Abstract
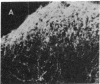
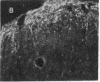
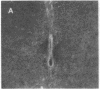
Antisera generated to substance P-Gly (SP-G) and substance P-Gly-Lys (SP-G-K), the likely unamidated COOH-terminally extended forms of substance P, were used to quantify and localize substance P precursor forms in hamster brain stem and spinal cord. The precursor determinant SP-G-K was liberated from larger heterogeneous forms by mild trypsinization of tissue extracts and was converted into the second precursor determinant, SP-G, by subsequent treatment with carboxypeptidase B. The basal levels of SP-G-K in brain stem and spinal cord were approximately equal to 0.5 pg/mg of tissue and rose 43- to 64-fold after trypsinization. Basal levels of SP-G were comparable to those of SP-G-K and rose 10- to 29-fold after combined enzyme treatments. Immunohistochemical labeling of axons and somata with anti-SP-G-K increased dramatically after trypsinization. This labeling was eliminated by preadsorption with authentic SP-G-K but not substance P or SP-G. Gel-permeation chromatography revealed SP-G-K-like immunoreactivity in fractions corresponding to considerably higher molecular weight than mature substance P. Collectively, these results support the hypothesis that substance P is synthesized from larger precursors and demonstrate that extended precursor forms are normally present in the axons and somata of neural systems that synthesize substance P.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Smyth D. G. Substrate specificity of an amidating enzyme in porcine pituitary. Biochem Biophys Res Commun. 1983 Apr 29;112(2):372–377. doi: 10.1016/0006-291x(83)91473-0. [DOI] [PubMed] [Google Scholar]
- Chang M. M., Leeman S. E. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem. 1970 Sep 25;245(18):4784–4790. [PubMed] [Google Scholar]
- Davis B. J., Burd G. D., Macrides F. Localization of methionine-enkephalin, substance P, and somatostatin immunoreactivities in the main olfactory bulb of the hamster. J Comp Neurol. 1982 Feb 1;204(4):377–383. doi: 10.1002/cne.902040408. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Torrens Y., Beaujouan J. C. The striatonigral substance P pathway and dopaminergic mechanisms. Ciba Found Symp. 1982;(91):281–295. doi: 10.1002/9780470720738.ch16. [DOI] [PubMed] [Google Scholar]
- Harmar A., Keen P. Synthesis, and central and peripheral axonal transport of substance P in a dorsal root ganglion-nerve preparation in vitro. Brain Res. 1982 Jan 14;231(2):379–385. doi: 10.1016/0006-8993(82)90374-2. [DOI] [PubMed] [Google Scholar]
- Harmar A., Schofield J. G., Keen P. Cycloheximide-sensitive synthesis of substance P by isolated dorsal root ganglia. Nature. 1980 Mar 20;284(5753):267–269. doi: 10.1038/284267a0. [DOI] [PubMed] [Google Scholar]
- Harmar A., Schofield J. G., Keen P. Substance P biosynthesis in dorsal root ganglia: an immunochemical study of [35S]methionine and [3H]proline incorporation in vitro. Neuroscience. 1981;6(10):1917–1922. doi: 10.1016/0306-4522(81)90031-2. [DOI] [PubMed] [Google Scholar]
- Ho R. H., DePalatis L. R. Substance P immunoreactivity in the median eminence of the North American opossum and domestic fowl. Brain Res. 1980 May 12;189(2):565–569. doi: 10.1016/0006-8993(80)90370-4. [DOI] [PubMed] [Google Scholar]
- Keen P., Harmar A. J., Spears F., Winter E. Biosynthesis, axonal transport and turnover of neuronal substance P. Ciba Found Symp. 1982;(91):145–164. doi: 10.1002/9780470720738.ch9. [DOI] [PubMed] [Google Scholar]
- Krause J. E., Reiner A. J., Advis J. P., McKelvy J. F. In vivo biosynthesis of [35S]- and [3H]substance P in the striatum of the rat and their axonal transport to the substantia nigra. J Neurosci. 1984 Mar;4(3):775–785. doi: 10.1523/JNEUROSCI.04-03-00775.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kream R. M., Davis B. J., Kawano T., Margolis F. L., Macrides F. Substance P and catecholaminergic expression in neurons of the hamster main olfactory bulb. J Comp Neurol. 1984 Jan 1;222(1):140–154. doi: 10.1002/cne.902220112. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Sandberg B. E., Hanley M. R., Iversen L. L. Purification and characterisation of a membrane-bound substance-P-degrading enzyme from human brain. Eur J Biochem. 1981 Feb;114(2):315–327. doi: 10.1111/j.1432-1033.1981.tb05151.x. [DOI] [PubMed] [Google Scholar]
- Lewis R. V., Stern A. S. Biosynthesis of the enkephalins and enkephalin-containing polypeptides. Annu Rev Pharmacol Toxicol. 1983;23:353–372. doi: 10.1146/annurev.pa.23.040183.002033. [DOI] [PubMed] [Google Scholar]
- Ljungdahl A., Hökfelt T., Nilsson G., Goldstein M. Distribution of substance P-like immunoreactivity in the central nervous system of the rat--II. Light microscopic localization in relation to catecholamine-containing neurons. Neuroscience. 1978;3(10):945–976. doi: 10.1016/0306-4522(78)90117-3. [DOI] [PubMed] [Google Scholar]
- Nawa H., Hirose T., Takashima H., Inayama S., Nakanishi S. Nucleotide sequences of cloned cDNAs for two types of bovine brain substance P precursor. Nature. 1983 Nov 3;306(5938):32–36. doi: 10.1038/306032a0. [DOI] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol Rev. 1983 Jun;35(2):85–141. [PubMed] [Google Scholar]
- Sperk G., Singer E. A. In vivo synthesis of substance P in the corpus striatum of the rat and its transport to the substantia nigra. Brain Res. 1982 Apr 22;238(1):127–135. doi: 10.1016/0006-8993(82)90776-4. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Konishi S., Powell D., Leeman S. E., Otsuka M. Identification of the motoneuron-depolarizing peptide in bovine dorsal root as hypothalamic substance P. Brain Res. 1974 Jun 14;73(1):59–69. doi: 10.1016/0006-8993(74)91007-5. [DOI] [PubMed] [Google Scholar]
- Torrens Y., Michelot R., Beaujouan J. C., Glowinski J., Bockaert J. In vivo biosynthesis of 35S-substance P from [35S]methionine in the rat striatum and its transport to the substantia nigra. J Neurochem. 1982 Jun;38(6):1728–1734. doi: 10.1111/j.1471-4159.1982.tb06655.x. [DOI] [PubMed] [Google Scholar]
- Winter E., Keen P. Effects of synthesis inhibition and nervous activity on concentrations of neuronal substance P. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jun;323(2):173–175. doi: 10.1007/BF00634267. [DOI] [PubMed] [Google Scholar]