Abstract
Objectives. We investigated associations of smoking and coronary heart disease (CHD) by age.
Methods. Data came from the Pooling Project on Diet and Coronary Heart Disease (8 prospective studies, 1974–1996; n = 192 067 women and 74 720 men, aged 40–89 years).
Results. During follow-up, 4326 cases of CHD were reported. Relative to never smokers, CHD risk among current smokers was highest in the youngest and lowest in the oldest participants. For example, among women aged 40 to 49 years the hazard ratio was 8.5 (95% confidence interval [CI] = 5.0, 14) and 3.1 (95% CI = 2.0, 4.9) among those aged 70 years or older. The largest absolute risk differences between current smokers and never smokers were observed among the oldest participants. Finally, the majority of CHD cases among smokers were attributable to smoking. For example, attributable proportions of CHD by age group were 88% (40-49 years), 81% (50-59 years), 71% for (60-69 years), and 68% (≥ 70 years) among women who smoked.
Conclusions. Among smokers, the majority of CHD cases are attributable to smoking in all age groups. Smoking prevention is important, irrespective of age.
Despite decades of attempts to reduce smoking prevalence, 20% of persons living in the United States still smoke, and smoking remains the number one cause of preventable mortality.1,2 A leading cause of death attributable to smoking is coronary heart disease (CHD).3
CHD etiology differs across age groups. For instance, relatively more cases of CHD among young adults may be attributable to genetic causes.4,5 Hence, among young adults, who are at low absolute risk for CHD, smoking may considered a risk factor that does not cause disease until later in life.
At the other end of the age scale, research suggests that the relative risk of CHD associated with smoking attenuates in old age.6 This finding could erroneously suggest that smoking is only a weak risk factor for the elderly and that smoking prevention should therefore be of low priority because quality-of-life issues outweigh the net gain in health. With an increasingly older population, understanding patterns in the strength of risk factors by age is of considerable interest.
The incidence of CHD varies considerably by age; it is very low in women younger than 40 years and in men younger than 50 years.7 For this reason, the statistical power to investigate effects of smoking on CHD in young adults is limited. We pooled the data from 8 prospective cohort studies with information on smoking and potential confounders, including diet, to gain a sufficient sample size to investigate associations between smoking and CHD in subsets of populations defined by age.
METHODS
The Pooling Project on Diet and Coronary Heart Disease comprised data from 12 prospective cohort studies. Each study had at least 150 incident CHD cases, assessed usual dietary intake, and conducted a validation study of the diet assessment method. The project’s studies were the Adventists Health Study,8 Atherosclerosis Risk in Communities Study,9 Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC),10 Finnish Mobile Clinic Health Examination,11 Glostrup Population Study,12 Health Professionals Follow-up Study,13 Israeli Ischemic Heart Disease Study,14 Iowa Women’s Health Study,15 Nurses’ Health Study (NHS),16 Västerbotten Intervention Program,17 and Women’s Health Study.18 For our analysis, we excluded data from the Adventists, Finnish, and Israeli studies because of missing information on 1 or more potential confounders (education, physical activity, alcohol intake). We also excluded the Iowa study because of self-reported information on CHD. Characteristics of the 8 included studies are presented in Table 1. We divided NHS data into 2 segments: NHSa (1980–1986) and NHSb (1986–1996) to take advantage of repeated-exposure assessment. The ATBC included only men who smoked. To verify that this did not affect our results, we repeated the analyses without the ATBC participants, without significant changes to our results.
