Abstract
Over-expression of N-methylpurine DNA glycosylase (MPG) has been suggested as a possible gene therapy approach to sensitize tumor cells to the cell killing effects of temozolomide, an imidazotetrazine-class chemotherapeutic alkylating agent. In the present study, we show that both elevated MPG expression and shRNA-mediated loss of Pol ß expression in human breast cancer cells increases cellular sensitivity to temozolomide. Resistance to temozolomide is restored by complementation of either wild-type human Pol ß or human Pol ß with an inactivating mutation specific to the polymerase active site yet functional for 5′dRP lyase activity. These genetic and cellular studies uniquely demonstrate that over-expression of MPG causes an imbalance in BER leading to an accumulation of cytotoxic 5′dRP lesions and that the 5′dRP lyase activity of Pol ß is required to restore resistance to temozolomide. These results imply that Pol ß dependent 5′dRP lyase activity is the rate-limiting step in BER in these cells and suggests that BER is a tightly balanced pathway for the repair of alkylated bases such as N7-MeG and N3-MeA. Further, we find that 5′dRP-mediated cell death is independent of caspase-3 activation and does not induce the formation of autophagosomes, as measured by GFP-LC3 localization. The experiments presented herein suggest that it will be important to investigate whether an active BER pathway could be partially responsible for the temozolomide-mediated resistance seen in some tumors and that balanced BER protein expression and overall BER capacity may help predict sensitivity to temozolomide.
Base excision repair (BER) is the predominant pathway for the repair of base damage mediated by endogenous and exogenous stressors (Almeida and Sobol, 2007; Lindahl and Wood, 1999). The repair of DNA bases damaged by alkylation is initiated in mammalian cells by N-methylpurine DNA glycosylase (MPG), also known as alkyladenine DNA glycosylase (AAG) (Wood et al., 2001). The majority of repair that is initiated by MPG occurs via short-patch BER, a mechanism whereby only 1 nucleotide is replaced. Once the modified base is removed by MPG, the resulting abasic site is hydrolyzed by AP endonuclease (APE1) (Wood et al., 2001), catalyzing the incision of the damaged strand, leaving a 3′OH and a 5′deoxyribose-phosphate moiety (5′dRP) at the margins of the repair site. DNA polymerase ß (Pol ß) subsequently hydrolyzes the 5′dRP moiety and fills the single nucleotide gap, preparing the strand for ligation by either DNA Ligase I or a complex of DNA Ligase IIIα and XRCC1.
As with many DNA repair processes, BER functions via a series of repair complexes that assemble at the site of the DNA lesion. For the repair of DNA damaged by alkylation, MPG, APE1, POL ß and XRCC1 are essential, with little evidence of effective complementary repair capacity (Almeida and Sobol, 2007). This would suggest that inhibition of or a deficiency in one or more of these essential BER proteins will lead to DNA repair-intermediate induced cell death (e.g., alkylated bases, abasic sites, 5′dRP-containing lesions or DNA single-strand breaks). When cells or in some cases, mice are deficient in Mpg, Xrcc1 or Pol ß, they are hypersensitive to alkylating agents (Elder et al., 1998; Engelward et al., 1996; Horton et al., 2008; Paik et al., 2005; Sobol et al., 1996). In addition, decreased APE1 expression can also lead to an increase in sensitivity to alkylating agents (Ono et al., 1994; Walker et al., 1994). We have extended our characterization of the role of Pol ß in the repair of alkylation damage in mouse cells, demonstrating that Pol ß provides cellular resistance to the clinical alkylating agent temozolomide (TMZ; an imidazotetrazine-class chemotherapeutic alkylating agent) by repairing lesions that ultimately trigger activation of the DNA damage response checkpoint (Trivedi et al., 2005). Interestingly, both MPG knockdown (Paik et al., 2005) and increased expression of MPG have been found to sensitize cells to alkylators (Fishel et al., 2007; Rinne et al., 2004). To avoid alkylation-damage induced mutations that accumulate in the absence of MPG expression, forced over-expression of MPG has been suggested as a strategic and viable gene therapy approach to sensitize tumor cells to TMZ.
To directly evaluate the role of MPG and Pol ß in human tumor cells with regard to the cellular response to alkylation damage, we developed human Pol ß specific shRNA-expressing lentiviruses to completely deplete human tumor cells of Pol ß, as well as vectors for ectopic expression of MPG and RNAi-resistant human Pol ß transgenes so as to define the enzymatic activity of Pol ß (5′dRP lyase or DNA polymerase activity) that confers TMZ resistance. We show that both elevated MPG expression and shRNA-mediated loss of Pol ß expression increases cellular sensitivity to TMZ in human breast cancer cells. In both cases, resistance to TMZ is restored by complementation of either wild-type human Pol ß or human Pol ß with an inactivating mutation specific to the polymerase active site yet functional for 5′dRP lyase activity. These genetic and cellular studies uniquely demonstrate that over-expression of MPG causes an imbalance in BER by saturating the Pol ß dependent removal of the cytotoxic 5′dRP lesion. Failure to remove this cytotoxic lesion does not induce caspase-3 activation and does not induce the formation of autophagosomes, as measured by GFP-LC3 localization. These studies support the possibility that the lyase activity of Pol ß is the rate-limiting step in BER in human cells and suggests that BER is a tightly balanced pathway for the repair of alkylated bases such as N7-MeG and N3-MeA.
Materials and methods
Chemicals and reagents
RPMI 1640 and heat inactivated fetal bovine serum were from Cambrex Biosciences Group and InVitrogen. Temozolomide (NSC# 362856; IUPAC name: 3-methyl-2-oxo-1,3,4,5,8-pentazabicyclo[4.3.0]nona-4,6,8-triene-7-carbo oxamide; CAS number: 85622-93-1) (Sobol, 2008b) was from the National Cancer Institute Developmental Therapeutics Program and prepared as 100 mM stock in DMSO. We used the following primary antibodies: anti-Pol ß (Mab clone 61; Thermo Fisher Scientific); anti-human MPG (Mab; clone 506-3D, kindly provided by Dr. S.J. Kennel, ORNL); anti-APE1 (EMD Biosciences); anti-PCNA (Santa Cruz); and anti-Flag (M2 Mab; Sigma-Aldrich). All electrophoresis reagents were from Bio-Rad. Neomycin and Dynabeads Protein G were purchased from InVitrogen. Puromycin, Gentamicin sulfate (10mg/ml) and 3x Flag peptide were from Clontech Laboratories, Irvine Scientific and Sigma-Aldrich, respectively. Etoposide and 3-Methyladenine were obtained from Sigma-Aldrich. Z-VAD-FMK was from Calbiochem and the caspase-3 colorimetric activity assay kit was from Millipore. pEGFP-LC3 plasmid was kindly provided by Dr. Tamotsu Yoshimori, National Institute of Genetics, Mishima, Japan.
Plasmid expression vectors and RNAi development
We used the following mammalian expression vectors: Human MPG: pRS1422 (Sobol et al., 2003); human Pol ß: pIRES-Neo/Flag-Pol ß (WT), pIRES-Puro/Flag-Pol ß (WT) and pIRES-Neo/Flag-Pol ß (D256A) (Sobol et al., 2000). Human Pol ß targeted FIV-based lentiviral shRNA expression vectors (pFIV-H1-Puro-hpolß.1, pFIV-H1-Puro-hpolß.2 and pFIV-H1-Purohpolß.3), pFIV-34N and pVSV-G constructs were from System Biosciences. pSuper-Retro shRNA (pSuper-Retro shRNA-1 and pSuper-Retro shRNA-2) expression vectors were from Oligoengine.
Cell line transfection and viral transduction conditions
Human MPG over-expression, Pol ß knockdown (KD), Flag Pol ß (WT) and Flag Pol ß (D256A) over-expressing cell lines were prepared by transfection using FuGene 6 Transfection Reagent (Roche) according to the manufacturer’s instructions. Stable cell lines (Table 1) were selected in G418 [800 μg/ml for human MPG expression plasmids, 700 μg/ml for pIRES-Neo/Flag-Pol ß (WT) and pIRES-Neo/Flag-Pol ß (D256A)] and puromycin [0.5 μg/ml for pIRES-Puro/Flag-Pol ß (WT)] for 2 weeks, individual clones (stably expressing human MPG or Pol ß) were amplified and 30 μg of nuclear extract was analyzed by immunoblotting for the expression of human MPG or human Pol ß protein and also probed for expression of APE1 and PCNA.
