender18, 19. Male subjects self-determined gonadal and pubic development on a scale of 1 to 5. Female subjects self-determined breast and pubic development on a scale of 1 to 5. Female subjects answered two additional questions regarding menses. The two questions were whether or not they had had their first period, and if so, when was the first day of their last period.
Leptin
Serum leptin was measured in duplicate using ELISAs constructed with antibodies purchased from R & D Systems (Minneapolis, MN). Monoclonal mouse anti-human antibodies (R&D Systems, MAB 398) were coated on 96-well polystyrene plates (Corning #25805–96, Corning, NY). The standard curves consisted of two-fold serial dilutions of recombinant cytokines (R&D Systems, 398-LP). Detection was carried out by sequential incubation with biotinylated goat anti-human polyclonal antibodies (R&D Systems, BAM 398)), streptavidin-conjugated horseradish peroxidase (Pierce #21126, Rockford, IL), and 2,2′-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid) (Sigma #1888, St. Louis, MO) in 0.1 M citric acid. Absorbance at 405 nm was measured with a Labsystems Multiskan MCC/340 plate reader (Needham Heights, MA). Assay sensitivity is 0.06 ng/mL. The intra-assay coefficient of variation was 2% and the inter-assay coefficient of variation was 5%.
Adiponectin
Plasma adiponectin was measured in duplicate in the Medical College of Georgia Vascular Biology Center using an ELISA kit (Linco Research Inc., St-Charles, MO). Assay sensitivity is 0.78 ng/mL. The intra-assay coefficient of variation was 7.4% and the inter-assay coefficient of variation was 8.4%
Statistical Analyses
All variables were checked for normality of distribution using normal probability plots and appropriate transformations were used when necessary. The distribution for fasting leptin was normalized by using a log transformation. Medians and ranges were reported for this variable based on the skewness of the distribution. Group comparisons for sample description were made using analysis of covariance (ANCOVA) to assess pubertal stage, race and gender comparisons while adjusting for age. There were no differences for pubertal stage, so it was dropped from the model. ANCOVA analysis was used to assess the relationship between %BF and log leptin while also adjusting for age, race, and sex. The interaction of %BF with gender or %BF with race was added to the model in order to determine if the relationship between %BF and log leptin was the same for these groups. Only significant effects were retained in the model which was then determined to best describe the relationship between %BF and leptin for these data. Adiponectin was then added to this model along with the interaction of adiponectin and %BF to determine whether it had a moderation effect on the %BF relationship with log leptin. Adiponectin and %BF were dichotomized at the median and the least squares means were used for ease of visualization and interpretation of the interaction. Statistical significance was determined at p<0.05. The statistical analyses were conducted with SAS 9.2.
Results
Clinical characteristics
Participants in this cross-sectional study included a total of 441 adolescents, 14 to 18 years of age (44% black; 56% white; 50% female; 50% male). The descriptive means for age, height, weight, BMI, %BF, leptin and adiponectin for each race-gender group and significant differences between groups are presented in Table 1. Although black girls were significantly older than the other groups in the sample (p=0.0058), the actual size of the difference between means was clinically insignificant at ≤ 0.5 years. Both height and weight increased significantly as age increased. Boys were taller (p<0.0001) and heavier than girls (p<0.0001), and blacks were heavier than whites (p=0.0010). Black girls had higher BMI values than black and white boys; black girls, black boys and white boys had higher BMI values than white girls (p=0.0041). Girls had higher %BF than white boys, and white boys had higher %BF than the black boys (p=0.0242). White girls had significantly higher levels of adiponectin than all other groups (p=0.0017). Additionally, black girls had higher leptin than white girls, and white girls had higher leptin than boys (p=0.0164).
Table 1.