TABLE 1—
Characteristics of Studies and Participants: Pooling Project on Diet and Coronary Heart Disease, 1974–1996
| Participants |
CHD Cases |
|||||||||
| Study | Enrolled, No. | Step I,a No. | Final,b No. | Study Years | Women, % | Age, Years, Mean (Range) | Current Smoker, % | Follow-Up, Person-Years | Deaths, No. | Total Events, No. |
| ARIC | 15 732 | 11 721 | 11 572 | 1987–1989 | 55 | 53.8 (44–67) | 25 | 102 744 | 68 | 381 |
| ATBC | 29 133 | 21 141 | 21 120 | 1984–1988 | 0 | 56.5 (50–70) | 100 | 121 692 | 534 | 1338 |
| GPS | 4072 | 3324 | 2803 | 1974–1995 | 54 | 50.0 (35–70) | 43 | 25 021 | 93 | 107 |
| HPFS | 51 529 | 41 754 | 38 654 | 1986–1988 | 0 | 52.6 (39–77) | 29 | 354 990 | 384 | 1167 |
| NHSa | 92 468 | 81 415 | 79 479 | 1980–1982 | 100 | 46.8 (35–67) | 29 | 501 752 | 95 | 390 |
| NHSb | 73 666 | 61 706 | 60 083 | 1986–1988 | 100 | 52.4 (40–67) | 21 | 591 107 | 200 | 672 |
| VIP | 25 732 | 20 076 | 18 244 | 1992–1996 | 54 | 50.0 (39–70) | 21 | 75 420 | 37 | 134 |
| WHS | 39 876 | 37 272 | 34 832 | 1992–1995 | 100 | 52.0 (38–89) | 13 | 178 192 | 9 | 137 |
| Total | 332 208 | 278 409 | 266 787 | 1974–1996 | 58 | 51.8 (35–89) | 35 | 1 950 918 | 1420 | 4326 |
Note. ARIC = Atherosclerosis Risk in Communities Study; ATBC =Alpha-Tocopherol Beta-Carotone Cancer Prevention Study; CHD = coronary heart disease; GPS = Glostrup Population Study; HPFS = Health Professionals Follow-up Study; NHSa = Nurses’ Health Study (1980–1986); NHSb Nurses' Health Study (1986–1996); VIP = Västerbotten Intervention Program; WHS = Women’s Health Study.
Sample size after exclusion of participants with baseline cardiovascular disease, cancer, and diabetes.
Sample size after exclusion of participants with missing information on smoking status and other covariates.
We excluded participants younger than 40 years. Because the presence of clinical disease itself may change smoking habits, we also excluded participants who reported a history of cardiovascular disease, diabetes or cancer (except nonmelanoma skin cancer) at baseline. We excluded participants with missing information on smoking or potential confounders from further analyses.
Measurements
We harmonized variables from the individual studies. We harmonized information on smoking into 2 variables: smoking status (never, former, current) and amount of smoking for current smokers (assuming 1 cigarette to be equivalent to 1 g of tobacco, 1 cheroot or 1 cigar to be equivalent to 3 g of tobacco, and 1 pipe to be equivalent to 5 g of tobacco). We assessed average daily alcohol intake from a question about typical intake of alcohol-containing beverages. For each beverage, we calculated grams of daily alcohol intake from responses on the amount and frequency of intake and the alcohol content of the beverage (according to study-specific conversion factors). We calculated total alcohol intake (g/day) by adding the beverage-specific intakes.
Physical activity measures varied across cohorts, calculated either according to an energy expenditure score of weekly time spent on various activities during the past year (Atherosclerosis Risk in Communities Study, Health Professionals Follow-up Study, NHS, Västerbotten Intervention Program, Women’s Health Study) or according to the intensity of the average weekly physical activity during the past year (ATBC, Glostrup Population Study). We harmonized these measures to a 5-level variable from 1 (least active) to 5 (most active).19–21 We calculated body mass index (defined as weight in kilograms divided by the square of height in meters) from self-reported height and weight (NHS, Women’s Health Study, Health Professionals Follow-Up Study) or from measurements taken during physical examinations (Glostrup Population Study, ATBC, Atherosclerosis Risk in Communities Study, Västerbotten Intervention Program).
Studies determined dietary intake at baseline with a food-frequency questionnaire or a dietary history interview. We calculated total energy intake as the sum of energy intake derived from fat, carbohydrates, and protein. Derived exposure measures were dietary intake of saturated, monounsaturated, and polyunsaturated fatty acids; fiber; and cholesterol. We evaluated the validation and repeatability of the diet assessment methods and found them to be reasonable for population studies of the nutrients of interest.
Our outcome of interest was incident CHD events (fatal and nonfatal). We applied standardized criteria for case ascertainment.22
Statistical Analysis
We identified all known and suspected risk factors not believed to be on the causal pathway between exposure and disease as potential confounders. Hence, our multivariate models incorporated educational level (< high school, high school, > high school), body mass index (< 18.5, 18.5–24.9, 25.0–29.9, and ≥ 30 kg/m2), total energy intake (kcal/day), and energy-adjusted quintiles of cholesterol, dietary fiber, saturated fat, monounsaturated fat, and polyunsaturated fat intakes. We modeled alcohol intake continuously and included a linear and root-squared term to account for the U-shaped association between alcohol and risk of CHD.23
We combined individual studies with an aggregated pooled-analysis technique allowing for calculation of a single pooled exposure–effect estimate.24 We estimated hazard ratios (HRs) of CHD by Cox proportional hazards regression with age as underlying time scale, allowing for delayed entry and including study as a fixed effect. We followed participants from baseline to date of CHD (fatal or nonfatal), death, or end of follow-up, whichever occurred first. We truncated follow-up periods longer than 10 years (Glostrup Population Study, Atherosclerosis Risk in Communities Study) to reduce heterogeneity between studies. We estimated multivariate adjusted absolute risks (incidence rates) by Poisson regression. We calculated risk differences and derived 95% confidence intervals (CIs) by bootstrap estimation (5000 replications), with the 2.5 and 97.5 percentiles of the distribution as lower and upper limits. We adjusted all models for year of baseline questionnaire, education, alcohol intake, body mass index, physical activity, total energy intake, and intakes of polyunsaturated fat, monounsaturated fat, saturated fat, fiber, and cholesterol.