Table 1.
Cell lines developed and used in this study
| Cell Line Name | Clone # |
TMZ IC50 (mM) |
Cell line Description | Growth Media |
|---|---|---|---|---|
| MDA-MB-231 (ATCC # HTB26) |
- | 2.0 | Human, Caucasian, breast, adenocarcinoma, epithelial cell |
RPMI 1640 (450 ml), Heat Inactivated FBS 10%, Gentamycin (10 μg/ml) |
| MDA-MB-231/Pol ß-KD | 2 | 0.8 | Human Breast cancer cells expressing Pol ß lentiviral shRNA. |
Growth media supplemented with Puromycin (0.5 μg/ml) |
| 10 | 0.8 | |||
| 18 | 0.7 | |||
| MDA-MB-231/MPG | 4 | 0.57 | MPG over-expression in human Breast cancer cells |
Growth media supplemented with Geneticin (800 μg/ml) |
| 5 | 0.50 | |||
| 6 | 0.90 | |||
| MDA-MB-231/Pol ß- KD(10)/MPG |
1 | 0.27 | MPG over-expression in Pol ß down-regulated human Breast cancer cells |
Growth media supplemented with Puromycin (0.5 μg/ml) & Geneticin (750 μg/ml) |
| 2 | 0.27 | |||
| MDA-MB-231/Pol ß- KD(10)/Flag-Pol ß-WT |
2 | 1.6 | WT Flag-Pol ß reconstituted in Pol ß down-regulated human Breast cancer cells |
Growth media supplemented with Puromycin (0.5 μg/ml) & Geneticin (700 μg/ml) |
| 3 | 2.0 | |||
| 4 | 1.5 | |||
| MDA-MB-231/Pol ß- KD(10)/Flag-Pol ß-D25A |
3 | 1.63 | D256A mutant Flag-Pol ß reconstituted in Pol ß down-regulated human Breast cancer cells |
Growth media supplemented with Puromycin (0.5 μg/ml) & Geneticin (700 μg/ml) |
| 4 | 1.27 | |||
| 5 | 1.66 | |||
| MDA-MB-231/MPG(4)/Flag- Pol ß-WT |
19 | 1.31 | WT Flag-Pol ß reconstituted in MPG over-expressing human Breast cancer cells |
Growth media supplemented with Puromycin (0.5 μg/ml) & Geneticin (700 μg/ml) |
| 20 | 2.0 | |||
| 21 | 1.88 |
Lentiviral particles were generated by transfection of three plasmids (the expression plasmid, e.g., pFIV-H1-puro-hPOLB.1; plus pFIV-34N and pVSV-G) into 293-FT cells (Poeschla et al., 1998) using FuGene 6. Culture media from transfected cells was collected 48 hours after transfection to isolate the viral particles, passed through 0.45 filters, used immediately or stored at −80°C in single-use aliquots. Lentiviral transduction was completed as follows: Briefly, 6.0 × 104 cells were seeded into 6-well plate and incubated for 24-30 hours at 5% CO2 at 37°C. Cells were transduced for 18 hours with shRNA-expression lentiviral stocks at 32°C and cultured for 72 h at 37°C. Stable cell lines were isolated following transduction as above followed by selection in puromycin (0.5 μg/ml) for 2 weeks. Individual clones (stable knockdown of human Pol ß protein) were amplified and 30 μg of nuclear extract was analyzed by immunoblotting for the expression of endogenous human Pol ß protein and also probed for expression of MPG, APE1 and PCNA. A descriptive list of the cell lines developed for and used in this study is detailed in Table 1.
Culture conditions and cell cytotoxicity assays
MDA-MB-231 breast cancer cells were obtained from ATCC (HTB-26). Human MPG over-expression, human Pol ß knockdown and human Pol ß over-expressing MDA-MB-231 cell lines were cultured at 37°C in a humidified incubator with 5% CO2 in RPMI 1640 supplemented with 10% heat inactivated fetal bovine serum and gentamicin (10 μg/ml). TMZ, etoposide (ETO) or 3-Methyladenine (3-MA) induced cytotoxicity was determined by a modified MTT assay, as we described previously (Trivedi et al., 2005). Results were calculated from the average of four separate experiments and are reported as the % of treated cells relative to the cells in control wells (% Control).
Cell extract preparation, immunoblot and immunoprecipitation assays
Nuclear extracts were prepared and protein concentration was determined as we described previously (Trivedi et al., 2005). Nuclear protein (30 μg) was separated by electrophoresis in a 4-20% Tris-Glycine SDS-polyacrylamide gel (InVitrogen) and electro-transferred to a 0.45 μM nitrocellulose membrane (Trans-Blot, Bio-Rad; Hercules, CA). Antigens were detected using standard protocols. Primary antibodies (anti-Pol ß, 500x; anti-hMPG, 1000x; anti-APE-1, 3000x; anti-Flag 2000x and anti-PCNA, 1000x) and the horseradish peroxidase (HRP)–conjugated secondary antibody (goat anti-mouse HRP; Bio-Rad, Hercules, CA) were diluted in TBST 5% milk.
For immunoprecipitation (IP), cell lysate from the above cell lines was prepared in RIPA buffer, incubated overnight with anti-Flag M2 antibodies at 4°C followed by one hour incubation with Protein G Dynabeads at 4°C. The immunoprecipitated material was washed with RIPA buffer and eluted with 3x Flag peptide, separated on SDS-PAGE, transferred to nitrocellulose filters and probed with anti-Pol ß antibody.
Induction of apoptosis and caspase-3 activation assay
24 hours after seeding duplicate plates for each cell line (2000 cells per well in a 96-well plate), each plate was pretreated with media alone or media supplemented with Z-VAD-FMK at a final concentration of 50 μM. After one hour Z-VAD-FMK treatment, cells were treated with ETO (15 μM) or various doses of TMZ (0.25 mM - 2.5 mM) and then incubated at 37°C for 48 hours. Cell cytotoxicity was determined by a modified MTT assay, as described above.
Caspase-3 activation was measured using a caspase-3 colorimetric activity assay kit as per the manufacturer’s instructions. Briefly, the assay is based on the spectophotometric identification of p-nitroaniline (pNA) after cleavage of a labeled DEVD-pNA substrate. For the caspase-3 activation assay, 0.75 ×106 cells were seeded in 100mm dishes. 24 hours after seeding, the cells [MDA-MB-231, MDA-MB-231/Pol ß-KD(10), MDA-MB-231/MPG(4), and MDA-MB-231/Pol ß-KD(10)/MPG(4)] were treated with 2.0 mM, 0.8 mM, 0.6 mM and 0.3 mM TMZ (the corresponding TMZ IC50 for each cell line; see Table 1) or media for 6, 12, 24 or 48 hours. Separately, cells were treated with 15 μM ETO or media for 24 hours. After induction of apoptosis with TMZ or ETO, the cell pellet (containing approximately 2×106 cells) was resuspended in 1x cell lysis buffer and the supernatant (cytosolic extract) was used to measure caspase-3 activation. Samples were mixed with assay mixture in a 96-well plate and incubated for 2 hours at 37°C and absorbance was measured at 405nm. The fold increase in caspase-3 activation was calculated by comparing the OD from the TMZ or ETO samples with the OD from the untreated control samples.
Inhibition of autophagy by 3-Methyladenine
24 hours after seeding duplicate plates for each cell line (2000 cells per well in a 96-well plate), each plate was pretreated with media alone or media supplemented with 3-MA at a final concentration of 5 mM. After 30 minutes of 3-MA treatment, cells were treated with various ranges of TMZ (0.25 mM- 2.5 mM) and then incubated at 37°C for 48 hours. Cell cytotoxicity was determined by a modified MTT assay, as described above.