Descriptive statistics for each race-gender group, mean (SD)
| Whites | Blacks | Significance | |||
|---|---|---|---|---|---|
|
| |||||
| Boys (n=120) | Girls (n=127) | Boys (n=102) | Girls (n=92) | ||
| Age (y) | 16.3 (1.2) | 16.0 (1.3) | 16.0 (1.2) | 16.5 (1.2) | Race × Gender, p=0.0058 BG > Whites & BB |
| Height (cm) | 174.1 (6.8) | 163.2 (6.0) | 175.0 (7.4) | 162.5 (6.0) | Gender, p<0.0001 |
| Weight (kg) | 70.1 (15.0) | 58.2 (10.6) | 71.4 (16.2) | 65.5 (15.7) | Race, p=0.0010 Gender, p<0.0001 |
| BMI (kg/m2) | 23.1 (4.4) | 21.8 (3.5) | 23.2 (4.6) | 24.8 (6.0) | Race × Gender, p=0.0041 BG > Boys > WG |
| % Body Fat | 19.6 (8.6) | 29.4 (6.9) | 16.9 (8.4) | 30.0 (8.4) | Race × Gender, p=0.0242 Girls > WB > BB |
| Adiponectin (μg/mL) | 15.7 (7.9) | 21.7 (9.8) | 14.9 (10.0) | 15.6 (8.5) | Race × Gender, p=0.0017 WG > Blacks & WB |
| * Leptin (ng/mL) | 2.8 (0.3–61.2) | 12.1 (1.3–57.5) | 2.3 (0.2–37.0) | 17.3 (2.8–77.3) | Race × Gender, p=0.0164 BG>WG>Boys |
WB=white boys WG=white girls BB=black boys BG=black girls
The distribution for fasting leptin was normalized by using a log transformation. Medians and ranges were reported for this variable based on the skewness of the distribution.
Associations between %BF and leptin
Percent BF was significantly associated with leptin (after adjusting for age, race and gender and the interaction between race and gender), such that higher %BF was associated with higher leptin in all groups (Model R2=0.36; p=0.0164). Furthermore, the strength of this relationship differed by gender. Adding the interaction of %BF with gender to the model accounted for a significant portion of the variance in leptin. The slope between %BF and leptin was greater in boys than girls (Model R2=0.79; p<0.0001). The increase of leptin in boys was 0.33 ng/ml for every 1% change in %BF, while increase of leptin in girls was 0.22 ng/ml for every 1% change in %BF. However, adding the interaction of %BF with race to the model did not account for a significant portion of the variance in leptin.
Influence of adiponectin on %BF-leptin relationship
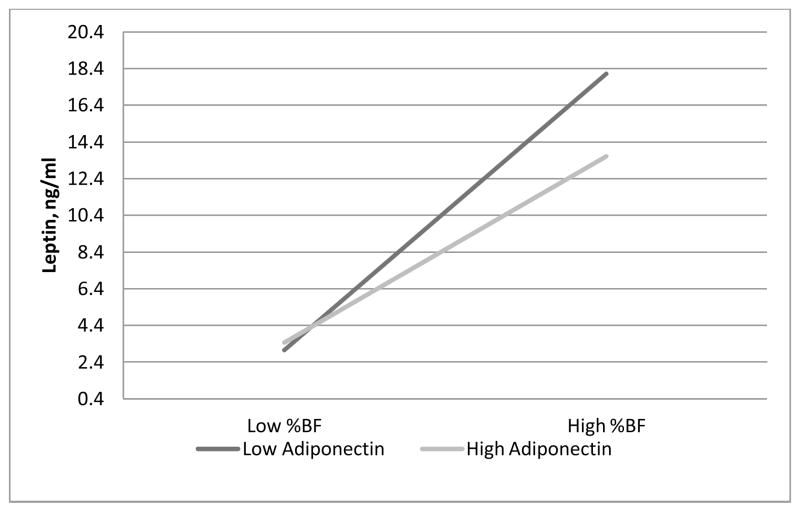
Adiponectin was also significantly associated with leptin (after adjusting for age, race and gender and the interaction between race and gender). Lower adiponectin was associated with higher leptin (Model R2=0.38; p=0.0024). When we added adiponectin and the interaction between %BF and adiponectin into the model including age, race, gender, the interaction between race and gender, %BF and the interaction between %BF and gender, we found a significant interaction of adiponectin with %BF, such that the positive relationship of %BF with leptin was stronger in those with lower adiponectin levels compared to those with higher adiponectin levels (Model R2=0.80; p=0.0220) (Figure 1).
Figure 1.
Plot of the % body fat by adiponectin interaction for leptin (Model R2=0.80; p=0.0220). For graphical purposes, %BF and adiponectin were classified as high if levels fell above the median and low if below. At higher %BF, those with high adiponectin had significantly lower leptin than those with low adiponectin.
Discussion
This cross-sectional study is one of only a few to investigate gender-specific and race-specific relationships of %BF with leptin in healthy black and white, male and female adolescents. To our knowledge, it is also the first to address the influence of adiponectin on the %BF-leptin relationship. Our data demonstrate that the relationship between %BF and leptin is stronger in boys than girls in this population, with no significant differences between blacks and whites. Moreover, high levels of adiponectin attenuate the %BF-leptin relationship in all groups.