We estimated the attributable fractions among current smokers as (RR-1)/RR, where RR (relative risk) was substituted with HR for CHD among current smokers relative to never smokers.25 We analyzed the study population separately for 4 age groups: 40 to 49 years, 50 to 59 years, 60 to 69 years, and 70 years and older. We updated all variables during follow-up and assigned participants to the appropriate age category; thus an individual could contribute person-time at risk to more than 1 age category. We assessed heterogeneity between study-specific effects by including an interaction term between smoking and study origin under the null hypothesis of no between-study differences in the HRs of CHD by smoking. We also compared the pooled risk estimates after systematically excluding each study from the analysis, 1 at a time.
RESULTS
The population of the 8 included studies comprised 192 067 women and 74 720 men (Table 1). The mean age was 51.8 years (range = 38–77 years). At baseline, 35% were current smokers.
Distributions of covariates according to smoking status were comparable across cohorts. For example, current smokers generally drank more alcohol than never smokers, were less physically active, and had lower intakes of fiber and saturated fat (Table 2).
TABLE 2—
Baseline Characteristics of Participants by Gender and Smoking Status: Pooling Project on Diet and Coronary Heart Disease, 1974–1996
| Characteristic | Total | Never Smokers | Past Smokers | Current Smokers, 1–14 Grams/Day | Current Smokers, ≥ 15 Grams/Day |
| Women | |||||
| Participants, no. | 192 067 | 87 855 | 59 699 | 26 6601 | 17 912 |
| Age, y, mean (SD) | 50.4 (7.6) | 50.5 (7.7) | 50.8 (7.6) | 50.8 (7.3) | 47.4 (6.8) |
| Education < high school, no. (%) | 8276 (4) | 3978 (6) | 1844 (3) | 1937 (7) | 517 (3) |
| Alcohol intake, g/d (5th, 95th percentile) | 1.8 (0.0, 28) | 0.9 (0.0, 16) | 2.7 (0.0, 29) | 2.2 (0.0, 35) | 2.7 (0.0, 37) |
| BMI, kg/m2, mean (SD) | 25.0 (4.7) | 25.3 (4.8) | 25.2 (4.7) | 24.4 (4.3) | 23.9 (4.1) |
| Physical inactivity, No. (%) | 66 248 (25) | 28 742 (33) | 17 718 (30) | 8878 (33) | 10 910 (61) |
| Polyunsaturated fat intake,a g/d (5th, 95th percentile) | 5.4 (3.3, 8.4) | 5.4 (3.4, 8.3) | 5.5 (3.3, 8.5) | 5.5 (3.4, 8.7) | 5.0 (3.0, 8.2) |
| Monounsaturated fat intake,a g/d (5th, 95th percentile) | 13.1 (8.1, 20.5) | 12.9 (8.1, 20.3) | 12.6 (7.8, 19.9) | 12.9 (8.5, 19.2) | 16.1 (9.7, 22.7) |
| Saturated fat intake,a g/d (5th, 95th percentile) | 12.8 (7.7, 20.0) | 12.6 (7.6, 19.8) | 12.3 (7.4, 19.5) | 12.8 (8.1, 19.5) | 15.9 (9.7, 22.1) |
| Fiber intake, g/d (5th, 95th percentile) | 15.4 (8.4, 25.7) | 15.9 (8.9, 26.2) | 16.0 (8.9, 26.3) | 14.7 (8.4, 24.1) | 11.7 (6.4, 20.3) |
| Cholesterol intake, mg/d (5th, 95th percentile) | 254 (139-458) | 250 (136-452) | 249 (137-454) | 243 (139-263) | 310 (173-519) |
| Total energy intake, Mcal/d (5th, 95th percentile) | 1.60 (0.90, 2.63) | 1.63 (0.92, 2.65) | 1.60 (0.91, 2.61) | 1.60 (0.89, 2.66) | 1.49 (0.81, 2.49) |