Identification of autophagosome formation with GFP-LC3
MDA-MB-231 and derived cell lines were seeded at 4,900 cells per well in 8-well chamber slides and cultured for 36 hours at 37°C and 5% CO2. Cells were then transfected with pEGFPLC3 for 36 hours, using FuGene as described above. Cells were pretreated with media alone or media supplemented with 5mM 3-MA for 30 minutes and then treated with or without the IC50 dose of TMZ for 24 hours (see Table 1). Cells were washed twice in PBS, fixed in 4% formaldehyde for 30 minutes at room temperature and counterstained with DAPI (0.5μg/mL) for 5 minutes.
Results
Over-expression of human MPG shifts the rate-limiting step in the BER pathway and induces a TMZ hypersensitive phenotype
In line with our hypothesis that the BER pathway repairs TMZ-induced lesions (Trivedi et al., 2005) and therefore variations in BER protein expression will impact TMZ-mediated cell death, we have analyzed the involvement of MPG in TMZ-responsiveness in human tumor cells (see Table 1) by increasing MPG expression so as to enhance BER initiation. Our MPG expression system allows us to significantly over-express MPG in MDA-MB-231 human breast cancer cells (Fig. 1A), as much as 10-fold, as determined by immunoblot analysis and quantification by NIH Image (v 1.62) analysis. Note the almost undetectable expression of MPG in the parental cells as compared to the over-expression cell lines (Fig. 1A, Compare lane 1 with lanes 2-4) and that over-expression of MPG does not alter the expression of the other BER proteins Pol ß or APE1 (Fig. 1A). It is our hypothesis that MPG over-expression shifts the rate-limiting step in the BER pathway, significantly enhancing BER initiation that may result in the accumulation of BER intermediates such as 5′dRP. In support of this hypothesis, an increased sensitivity to TMZ is observed when MPG is over-expressed in these cells (Fig. 1B), in-line with that of Rinne et al (Rinne et al., 2004). Since MPG performs the first step in the BER pathway and is essential to manifest the Pol ß null phenotype observed in mouse cells (Sobol et al., 2003), we reasoned that MPG over-expression saturates endogenous Pol ß and yields an apparent Pol ß deficiency, since glycosylase over-expression could cause an overall BER imbalance (Coquerelle et al., 1995). To determine if the endogenous level of Pol ß is rate limiting when MPG is over-expressed, the MPG over-expressed MDA-MB-231 human breast cancer cells were modified to also over-express Pol ß, increasing expression several-fold as compared to endogenous levels. For this, the MPG over-expression cells were complemented with human epitope-tagged Pol ß (Fig. 2A). There is no change in expression of endogenous Pol ß protein nor in expression of the other BER proteins but these cells exhibit robust expression of the transgenic Flag-Pol ß (Fig. 2A). By immunoblot analysis, we estimate that these cells express 5X greater transgenic Pol ß than the endogenous level of Pol ß in these cells. However, over-expression of wild-type Pol ß in our MPG over-expression cells completely restores resistance to TMZ (Fig. 2B) whereas cells with vector control are just as sensitive to TMZ as the original cells, demonstrating that the hypersensitivity of cells to TMZ that over-express MPG is due to un-repaired Pol ß substrates such as the cytotoxic 5′dRP lesions that would accumulate following TMZ exposure if the Pol ß step in BER was rate-limiting.
Fig. 1.
Over-expression of human MPG in MDA-MB-231 cells shifts the rate-limiting step in the BER pathway and induces a temozolomide hypersensitive phenotype. (A) MPG over-expression as determined by immunoblot analysis of nuclear proteins isolated from the MDA-MB-231 or MPG over-expressing MDA-MB-231 cells. Proteins isolated from three separate MPG over-expressing clones are shown [MDA-MB-231/MPG(Clone 4); MDAMB-231/MPG(Clone 5) and MDA-MB-231/MPG(Clone 6); lanes 2-4] as compared to proteins isolated from control cells (MDA-MB-231; lane 1). Pol ß and APE1 expression as determined by immunoblot. PCNA expression is shown as a loading control (lower panel). (B) MDA-MB-231 cells (open circle) or MPG over-expression MDA-MB-231 cells (MDA-MB-231/MPG(Clone 4), filled circle; MDA-MB-231/MPG(Clone 5), filled square and MDA-MB-231/MPG(Clone 6), filled diamond) were cultured in 96-well plates for 24 hours prior to exposure to TMZ. Viable cells were determined using a modified MTT assay. Plots show the % viable cells as compared to untreated (control) cells. Means are calculated from quadruplicate values in each experiment. Results indicate the mean ± S.E. of four independent experiments.
Fig. 2.
Reconstitution of human Flag-Pol ß in MPG over-expressing MDA-MB-231 cells restores resistance to temozolomide. (A) DNA Pol ß expression as determined by immunoblot analysis of nuclear proteins isolated from the MDA-MB-231 or MPG over-expressing MDA-MB-231 cells transfected with Flag-Pol ß. Proteins isolated from three separate Flag-Pol ß expressing clones are shown [MDA-MB-231/MPG(4)/Flag-Pol ß-WT(19), MDA-MB-231/MPG(4)/Flag-Pol ß-WT(20) and MDA-MB-231/MPG(4)/Flag-Pol ß-WT(21); lanes 4-6] as compared to proteins isolated from control cells (MDA-MB-231; lane 1), MPG over-expressing/MDA-MB-231 cells (MDA-MB-231/MPG(4); lane 2) or pIRES-Puro transfected in MPG over-expressing/MDA-MB-231 cells (MDA-MB-231/MPG(4)/Puro(1); lane 3). Flag, MPG and APE1 expression was determined by immunoblot. PCNA expression is shown as a loading control (lower panel). For immunoprecipitation (IP), cell lysate from the above cell lines was incubated overnight with anti-Flag antibodies followed by 1-h incubation with Protein G Dynabeads. Immunoprecipitates were eluted with 3x Flag peptide, separated on SDS-PAGE and transferred to nitrocellulose filters and probed with anti-Pol ß antibody. (B) MDA-MB-231 cells (open circle) or MPG over-expressing MDA-MB-231 cells (MDA-MB-231/MPG(4), filled circle) or pIRES-Puro transfected in MPG over-expressing/MDA-MB-231 cells (MDA-MB-231/MPG(4)/Puro(1), circle within square) or MPG over-expressing/MDA-MB-231 cells expressing Flag-Pol ß [MDA-MB-231/MPG(4)/Flag-Pol ß-WT(19), half square; MDA-MB-231/MPG(4)/Flag-Pol ß-WT(20), filled half square and MDA-MB-231/MPG(4)/Flag-Pol ß-WT(21), inverted half square] were cultured in 96-well plates for 24 hours prior to exposure to TMZ. Viable cells were determined using a modified MTT assay as described in Fig. 1.
Human DNA Pol ß protein is required for resistance to the cytotoxicity of the alkylating agent TMZ
To facilitate the analysis of the BER pathway and Pol ß in particular, in human tumor cells, we designed shRNA expressing vectors, specific for different regions of Pol ß mRNA. As shown in Fig. 3A, five shRNA expression plasmids specific to human Pol ß were developed based on Genbank sequence NM_002690. Of those, four sequences were specific for the human Pol ß open reading frame (ORF) and one sequence was specific for a region outside the ORF, within the 3′UTR. Interestingly, the 3′UTR targeted shRNA proved most effective. The sequence for each shRNA target is shown in Fig. 3B. Each vector (either plasmid or lentivirus) was used to develop single-cell clones and each was analyzed for effectiveness of Pol ß knockdown by probing Pol ß expression via immunoblot analysis of nuclear proteins. The pSuper-Retro shRNA expression vectors were not effective in Pol ß knockdown (data not shown). Interestingly, the ORF specific shRNA lentiviral vectors were only partially or minimally effective (pFIV-H1-Puro-hpolß.2 and pFIV-H1-Puro-hpolß.3) in leading to a decrease in Pol ß expression (Fig. 3C). Although some clones were identified that had lost most expression of Pol ß, many presented with partial expression and some with as much as 50% expression as compared to the parental cell line or an empty vector control (not shown). However, all cell clones expressing the 3′UTR specific Pol ß shRNA (pFIV-H1-Puro-hpolß.1) led to complete loss of Pol ß protein expression (Fig. 3D). Note that the expression of human Pol ß specific shRNA does not impact expression of other BER proteins (Fig. 3D). PCNA expression is shown as a loading control (Fig. 3D). This loss of Pol ß expression has been observed for cells in culture as long as 4 months, we have not analyzed them after longer periods in culture. We next tested the cells for TMZ sensitivity, so as to evaluate the impact of loss of Pol ß. Human tumor cells that have lost Pol ß expression are significantly more sensitive to TMZ than the parental cells (Fig. 3E).