Gender and race differences in clinical characteristics
Excess adiposity compromises health in pediatric populations by promoting premature development of metabolic disease6, 20–22. Adolescence is a time of rapid growth and maturation, when gender and race differences in body composition, metabolism and risk factors begin to emerge. Overall, adolescent males have higher rates of metabolic disease than adolescent females23, 24 and blacks have a higher risk than whites25–27. Adipokines such as leptin and adiponectin have recently been studied as potential serum biomarkers linking excess adiposity to elevated metabolic risk. However, adolescent studies have had mixed findings and black subjects are largely underrepresented.
We chose to study a sample of subjects during late adolescence to minimize differential hormonal influence seen in younger populations and chronic illnesses or medication use observed in adult cohorts. Moreover, we included girls and boys, blacks and whites to help elucidate variations in this age group which potentially contribute to the noteworthy gender and race divergence in adult disease prevalence. Because differences in the onset and progression of puberty potentially affect outcome measurements, we assessed pubertal stage, race and gender comparisons for age. We found no significant differences in pubertal stage, suggesting no extreme differences in hormonal status to influence study measures.
Results of our gender and race group comparisons for adiposity support the previous findings in the literature. Our findings that black girls had higher BMI than other groups and that girls had higher %BF than boys is consistent with previous studies from the National Health and Nutrition Examination Survey in 12–19 year olds28 and the National Heart, Lung and Blood Institute Growth and Health Study in 12–17 year olds29. While studies in adults consistently show females to have higher leptin than males30–32, reports of leptin gender distinctions are mixed in children and adolescents12, 33, 34. Our group has previously reported higher leptin in girls than boys in a separate cohort of black adolescents11. The present study supports these earlier findings with data showing that black girls had higher levels of circulating leptin than black boys. Moreover, this present study adds an additional piece by showing leptin to also be significantly higher in white girls than white boys. Of particular interest in our study is that white girls had significantly higher levels of circulating adiponectin than all other groups. Previous studies also provide evidence that serum adiponectin is higher in females than males, and higher in whites than blacks, even after adjusting for adiposity35, 36. Such gender disparities are believed to be primarily due to testosterone regulation37–40 and may help explain differences in disease risk between genders. However, mechanisms for adiponectin variation by race remain to be elucidated.
Relationship between %BF and leptin
Leptin is an adipocyte-derived protein, structurally classified as a cytokine, with functions spanning from energy and neuroendocrine modulation to immune regulation41, 42. Although the positive relationship between adiposity and circulating leptin has been well documented, the strength of associations varies by type of adiposity measurement17. We used %BF because it has been shown to explain a higher proportion of the variance for serum leptin13, 43 and is believed to be the only significant adiposity predictor of leptin in males44.
Not surprisingly, our data show a positive relationship between %BF and leptin in all groups. However, the finding that this relationship was stronger in boys than girls is of particular interest. At higher levels of %BF, boys had higher leptin than girls. This was somewhat unexpected, given that leptin levels are inversely correlated with testosterone in healthy adolescents33 and positively with estrogen in adult females45, 46. However, another study has reported similar findings of a stronger male %BF-leptin relationship in a population of rural Chinese 13–21 year olds13. Despite having lower body fat and lower overall leptin, adolescent boys have a higher prevalence of metabolic disease than girls23, 24, a trend that continues into adulthood47. Previous studies examining the impact of race on circulating leptin in blacks and whites have shown mixed results48, 49. Therefore, further studies are warranted to elucidate race differences and the influence of gender hormones on these relationships.
Adiponectin moderates the %BF-leptin relationship
Adiponectin is classified within the collagen family, yet shares several regulatory and homeostatic functions with leptin16, 50–52. Contrary to leptin, adiponectin levels decrease with adiposity53. Moreover, corresponding low adiponectin is associated with increased susceptibility to metabolic disease2–4. Although much is known independently about these two adipokines, the precise nature and clinical implication of their interaction remains to be elucidated. Not only did the adiponectin account for a significant amount of the variance in leptin, but it also altered the relationship between %BF and leptin. That is to say, we found a significant %BF by adiponectin interaction such that the positive relationship between %BF and leptin was attenuated in subjects who had higher adiponectin. This interaction was the same in all race/sex groups. These findings suggest that adiponectin may act to protect against the development of high leptin levels and corresponding risk of metabolic disease, even in adolescents as young as 14–18 years of age.