Fig. 3.
Down-regulation of endogenous human Pol ß protein expression in human breast cancer cells. (A) Diagram depicting the target sequence for 5 separate shRNA expressing vectors (plasmid and lentiviral-based), specific for different regions of Pol ß mRNA. Four predicted sequences of shRNA targets are within the open reading frame (ORF) and one sequence was specific for a region outside the ORF, within the 3′UTR. (B) Sequences of the target for five different Pol ß specific shRNA vectors. (C) Down-regulation of endogenous human Pol ß protein expression in human breast cancer cells (MDA-MB-231) after transduction of three different Pol ß shRNA specific lentiviral vectors. DNA Pol ß expression as determined by immunoblot analysis of nuclear proteins isolated from seven separate shRNA-expressing clones is shown. PCNA expression is shown as a loading control (lower panel). (D) DNA Pol ß expression as determined by immunoblot analysis of nuclear proteins isolated from the MDA-MB-231 or MDA-MB-231 cells transduced with a human Pol ß shRNA lentiviral vector. Proteins isolated from three separate shRNA-expressing clones are shown (MDA-MB-231/Pol ß-KD (Clone 2); MDA-MB-231/Pol ß-KD (Clone 10); MDA-MB-231/Pol ß-KD (Clone 18); lanes 2-4) as compared to proteins isolated from control cells (MDA-MB-231; lane 1). APE1 expression as determined by immunoblot. PCNA expression is shown as a loading control (lower panel). (E) MDA-MB-231 cells (open circle) or MDA-MB-231 cells expressing Pol ß specific shRNA [MDA-MB-231/Pol ß-KD (Clone 2), squares; MDA-MB-231/Pol ß-KD (Clone 10), diamonds and MDA-MB-231/Pol ß-KD (Clone 18), triangle] were cultured in 96-well plates for 24 hours prior to exposure to TMZ. Viable cells were determined using a modified MTT assay as described in Fig. 1.
We next evaluated the role of Pol ß in the breast cancer cells with elevated expression of MPG. For these analyses, we combined MPG over-expression and Pol ß knockdown to demonstrate that the Pol ß deficient hypersensitivity to TMZ is mediated by MPG-mediated repair initiation. Immunoblots are shown demonstrating that these human tumor cells can harbor both MPG over-expression plus the loss of Pol ß expression (Fig. 4A). Functionally, we find that over-expression of MPG combined with Pol ß knockdown drastically increases the sensitivity of human tumor cells to TMZ, a decrease in IC50 of almost 10-fold when compared to the parental cells (Fig. 4B). Pol ß therefore plays an important role in BER and protects from TMZ induced cell death, supporting our hypothesis that an active BER pathway could be partially responsible for the TMZ mediated resistance seen in some tumors. Most significantly, these results emphasize the intimate balance in repair protein expression that must be maintained to prevent accumulation of cytotoxic and genotoxic lesions such as repair intermediates (e.g., 5′dRP).
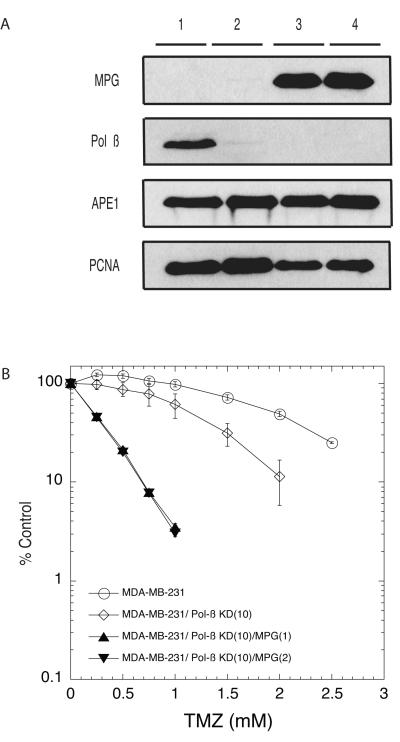
Fig. 4.
Over-expression of human MPG in Pol ß down-regulated MDA-MB-231 cells results in further increase in sensitivity to temozolomide. (A) MPG over-expression as determined by immunoblot analysis of nuclear proteins isolated from the MDA-MB-231 or MPG over-expressing MDA-MB-231 cells transduced with a Pol ß shRNA lentiviral vector. Proteins isolated from two separate MPG over-expressing clones are shown [MDA-MB-231/Pol ß-KD(10)/MPG(1) and MDA-MB-231/Pol ß-KD(10)/MPG(2); lanes 3-4] as compared to proteins isolated from control cells (MDA-MB-231; lane 1) or Pol ß knockdown cells (MDA-MB-231/Pol ß-KD (Clone 10); lane 2). Pol ß and APE1 expression was determined by immunoblot. PCNA expression is shown as a loading control (lower panel). (B) MDA-MB-231 cells (open circle) or MDA-MB-231 cells expressing Pol ß specific shRNA (MDA-MB-231/Pol ß-KD (Clone 10), diamonds) or MPG over-expression in Pol ß down-regulated MDA-MB-231 cells (MDA-MB-231/Pol ß-KD(10)/MPG(1), filled triangle and MDA-MB-231/Pol ß-KD(10)/MPG(2), inverted filled triangle) were cultured in 96-well plates for 24 hours prior to exposure to TMZ. Viable cells were determined using a modified MTT assay as described in Fig. 1.
Hypersensitivity of human Pol ß knockdown cells is due to un-repaired 5′dRP lesions
We have shown that loss of Pol ß expression, as mediated by shRNA expression, or saturation of the Pol ß step in the pathway by MPG over-expression, leads to hypersensitivity to TMZ. To demonstrate that the observed TMZ hypersensitivity is specific to Pol ß (e.g., not due to an aberrant RNAi-mediated non-specific effect), we complemented our Pol ß knockdown cells with RNAi-resistant human epitope-tagged Pol ß. This is feasible by taking advantage of our 3′UTR-specific Pol ß shRNA system (Fig. 3A) and to compliment Pol ß knockdown human tumor cells using expression vectors lacking the shRNA-targeted 3′UTR. As shown, there is no expression of endogenous Pol ß but robust expression of the transgenic Flag-Pol ß (Fig. 5A). Also, note that there is no change in the expression of APE1 (Fig. 5A). To further confirm that the observed expression of Pol ß is not by re-expression of endogenous Pol ß, we characterized the expression of the transgene by immunoprecipitation (IP) using the anti-Flag antibody and immunoblot (IB) analysis of the precipitated material with a Pol ß monoclonal antibody, confirming the presence of Flag epitope-tagged Pol ß only in the transfected cell lines (Fig. 5A, lanes 4-6). Importantly, expression of wild-type Flag-Pol ß in our human tumor Pol ß knockdown cells completely restores resistance to TMZ, whereas cells with a vector control are just as sensitive to TMZ as the original Pol ß knockdown cells (Fig. 5B).
Fig. 5.
Reconstitution of human Flag-Pol ß in Pol ß down-regulated MDA-MB-231 cells restores resistance to temozolomide. (A) DNA Pol ß expression as determined by immunoblot analysis of nuclear proteins isolated from the MDA-MB-231 cells or MDAMB-231/Pol ß KD cells transfected with the Flag-Pol ß. Proteins isolated from three separate Flag-Pol ß expressing clones are shown [MDA-MB-231/Pol ß-KD(10)/Flag-Pol ß-WT(2), MDA-MB-231/Pol ß-KD(10)/Flag-Pol ß-WT(3) and MDA-MB-231/Pol ß-KD(10)/Flag-Pol ß-WT(4); lanes 4-6] as compared to proteins isolated from control cells (MDA-MB-231; lane 1), MDA-MB-231/Pol ß KD cells (MDA-MB-231/Pol ß-KD (Clone 10); lane 2) or pIRES-Neo transfected MDA-MB-231/Pol ß KD cells (MDA-MB-231/Pol ß-KD(10)/Neo(1); lane 3). Flag and APE1 expression was determined by immunoblot. PCNA expression is shown as a loading control (lower panel). For immunoprecipitation (IP), cell lysate from the above cell lines was incubated overnight with anti-Flag antibodies followed by 1-h incubation with Protein G Dynabeads. Immunoprecipitates were eluted with 3x Flag peptide, separated on SDS-PAGE and transferred to nitrocellulose filters and probed with anti-Pol ß antibody. (B) MDA-MB-231 cells (open circle) or MDA-MB-231 cells expressing Pol ß specific shRNA (MDA-MB-231/Pol ß-KD (Clone 10), diamonds) or pIRES-Neo transfected Pol ß KD/MDA-MB-231 cells (MDAMB-231/Pol ß-KD(10)/Neo(1), dotted circle) or Pol ß KD/MDA-MB-231 cells expressing Flag-Pol ß (MDA-MB-231/Pol ß-KD(10)/Flag-Pol ß-WT(2), cross square; MDA-MB-231/Pol ß-KD(10)/Flag-Pol ß-WT(3), plus square and MDA-MB-231/Pol ß-KD(10)/Flag-Pol ß-WT(4), dotted square) were cultured in 96-well plates for 24 hours prior to exposure to TMZ. Viable cells were determined using a modified MTT assay as described in Fig. 1.
However, as we have shown previously in mouse cells, the Pol ß substrate and BER intermediate 5′dRP is highly toxic (Sobol et al., 1996; Sobol et al., 2000), suggesting that the observed hypersensitivity of human Pol ß knockdown cells to TMZ may be due to loss of 5′dRP lyase activity of Pol ß. To this end, we complemented our Pol ß knockdown cells with human epitope-tagged Pol ß encoding a D256A mutation in the polymerase active site that retains complete 5′dRP lyase activity yet is devoid of polymerase activity (Fig. 6A). As with the cells complemented with Pol ß, there is no expression of endogenous Pol ß but robust expression of the transgenic Flag-Pol ß D256A mutant (Fig. 6A). Also, there is no change in the expression of APE1 (Fig. 6A). As above, expression of the transgene was confirmed by a combined IP/IB (Fig. 6A, lanes 4-6). Most importantly, expression of the polymerase-defective/5′dRP lyase active D256A Pol ß mutant in our human tumor Pol ß knockdown cells completely restores resistance to TMZ (Fig. 6B), whereas cells with vector control are just as sensitive to TMZ as the original Pol ß knockdown cells, indicating that the hypersensitivity of cells to TMZ that have lost Pol ß expression (due to Pol ß specific shRNA) is due to un-repaired cytotoxic 5′dRP lesions.
Fig. 6.
Reconstitution of polymerase inactive mutant human Flag-Pol ß (D256A) in Pol ß down-regulated MDA-MB-231 cells restores resistance to temozolomide. (A) DNA Pol ß expression as determined by immunoblot analysis of nuclear proteins isolated from the MDA-MB-231 or Pol ß KD/MDA-MB-231 cells transfected with polymerase inactive mutant Flag-Pol ß (D256A). Proteins isolated from three separate Flag-Pol ß (D256A) expressing clones are shown [MDA-MB-231/Pol ß-KD(10)/Flag-Pol ß-D256A(3), MDAMB-231/Pol ß-KD(10)/Flag-Pol ß- D256A(4) and MDA-MB-231/Pol ß-KD(10)/Flag-Pol ß- D256A(5); lanes 4-6] as compared to proteins isolated from control cells (MDA-MB-231; lane 1), MDA-MB-231/Pol ß KD cells (MDA-MB-231/Pol ß-KD (Clone 10); lane 2) or pIRES-Neo transfected MDA-MB-231/Pol ß KD cells (MDA-MB-231/Pol ß-KD(10)/Neo(1); lane 3). Flag and APE1 expression was determined by immunoblot. PCNA expression is shown as a loading control (lower panel). For immunoprecipitation (IP), cell lysate from the above cell lines was incubated overnight with anti-Flag antibodies followed by 1-h incubation with Protein G Dynabeads. Immunoprecipitates were eluted with 3x Flag peptide, separated on SDS-PAGE and transferred to nitrocellulose filters and probed with anti-Pol ß antibody. (B) MDA-MB-231 cells (open circle) or MDA-MB-231 cells expressing Pol ß specific shRNA (MDA-MB-231/Pol ß-KD (Clone 10), diamonds). pIRES-Neo transfected MDA-MB-231/Pol ß KD cells (MDA-MB-231/Pol ß-KD(10)/Neo(1), dotted circle) or MDA-MB-231/Pol ß KD cells expressing Flag-Pol ß (D256A) [MDA-MB-231/Pol ß-KD(10)/Flag-Pol ß-D256A(3), inverted triangle; MDA-MB-231/Pol ß-KD(10)/Flag-Pol ß-D256A(4), lower shaded squares and MDA-MB-231/Pol ß-KD(10)/Flag-Pol ß-D256A(5), upper shaded square] were cultured in 96-well plates for 24 hours prior to exposure to TMZ. Viable cells were determined using a modified MTT assay as described in Fig. 1.
Minimal or no contribution of apoptosis and autophagy to 5′dRP lesion-induced cell death in MDA-MB-231 cells following temozolomide treatment
To investigate the involvement of apoptosis due to loss of Pol ß or MPG over-expression in MDA-MB-231 cells following TMZ treatment, we measured caspase-3 activation. Additionally we also determined if TMZ induced cell death in these cells could be prevented by pretreatment with Z-VAD-FMK, a pan-caspase inhibitor (Fig. 7). Etoposide (ETO) is a widely used DNA damaging agent to induce apoptosis and was used as an positive control in this study (Bockbrader et al., 2005). The dose response of ETO is essentially the same for all the cell lines used herein (Fig. 7A) and pretreatment with 50 μM Z-VAD-FMK prevented ETO-induced apoptosis in all these cells (Fig. 7B), indicating a functional apoptotic pathway. Interestingly, pretreatment with 50 μM Z-VADFMK failed to prevent TMZ induced cell death (Fig. 7C), suggesting that 5′dRP-induced cell death is not via apoptosis. This is supported by the results from the caspase-3 activation assay, in which we observed a 1-2 fold increase in caspase-3 activation at 6, 12 or 24 hours and a 3-fold increase at 48 hours after TMZ exposure in all the cell lines tested, suggesting a minor level of caspase-3 activation that is independent of 5′dRP-lesion accumulation. As expected, we observed a 6-8 fold increase in caspase-3 activation within 24 hours after ETO exposure (Fig. 7D). Recently Mhaidat et al reported that TMZ does not induce apoptosis in melanoma cells, similar to our results in the MDA-MB-231 cell line presented herein (Mhaidat et al., 2007). The results from the present study support the view that TMZ induced 5′dRP lesion-induced cell death in MDA-MB-231 cells is independent of caspase-3 activation and not via the onset of apoptosis.
Fig. 7.
Analyzing the contribution of apoptosis to 5′dRP lesion-induced cell death in MDA-MB-231 cells following temozolomide treatment. (A) Etoposide (ETO) induced dose response was determined by culturing cells (as labeled in the figure) in 96-well plates for 24 hours prior to exposure to ETO. Viable cells were determined using a modified MTT assay as described in Fig. 1. (B) Cells ⟨MDA-MB-231 cells [group 1]; MDA-MB-231 cells expressing Pol ß specific shRNA (MDA-MB-231/Pol ß-KD (Clone 10), [group 2]; MPG over-expression MDA-MB-231 cells (MDA-MB-231/MPG(Clone 4), [group 3] and MPG over-expression in Pol ß down-regulated MDA-MB-231 cells (MDA-MB-231/Pol ß-KD(10)/MPG(1) [group 4]⟩ were cultured in 96-well plates for 24 hours prior to exposure with media or Z-VAD-FMK (50 μM) for one hour. After Z-VAD-FMK pretreatment, cells were treated with ETO (15 μM) and viable cells were determined using a modified MTT assay as described in Fig. 1. (C) Cells (as labeled in the figure), were cultured in 96-well plates for 24 hours prior to exposure with media or Z-VAD-FMK (50 μM) for one hour. After Z-VAD-FMK pretreatment, cells were treated with TMZ (0.25-2.5 mM) and viable cells were determined using a modified MTT assay as described in Fig. 1. (D) Caspase-3 activation was determined as follows: MDA-MB-231 cells [group 1]; MDA-MB-231 cells expressing Pol ß specific shRNA (MDA-MB-231/Pol ß-KD (Clone 10), [group 2]; MPG over-expression MDA-MB-231 cells (MDA-MB-231/MPG(Clone 4), [group 3] and MPG over-expression in Pol ß down-regulated MDAMB-231 cells (MDA-MB-231/Pol ß-KD(10)/MPG(1) [group 4] were treated with 2.0 mM, 0.8 mM. 0.6 mM or 0.3 mM TMZ (the corresponding TMZ IC50 for each cell line; see Table 1) or media for 6, 12, 24 and 48 hours. These cells were also treated with 15 μM ETO or media for 24 hours. After drug treatment, the cell pellet was resuspended in 1x cell lysis buffer and cytosolic extract was used for the assay of caspase-3 activation.