Strengths, limitations and future directions
One key strength of this study is the sample of healthy black and white adolescent boys and girls. Few studies have examined gender and race differences in the relationship between adiposity and leptin, and this is the first study in adolescents to examine adiponectin as a potential modulator of this relationship. Moreover, several adolescent studies report only BMI or waist circumference, as they do not have DXA measurements available33, 35, 54. Our study used DXA to obtain more precise adiposity assessment of %BF. Lastly, leptin secretion is believed to be diurnal, accounting for higher circulating levels in the early morning and late night55. All subjects in this study were fasting and tested within a short timeframe, in the early morning, to help minimize variation.
Our data show higher circulating leptin as %BF increases, a relationship that was moderated by addition of adiponectin to the model. However, these findings are limited to a cross-sectional sample of black and white adolescents. Longitudinal and intervention studies will be necessary to better elucidate precise relationships between these measures and the influence of testosterone. A second possible limitation of this study is that the pubertal stages were self-reported and not verified by a physician. Measuring circulating pubertal hormones and examining the influence of diet, physical activity, and socioeconomic status were not included in this study. Additional investigations are needed to focus on the influence of these factors in the adolescent population since they all have potential to alter the relationships between adiposity and circulating adipokines.
In conclusion, our results show that there is a relationship between %BF and leptin, even as early as adolescence, that it varies by gender and may be influenced by adiponectin. As the development of metabolic disease begins early in life, a better understanding of physiologic events at this age is essential. Future studies are needed to investigate precise mechanisms and physiologic outcomes of these adiposity-related interactions, as well as how to develop effective obesity prevention strategies.
Acknowledgments
We would like to thank the adolescents who participated in this study, the local high schools and the Richmond and Columbia Boards of Education who facilitated subject recruitment. Additionally, we would like to acknowledge Dr. Paule Barbeau for study design assistance and support, Dr. Joe Cannon for performing the leptin assays, and Ms. Elizabeth Stewart for data management.
Funding for this study was provided by NIH HL64157, NIH 1T32HL076146-01.
References
- 1.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 2.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277(29):25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 3.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281(5):2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 4.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002;147(2):173–180. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]
- 5.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120(2):340–345. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 6.Berenson GS, Srinivasan SR. Emergence of obesity and cardiovascular risk for coronary artery disease: the Bogalusa Heart Study. Prev Cardiol. 2001;4(3):116–121. doi: 10.1111/j.1520-037x.2001.00537.x. [DOI] [PubMed] [Google Scholar]
- 7.Franks PW, Hanson RL, Knowler WC, Moffett C, Enos G, Infante AM, Krakoff J, Looker HC. Childhood predictors of young-onset type 2 diabetes. Diabetes. 2007;56(12):2964–2972. doi: 10.2337/db06-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152(2):201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Mayor RV, Andrade MA, Rios M, Lage M, Dieguez C, Casanueva FF. Serum leptin levels in normal children: relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. J Clin Endocrinol Metab. 1997;82(9):2849–2855. doi: 10.1210/jcem.82.9.4235. [DOI] [PubMed] [Google Scholar]