We next evaluated the impact of 3-Methyladenine (3-MA), a specific inhibitor of autophagy, on 5′dRP lesion mediated cell death in BER defective cells (due to loss of Pol ß or MPG over-expression) following TMZ treatment. 3-MA treatment alone has a similar effect on all the MDA-MB-231 cells used herein (Fig. 8A) and pretreatment with 5 mM 3-MA, as shown in Fig. 8B, failed to prevent TMZ induced cell death. Consistent with these results, we find that 5′dRP-mediated cell death does not induce the formation of autophagosomes, as measured by GFP-LC3 localization. Greater than 99% of all cells, regardless of BER status or TMZ exposure, presented a diffuse pattern of GFPLC3 staining (Fig. 8C). Less than 1% of the cells were observed with a punctate expression pattern of GFP-LC3, indicative of spontaneous formation of autophagosomes (Fig. 8D). However, this pattern was not altered by TMZ exposure or BER protein expression status (not shown), suggestive of a low background level of autophagosome formation in all the cell lines used herein.
Fig. 8.
Measuring the contribution of autophagy to 5′dRP lesion-induced cell death in MDA-MB-231 cells following temozolomide treatment. (A) 3-Methyladenine (3-MA) induced dose response was determined by culturing cells (as labeled in the figure) in 96-well plates for 24 hours prior to exposure to 3-MA. Viable cells were determined using a modified MTT assay as described in Fig. 1. (B) TMZ-induced cytotoxicity following 3-MA pre-treatment was evaluated by culturing cells (as labeled in the figure) in 96-well plates for 24 hours prior to exposure with media or 3-MA (5 mM) for 30 minutes. After 3-MA pretreatment, cells were then treated with TMZ (0.25-2.5 mM) for 48 hr and viable cells were determined using a modified MTT assay as described in Fig. 1. Representative image showing (C) diffuse pattern of pEGFP-LC3 expression observed in >99% of the cells and (D) a punctuate pattern of pEGFP-LC3 expression (see arrow) and localization observed in less than 1% of the cell population. This expression pattern was independent of BER status, TMZ treatment, or 3-MA treatment.
Discussion
Over-expression of methyl-specific DNA glycosylases has been reported to have varying effects in mammalian cells, ranging from no effect to eliciting an increase in or a decrease in sensitivity to alkylating agents (Habraken and Laval, 1993; Ibeanu et al., 1992; Klungland et al., 1992). We and others have found that MPG over-expression induces a DNA-damage sensitivity phenotype in mouse fibroblasts (Trivedi et al., 2005) as well as human breast and ovarian tumor cells (Fishel et al., 2007; Rinne et al., 2004). In this study we demonstrate that both MPG over-expression and Pol ß depletion (via shRNA-mediated knockdown) similarly affect the sensitivity of human breast cancer (MDA-MB-231) cells to alkylation damage. However, it is the collective expression of both of these DNA repair proteins that defines the sensitivity phenotype. Herein, we demonstrate that the Pol ß substrate 5′dRP (a BER intermediate) is the cytotoxic lesion in both MPG over-expressed and Pol ß knockdown (KD) human tumor cells. This TMZ-induced cell death (due to 5′dRP-lesion accumulation) is not likely via caspase-mediated apoptosis, as we do not observe caspase-3 activation following TMZ exposure. Although autophagy has been reported to play a role in TMZ induced cell death in some glioma cell lines when observed 72 hours after exposure (Kanzawa et al., 2004), this study suggests that 5′dRP-mediated cytotoxicity does not induce the formation of autophagosomes, as measured by GFP-LC3 localization, suggesting a possible role of a necrotic mechanism of cell death due to failed repair of the cytotoxic 5′dRP BER intermediate. It also appears that p53 is not involved in the genotoxin-induced cell death observed, as MDA-MB-231 cells express a mutant form of p53 (R280K; http://www.sanger.ac.uk/genetics/CGP/CellLines/), albeit at elevated levels (Hui et al., 2006), in-line with our earlier observations in p53-null mouse cells (Sobol et al., 2003). MPG is the BER initiating enzyme and DNA Pol ß is generally considered the rate-limiting enzyme in BER. In this study, we tested the hypothesis that MPG over-expression induces a BER imbalance that saturates the Pol ß 5′dRP lyase activity in the BER pathway, leading to 5′dRP-mediated cell death in cells that over-express MPG. If left un-repaired, 5′dRP moieties could give rise to an increase in genomic instability (Sobol et al., 2003), eventually leading to cell death (Sobol et al., 2000).
As the first enzyme in the BER process, MPG expression is required for the initiation of BER to repair alkylated bases (Wood et al., 2001). There is a wealth of information regarding the enzymology and structural biology of MPG (Wyatt and Pittman, 2006). However, little is known about the mechanism of MPG initiated BER in vivo. It has been reported that MPG expression levels vary considerably (Cerda et al., 1998; Kim et al., 2003). In addition, MPG is also reported to have multiple post-translational modifications and to form protein-protein interactions with many DNA repair proteins including XRCC1 and hR23A, among others (Almeida and Sobol, 2007). But the role of these post-translational modifications and protein-protein interactions are unknown. Due to the many binding partners of MPG, it might be possible that over-expression of MPG leads to cellular sensitivity by negatively effecting the function of these binding proteins. The studies described here suggest that MPG over-expression increases repair initiation of alkylated bases in vivo. Increased cellular sensitivity is only observed when cells are exposed to TMZ and the TMZ-induced cell sensitivity is altered by the expression level of Pol ß. The increased repair initiation then would lead to an imbalance in BER, in this case by saturating the Pol ß step in the repair pathway.
DNA pol ß has long been considered a DNA repair polymerase (Sobol, 2007). Pol ß is a 335 amino acid, bi-functional enzyme, facilitating both the DNA synthesis step in BER as well as the gap-tailoring step to remove the 5′dRP group formed during BER following APE1 hydrolysis of the abasic site (Almeida and Sobol, 2007). Extensive enzymology, protein biochemistry and structural biology of Pol ß has provided intimate details of the structure of the protein (Beard and Wilson, 2006) and the mechanisms of DNA synthesis and 5′dRP lyase activity conducted by Pol ß using both purified recombinant enzyme and extracts from mouse and human cells (Beard and Wilson, 2006; Prasad et al., 2005). Pol ß facilitates repair via the formation of protein complexes at the lesion site with several BER proteins including APE1, DNA ligase I, XRCC1, PCNA and PARP-1 and -2, among others (Almeida and Sobol, 2007). By genetic complementation of Pol ß null mouse fibroblasts, it was determined that the increased sensitivity of Pol ß null mouse cells to alkylation damage was the result of a failure to repair the 5′dRP BER intermediate (Sobol et al., 1996; Sobol et al., 2000). However, little is known about the significance of the 5′dRP lyase activity of Pol ß in human cells and the impact of Pol ß expression and function on the human cellular response to DNA damage. Cleary, theses studies demonstrate that variations in Pol ß expression affect cellular sensitivity to TMZ in an MPG dependent manner and due to a failure to repair the 5′dRP repair intermediate.
Expression of Pol ß is highly variable in human cells (Srivastava et al., 1999). Pol ß is up-regulated in adenocarcinoma (Srivastava et al., 1999), ovarian tumor (Bergoglio et al., 2001) and glioma cells (Gomi et al., 1996) and is found to be over-expressed in greater than 35% of human tumors (Starcevic et al., 2004). Further, Pol ß function may be altered by post-translational modification, including methylation, acetylation and ubiquitylation (Sobol, 2008a), suggesting a cellular requirement to regulate Pol ß function. In addition, many Pol ß variants with altered function have been isolated from human tumors (Lang et al., 2007; Tan et al., 2005).
DNA Pol ß appears to be the major BER polymerase in MDA-MB-231 human breast cancer cells. RNAi-mediated expression loss of Pol ß does not affect cell viability yet Pol ß KD cells are hypersensitive to the cytotoxic effects of the clinical alkylating agent TMZ and this is further enhanced when MPG expressed in increased. This increase in cellular sensitivity to TMZ is consistent with earlier studies using mouse knockout fibroblasts (Horton et al., 2005; Trivedi et al., 2005). By utilizing this knockdown/knock-in strategy, we show that the hypersensitive phenotype is a direct result of the loss of Pol ß expression, eliminating concerns that the knockdown phenotype may be the result of off-target effects of RNA interference-mediated gene silencing.
These studies address the problem of cellular resistance of alkylation damage induced by the chemotherapeutic agent TMZ yet may be considered a model for many types of base lesions that are repaired by BER. We focused these studies on biochemical and molecular mechanisms that mediate the repair of alkylation-induced DNA damage in human tumor cells. Overall, we show that MPG, Pol ß and base excision repair contributes significantly to the repair of alkylation-induced DNA damage in human cells. Therefore, modulating the BER pathway by MPG over-expression or by Pol ß inhibition / loss of expression could enhance the chemotherapeutic index of agents that damage DNA and initiate BER. Further, cellular conditions that impact Pol ß expression and/or BER balance can predispose a given human cell population to 5′dRP-mediated genome instability and cytotoxicity. However, the overall BER capacity must be considered when evaluating cellular response to TMZ and similar DNA damaging agents. The experiments presented herein suggest that it will be important to investigate whether an active BER pathway could be partially responsible for the TMZ-mediated resistance seen in some tumors and that overall BER capacity may help predict sensitivity to TMZ. Further, it will be important to evaluate whether a BER imbalance will predispose human primary cells to cellular and environmental genotoxins, inducing an increase in genome instability, cellular senescence or in the case of post-mitotic cells (neurons), neurodegeneration due to failed 5′dRP lesion repair. It is also interesting to speculate that the increase in genome instability due to a BER imbalance combined with chronic inflammation (ulcerative colitis) may be brought about by a deficiency in the repair of the BER intermediate 5′dRP (Hofseth et al., 2003). Finally, these findings may have clinical applicability towards the design of specific Pol ß and BER modulators as adjuvant treatments for reversing a TMZ resistant phenotype and suggest that alkylating agents such as TMZ combined with BER modulators may be explored as possible therapeutics in the treatment of breast cancer.
Acknowledgements
We would like to thank Drs. R.D. Wood (University of Pittsburgh), K.A. Eckert (The Pennsylvania State University College of Medicine) and K.H. Almeida (Rhode Island College) for providing helpful discussions.
This research was supported by a Research Scholar grant (RSG-05-246-01-GMC) from the American Cancer Society, grants from the Susan G. Komen Breast Cancer Foundation (Grant # BCTR0403276), NIH (1 R01 AG24364-01; P20 CA103730, 1 P20 CA132385-01 and 1P50 CA 097190 01A1), the Brain Tumor Society, the UPMC Health System Competitive Medical Research Fund and the University of Pittsburgh Cancer Institute to RWS. This project is also funded, in part, under a grant with the Pennsylvania Department of Health. The Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions.
Non-standard abbreviations
- Pol ß
DNA polymerase ß
- shRNA
small hairpin RNA
- BER
base excision repair
- 5′ dRP
5′ deoxyribose-phosphate
- MPG
N-methylpurine DNA glycosylase
- AAG
alkyladenine DNA glycosylase
- APE1
AP endonuclease 1
- TMZ
temozolomide
- WT
wild-type
- DMSO
dimethyl sulfoxide
- PCNA
proliferating cell nuclear antigen
- G418
Neomycin
- KD
knockdown
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide
- IP
immunoprecipitation
- IB
immunoblot
- ORF
open reading frame
- UTR
untranslated region
- Flag-Pol ß (D256A)
polymerase defective mutant of human pol ß
- Z-VAD-FMK
Pan-caspase inhibitor benzyloxycarbonyl-Ala-Asp-fluoromethylketone
- 3-MA
3-Methyladenine
- ETO
etoposide
- DAPI
4′,6-diamidino-2-phenylindole
References
- Almeida KH, Sobol RW. A unified view of base excision repair: lesion-dependent protein complexes regulated by post-translational modification. DNA Repair. 2007;6(6):695–711. doi: 10.1016/j.dnarep.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard WA, Wilson SH. Structure and mechanism of DNA polymerase Beta. Chem Rev. 2006;106(2):361–382. doi: 10.1021/cr0404904. [DOI] [PubMed] [Google Scholar]
- Bergoglio V, Canitrot Y, Hogarth L, Minto L, Howell SB, Cazaux C, Hoffmann JS. Enhanced expression and activity of DNA polymerase ß in human ovarian tumor cells: impact on sensitivity towards antitumor agents. Oncogene. 2001;20(43):6181–6187. doi: 10.1038/sj.onc.1204743. [DOI] [PubMed] [Google Scholar]
- Bockbrader KM, Tan M, Sun Y. A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene. 2005;24(49):7381–7388. doi: 10.1038/sj.onc.1208888. [DOI] [PubMed] [Google Scholar]
- Cerda SR, Turk PW, Thor AD, Weitzman SA. Altered expression of the DNA repair protein, N-methylpurine-DNA glycosylase (MPG) in breast cancer. FEBS Lett. 1998;431(1):12–18. doi: 10.1016/s0014-5793(98)00697-8. [DOI] [PubMed] [Google Scholar]
- Coquerelle T, Dosch J, Kaina B. Overexpression of N-methylpurine-DNA glycosylase in Chinese hamster ovary cells renders them more sensitive to the production of chromosomal aberrations by methylating agents--a case of imbalanced DNA repair. Mutation Research. 1995;336(1):9–17. doi: 10.1016/0921-8777(94)00035-5. [DOI] [PubMed] [Google Scholar]
- Elder RH, Jansen JG, Weeks RJ, Willington MA, Deans B, Watson AJ, Mynett KJ, Bailey JA, Cooper DP, Rafferty JA, Heeran MC, Wijnhoven SW, van Zeeland AA, Margison GP. Alkylpurine-DNA-N-glycosylase knockout mice show increased susceptibility to induction of mutations by methyl methanesulfonate. Molecular and Cellular Biology. 1998;18(10):5828–5837. doi: 10.1128/mcb.18.10.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelward BP, Dreslin A, Christensen J, Huszar D, Kurahara C, Samson L. Repair-deficient 3-methyladenine DNA glycosylase homozygous mutant mouse cells have increased sensitivity to alkylation-induced chromosome damage and cell killing. EMBO Journal. 1996;15(4):945–952. [PMC free article] [PubMed] [Google Scholar]
- Fishel ML, He Y, Smith ML, Kelley MR. Manipulation of base excision repair to sensitize ovarian cancer cells to alkylating agent temozolomide. Clin Cancer Res. 2007;13(1):260–267. doi: 10.1158/1078-0432.CCR-06-1920. [DOI] [PubMed] [Google Scholar]