- 10.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 11.Petty KH, Li K, Dong Y, Fortenberry J. Stallmann-Jorgensen I, Guo D, Zhu H. Sex dimorphisms in inflammatory markers and adiposity in African-American youth. Int J Pediatr Obes. 2010 doi: 10.3109/17477160903497019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagy TR, Gower BA, Trowbridge CA, Dezenberg C, Shewchuk RM, Goran MI. Effects of gender, ethnicity, body composition, and fat distribution on serum leptin concentrations in children. J Clin Endocrinol Metab. 1997;82(7):2148–2152. doi: 10.1210/jcem.82.7.4077. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Liu X, Brickman WJ, Christoffel KK, Zimmerman D, Tsai HJ, Wang G, Wang B, Li Z, Tang G, Yang J, Xu X, Wang X. Association of plasma leptin concentrations with adiposity measurements in rural Chinese adolescents. J Clin Endocrinol Metab. 2009;94(9):3497–3504. doi: 10.1210/jc.2009-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dridi S, Taouis M. Adiponectin and energy homeostasis: consensus and controversy. J Nutr Biochem. 2009;20(11):831–839. doi: 10.1016/j.jnutbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, Campbell-Thompson M, Crawford J, Shek EW, Scarpace PJ, Zolotukhin S. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci U S A. 2003;100(24):14217–14222. doi: 10.1073/pnas.2333912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coope A, Milanski M, Araujo EP, Tambascia M, Saad MJ, Geloneze B, Velloso LA. AdipoR1 mediates the anorexigenic and insulin/leptin-like actions of adiponectin in the hypothalamus. FEBS Lett. 2008;582(10):1471–1476. doi: 10.1016/j.febslet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Gutin B, Litaker M, Islam S, Manos T, Smith C, Treiber F. Body-composition measurement in 9–11-y-old children by dual-energy X-ray absorptiometry, skinfold-thickness measurements, and bioimpedance analysis. Am J Clin Nutr. 1996;63(3):287–292. doi: 10.1093/ajcn/63.3.287. [DOI] [PubMed] [Google Scholar]
- 18.Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child development. 1987;58(3):829–841. [PubMed] [Google Scholar]
- 19.Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66(6):918–920. [PubMed] [Google Scholar]
- 20.Fagot-Campagna A. Emergence of type 2 diabetes mellitus in children: epidemiological evidence. J Pediatr Endocrinol Metab. 2000;13 (Suppl 6):1395–1402. doi: 10.1515/jpem-2000-s613. [DOI] [PubMed] [Google Scholar]
- 21.Ehtisham S, Barrett TG. The emergence of type 2 diabetes in childhood. Ann Clin Biochem. 2004;41(Pt 1):10–16. doi: 10.1258/000456304322664654. [DOI] [PubMed] [Google Scholar]
- 22.Fagot-Campagna A, Pettitt DJ, Engelgau MM, Burrows NR, Geiss LS, Valdez R, Beckles GL, Saaddine J, Gregg EW, Williamson DF, Narayan KM. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136(5):664–672. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the International Diabetes Federation. Diabetes Care. 2008;31(3):587–589. doi: 10.2337/dc07-1030. [DOI] [PubMed] [Google Scholar]
- 24.Syme C, Abrahamowicz M, Leonard GT, Perron M, Pitiot A, Qiu X, Richer L, Totman J, Veillette S, Xiao Y, Gaudet D, Paus T, Pausova Z. Intra-abdominal adiposity and individual components of the metabolic syndrome in adolescence: sex differences and underlying mechanisms. Arch Pediatr Adolesc Med. 2008;162(5):453–461. doi: 10.1001/archpedi.162.5.453. [DOI] [PubMed] [Google Scholar]
- 25.Carroll JF, Fulda KG, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, Cardarelli R. Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity (Silver Spring) 2009;17(7):1420–1427. doi: 10.1038/oby.2008.657. [DOI] [PubMed] [Google Scholar]
- 26.Appel SJ, Oster RA, Floyd NA, Ovalle F. Cardiometabolic risk among African American women: a pilot study. J Cardiovasc Nurs. 2009;24(2):140–150. doi: 10.1097/JCN.0b013e318197aa3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, Bae S, Cardarelli R. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16(3):600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 28.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 29.Kimm SY, Barton BA, Obarzanek E, McMahon RP, Sabry ZI, Waclawiw MA, Schreiber GB, Morrison JA, Similo S, Daniels SR. Racial divergence in adiposity during adolescence: The NHLBI Growth and Health Study. Pediatrics. 2001;107(3):E34. doi: 10.1542/peds.107.3.e34. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81(9):3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 31.Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Jinagouda SD, el-Tawil K, Rude RK, Kamdar V. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997;82(2):579–584. doi: 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, Garvey WT. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82(4):1293–1300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- 33.Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Muller J, Skakkebaek NE, Heiman ML, Birkett M, Attanasio AM, Kiess W, Rascher W. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab. 1997;82(9):2904–2910. doi: 10.1210/jcem.82.9.4251. [DOI] [PubMed] [Google Scholar]
- 34.Demerath EW, Towne B, Wisemandle W, Blangero J, Chumlea WC, Siervogel RM. Serum leptin concentration, body composition, and gonadal hormones during puberty. Int J Obes Relat Metab Disord. 1999;23(7):678–685. doi: 10.1038/sj.ijo.0800902. [DOI] [PubMed] [Google Scholar]