- Gomi A, Shinoda S, Sakai R, Hirai H, Ozawa K, Masuzawa T. Elevated expression of DNA polymerase beta gene in glioma cell lines with acquired resistance to 1-(4-amino-2-methyl-5-pyrimidinyl)methyl-3-(2-chloroethyl)-3-nitrosourea. Biochem Biophys Res Commun. 1996;227(2):558–563. doi: 10.1006/bbrc.1996.1545. [DOI] [PubMed] [Google Scholar]
- Habraken Y, Laval F. Increased resistance of the Chinese hamster mutant irs1 cells to monofunctional alkylating agents by transfection of the E. coli or mammalian N3-methyladenine-DNA-glycosylase genes. Mutat Res. 1993;293(3):187–195. doi: 10.1016/0921-8777(93)90069-s. [DOI] [PubMed] [Google Scholar]
- Hofseth LJ, Khan MA, Ambrose M, Nikolayeva O, Xu-Welliver M, Kartalou M, Hussain SP, Roth RB, Zhou X, Mechanic LE, Zurer I, Rotter V, Samson LD, Harris CC. The adaptive imbalance in base excision-repair enzymes generates microsatellite instability in chronic inflammation. Journal of Clinical Investigation. 2003;112(12):1887–1894. doi: 10.1172/JCI19757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JK, Stefanick DF, Naron JM, Kedar PS, Wilson SH. Poly(ADP-ribose) polymerase activity prevents signaling pathways for cell cycle arrest following DNA methylating agent exposure. J Biol Chem. 2005;280(16):15773–15785. doi: 10.1074/jbc.M413841200. [DOI] [PubMed] [Google Scholar]
- Horton JK, Watson M, Stefanick DF, Shaughnessy DT, Taylor JA, Wilson SH. XRCC1 and DNA polymerase beta in cellular protection against cytotoxic DNA single-strand breaks. Cell Res. 2008;18(1):48–63. doi: 10.1038/cr.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Zheng Y, Yan Y, Bargonetti J, Foster DA. Mutant p53 in MDA-MB-231 breast cancer cells is stabilized by elevated phospholipase D activity and contributes to survival signals generated by phospholipase D. Oncogene. 2006;25(55):7305–7310. doi: 10.1038/sj.onc.1209735. [DOI] [PubMed] [Google Scholar]
- Ibeanu G, Hartenstein B, Dunn WC, Chang LY, Hofmann E, Coquerelle T, Mitra S, Kaina B. Overexpression of human DNA repair protein N-methylpurine-DNA glycosylase results in the increased removal of N-methylpurines in DNA without a concomitant increase in resistance to alkylating agents in Chinese hamster ovary cells. Carcinogenesis. 1992;13(11):1989–1995. doi: 10.1093/carcin/13.11.1989. [DOI] [PubMed] [Google Scholar]
- Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11(4):448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- Kim NK, Ahn JY, Song J, Kim JK, Han JH, An HJ, Chung HM, Joo JY, Choi JU, Lee KS, Roy R, Oh D. Expression of the DNA repair enzyme, N-methylpurine-DNA glycosylase (MPG) in astrocytic tumors. Anticancer Res. 2003;23(2B):1417–1423. [PubMed] [Google Scholar]
- Klungland A, Fairbairn L, Watson AJ, Margison GP, Seeberg E. Expression of the E.coli 3-methyladenine DNA glycosylase I gene in mammalian cells reduces the toxic and mutagenic effects of methylating agents. Embo J. 1992;11(12):4439–4444. doi: 10.1002/j.1460-2075.1992.tb05544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Dalal S, Chikova A, Dimaio D, Sweasy JB. The E295K DNA polymerase beta gastric cancer-associated variant interferes with base excision repair and induces cellular transformation. Mol Cell Biol. 2007;27(15):5587–5596. doi: 10.1128/MCB.01883-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286(5446):1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- Mhaidat NM, Zhang XD, Allen J, Avery-Kiejda KA, Scott RJ, Hersey P. Temozolomide induces senescence but not apoptosis in human melanoma cells. Br J Cancer. 2007;97(9):1225–1233. doi: 10.1038/sj.bjc.6604017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Furuta T, Ohmoto T, Akiyama K, Seki S. Stable expression in rat glioma cells of sense and antisense nucleic acids to a human multifunctional DNA repair enzyme, APEX nuclease. Mutat Res. 1994;315(1):55–63. doi: 10.1016/0921-8777(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Paik J, Duncan T, Lindahl T, Sedgwick B. Sensitization of human carcinoma cells to alkylating agents by small interfering RNA suppression of 3-alkyladenine-DNA glycosylase. Cancer Res. 2005;65(22):10472–10477. doi: 10.1158/0008-5472.CAN-05-1495. [DOI] [PubMed] [Google Scholar]
- Poeschla EM, Wong-Staal F, Looney DJ. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med. 1998;4(3):354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- Prasad R, Batra VK, Yang XP, Krahn JM, Pedersen LC, Beard WA, Wilson SH. Structural insight into the DNA polymerase beta deoxyribose phosphate lyase mechanism. DNA Repair (Amst) 2005;4(12):1347–1357. doi: 10.1016/j.dnarep.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Rinne M, Caldwell D, Kelley MR. Transient adenoviral N-methylpurine DNA glycosylase overexpression imparts chemotherapeutic sensitivity to human breast cancer cells. Mol Cancer Ther. 2004;3(8):955–967. [PubMed] [Google Scholar]
- Sobol RW. DNA polymerase ß null mouse embryonic fibroblasts harbor a homozygous null mutation in DNA polymerase iota. DNA Repair (Amst) 2007;6(1):3–7. doi: 10.1016/j.dnarep.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobol RW. CHIPping Away at Base Excision Repair. Mol Cell. 2008a;29(4):413–415. doi: 10.1016/j.molcel.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Sobol RW. Temozolomide. In: Schwab M, editor. Encyclopedia of Cancer. Springer; Berlin, Heidelberg, New York: 2008b. In Press. [Google Scholar]
- Sobol RW, Horton JK, Kuhn R, Gu H, Singhal RK, Prasad R, Rajewsky K, Wilson SH. Requirement of mammalian DNA polymerase-ß in base-excision repair. Nature. 1996;379(6561):183–186. doi: 10.1038/379183a0. [DOI] [PubMed] [Google Scholar]
- Sobol RW, Kartalou M, Almeida KH, Joyce DF, Engelward BP, Horton JK, Prasad R, Samson LD, Wilson SH. Base Excision Repair Intermediates Induce p53-independent Cytotoxic and Genotoxic Responses. J Biol Chem. 2003;278(41):39951–39959. doi: 10.1074/jbc.M306592200. [DOI] [PubMed] [Google Scholar]
- Sobol RW, Prasad R, Evenski A, Baker A, Yang XP, Horton JK, Wilson SH. The lyase activity of the DNA repair protein ß-polymerase protects from DNA-damage-induced cytotoxicity. Nature. 2000;405(6788):807–810. doi: 10.1038/35015598. [DOI] [PubMed] [Google Scholar]
- Srivastava DK, Husain I, Arteaga CL, Wilson SH. DNA polymerase ß expression differences in selected human tumors and cell lines. Carcinogenesis. 1999;20(6):1049–1054. doi: 10.1093/carcin/20.6.1049. [DOI] [PubMed] [Google Scholar]
- Starcevic D, Dalal S, Sweasy JB. Is there a link between DNA polymerase beta and cancer? Cell Cycle. 2004;3(8):998–1001. [PubMed] [Google Scholar]
- Tan XH, Zhao M, Pan KF, Dong Y, Dong B, Feng GJ, Jia G, Lu YY. Frequent mutation related with overexpression of DNA polymerase beta in primary tumors and precancerous lesions of human stomach. Cancer Lett. 2005;220(1):101–114. doi: 10.1016/j.canlet.2004.07.049. [DOI] [PubMed] [Google Scholar]
- Trivedi RN, Almeida KH, Fornsaglio JL, Schamus S, Sobol RW. The Role of Base Excision Repair in the Sensitivity and Resistance to Temozolomide Mediated Cell Death. Cancer Res. 2005;65(14):6394–6400. doi: 10.1158/0008-5472.CAN-05-0715. [DOI] [PubMed] [Google Scholar]
- Walker LJ, Craig RB, Harris AL, Hickson ID. A role for the human DNA repair enzyme HAP1 in cellular protection against DNA damaging agents and hypoxic stress. Nucleic Acids Res. 1994;22(23):4884–4889. doi: 10.1093/nar/22.23.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RD, Mitchell M, Sgouros J, Lindahl T. Human DNA repair genes. Science. 2001;291(5507):1284–1289. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- Wyatt MD, Pittman DL. Methylating agents and DNA repair responses: Methylated bases and sources of strand breaks. Chem Res Toxicol. 2006;19(12):1580–1594. doi: 10.1021/tx060164e. [DOI] [PMC free article] [PubMed] [Google Scholar]