- 35.Woo JG, Dolan LM, Daniels SR, Goodman E, Martin LJ. Adolescent sex differences in adiponectin are conditional on pubertal development and adiposity. Obes Res. 2005;13(12):2095–2101. doi: 10.1038/oby.2005.260. [DOI] [PubMed] [Google Scholar]
- 36.Patel DA, Srinivasan SR, Xu JH, Chen W, Berenson GS. Adiponectin and its correlates of cardiovascular risk in young adults: the Bogalusa Heart Study. Metabolism. 2006;55(11):1551–1557. doi: 10.1016/j.metabol.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 37.Laughlin GA, Barrett-Connor E, May S. Sex-specific determinants of serum adiponectin in older adults: the role of endogenous sex hormones. Int J Obes (Lond) 2007;31(3):457–465. doi: 10.1038/sj.ijo.0803427. [DOI] [PubMed] [Google Scholar]
- 38.Shand BI, Scott RS, Elder PA, George PM. Plasma adiponectin in overweight, nondiabetic individuals with or without insulin resistance. Diabetes Obes Metab. 2003;5(5):349–353. doi: 10.1046/j.1463-1326.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 39.Kwon K, Jung SH, Choi C, Park SH. Reciprocal association between visceral obesity and adiponectin: in healthy premenopausal women. Int J Cardiol. 2005;101(3):385–390. doi: 10.1016/j.ijcard.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 40.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 42.Chan JL, Moschos SJ, Bullen J, Heist K, Li X, Kim YB, Kahn BB, Mantzoros CS. Recombinant methionyl human leptin administration activates signal transducer and activator of transcription 3 signaling in peripheral blood mononuclear cells in vivo and regulates soluble tumor necrosis factor-alpha receptor levels in humans with relative leptin deficiency. J Clin Endocrinol Metab. 2005;90(3):1625–1631. doi: 10.1210/jc.2004-1823. [DOI] [PubMed] [Google Scholar]
- 43.Mahabir S, Baer D, Johnson LL, Roth M, Campbell W, Clevidence B, Taylor PR. Body Mass Index, percent body fat, and regional body fat distribution in relation to leptin concentrations in healthy, non-smoking postmenopausal women in a feeding study. Nutr J. 2007;6:3. doi: 10.1186/1475-2891-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peltz G, Sanderson M, Perez A, Sexton K, Ochoa Casares D, Fadden MK. Serum leptin concentration, adiposity, and body fat distribution in Mexican-Americans. Arch Med Res. 2007;38(5):563–570. doi: 10.1016/j.arcmed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Mannucci E, Ognibene A, Becorpi A, Cremasco F, Pellegrini S, Ottanelli S, Rizzello SM, Massi G, Messeri G, Rotella CM. Relationship between leptin and oestrogens in healthy women. Eur J Endocrinol. 1998;139(2):198–201. doi: 10.1530/eje.0.1390198. [DOI] [PubMed] [Google Scholar]
- 46.Quinton ND, Laird SM, Okon MA, Li TC, Smith RF, Ross RJ, Blakemore AI. Serum leptin levels during the menstrual cycle of healthy fertile women. Br J Biomed Sci. 1999;56(1):16–19. [PubMed] [Google Scholar]
- 47.Berenson GS, Wattigney WA, Tracy RE, Newman WP, 3rd, Srinivasan SR, Webber LS, Dalferes ER, Jr, Strong JP. Atherosclerosis of the aorta and coronary arteries and cardiovascular risk factors in persons aged 6 to 30 years and studied at necropsy (The Bogalusa Heart Study) Am J Cardiol. 1992;70(9):851–858. doi: 10.1016/0002-9149(92)90726-f. [DOI] [PubMed] [Google Scholar]
- 48.Danadian K, Suprasongsin C, Janosky JE, Arslanian S. Leptin in African-American children. J Pediatr Endocrinol Metab. 1999;12(5):639–644. doi: 10.1515/JPEM.1999.12.5.639. [DOI] [PubMed] [Google Scholar]
- 49.Wong WW, Nicolson M, Stuff JE, Butte NF, Ellis KJ, Hergenroeder AC, Hill RB, Smith EO. Serum leptin concentrations in Caucasian and African-American girls. J Clin Endocrinol Metab. 1998;83(10):3574–3577. doi: 10.1210/jcem.83.10.5154. [DOI] [PubMed] [Google Scholar]
- 50.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 51.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10(5):524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 52.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221(2):286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 53.Park KG, Park KS, Kim MJ, Kim HS, Suh YS, Ahn JD, Park KK, Chang YC, Lee IK. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract. 2004;63(2):135–142. doi: 10.1016/j.diabres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 54.Prevalence of the metabolic syndrome among a racially/ethnically diverse group of U S. eighth-grade adolescents and associations with fasting insulin and homeostasis model assessment of insulin resistance levels. Diabetes Care. 2008;31(10):2020–2025. doi: 10.2337/dc08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JF. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97(5):1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]