| Hypertension, no. (%) | 35 637 (19) | 16 839 (19) | 11 542 (19) | 4786 (18) | 2470 (14) |
| Dyslipidaemia, no. (%) | 20 850 (11) | 9637 (11) | 7109 (12) | 2922 (11) | 1182 (7) |
| Men | |||||
| Participants, no. | 74 720 | 24 594 | 21 982 | 7403 | 20 741 |
| Age, y, mean (SD) | 54.0 (8.4) | 51.7 (9.1) | 54.1 (9.1) | 55.4 (7.8) | 56.3 (5.7) |
| Education < high school, no. (%) | 20 773 (28) | 2588 (11) | 2775 (13) | 4082 (55) | 11 328 (55) |
| Alcohol intake, g/d (5th, 95th percentile) | 6.5 (0.0, 47) | 3.1 (0.0, 32) | 7.1 (0.0, 44) | 6.8 (0.0, 45) | 12.2 (0.0, 64) |
| BMI, kg/m2, mean (SD) | 25.8 (3.5) | 25.5 (3.4) | 26.0 (3.4) | 25.9 (3.5) | 26.1 (3.8) |
| Physical inactivity, no. (%) | 18 850 (25) | 4466 (18) | 4021 (18) | 2096 (28) | 8267 (40) |
| Polyunsaturated fat intake,a g/d (5th, 95th percentile) | 5.4 (3.3, 8.9) | 5.5 (3.6, 8.4) | 5.6 (3.6, 8.6) | 5.0 (3.2, 9.4) | 4.8 (3.0, 9.8) |
| Monounsaturated fat intake,a g/d (5th, 95th percentile) | 12.8 (8.5, 16.8) | 12.4 (7.9, 16.6) | 12.5 (8.0, 17.0) | 13.1 (9.7, 16.9) | 13.4 (10.1, 16.8) |
| Saturated fat intake,a g/d (5th, 95th percentile) | 13.2 (7.5, 23.8) | 11.5 (6.8, 17.7) | 11.6 (6.9, 17.8) | 16.7 (8.9, 25.2) | 18.3 (10.5, 26.4) |
| Fiber intake, g/d (5th, 95th percentile) | 19.8 (11.5, 32.3) | 21.0 (12.6, 34.5) | 20.2 (12.0, 33.1) | 19.5 (11.4, 30.2) | 18.1 (10.3, 29.0) |
| Cholesterol intake, mg/d (5th, 95th percentile) | 322 (174-573) | 283 (160-477) | 291 (166-491) | 352 (182-591) | 403 (243-660) |
| Total energy intake, Mcal/d (5th, 95th percentile) | 2.14 (1.15, 3.67) | 1.94 (1.10, 3.20) | 1.90 (1.08, 3.17) | 2.41 (1.27, 3.91) | 2.63 (1.45, 4.17) |
| Hypertension, no. (%) | 14 388 (19) | 4479 (18) | 5038 (23) | 1350 (18) | 3521 (17) |
| Dyslipidaemia, no. (%) | 5076 (9) | 2052 (8) | 2294 (10) | 256 (8) | 474 (12) |
Energy adjusted.
During a mean follow-up time of 7.2 years among women and 8.0 years among men, studies reported 1365 and 2961 CHD cases, respectively. In pooled analysis, the multivariate adjusted HR (relative to female never smokers) was 1.30 (95% CI = 1.11, 1.51) among women who were past smokers, 4.12 (95% CI = 3.57, 4.76) among women who smoked 1 to 14 grams per day, and 4.85 (95% CI = 4.00, 5.88) among women who smoked more than 15 grams per day. Corresponding HRs among men were 1.16 (95% CI = 1.03, 1.30), 1.95 (95% CI = 1.66, 2.28), and 2.35 (95% CI = 2.03, 2.71; Table 3). We detected no sign of heterogeneity between studies (P = .3 in women and P = .11 in men). Regressions that excluded each study in turn confirmed that no single study had particular influence on the pooled estimates. Hence, we considered the pooled HRs to be appropriate summaries of the study-specific data.
TABLE 3—
Study-Specific and Adjusted Pooled Hazard Ratios of Coronary Heart Disease by Smoking Status: Pooling Project on Diet and Coronary Heart Disease, 1974–1996
| Study | Total | Never Smokers | Past Smokers | Current Smokers, 1–14 g/d | Current Smokers, ≥ 15 g/d |
| Women, No. or HR (95% CI) | |||||
| CHD cases | 378 | 306 | 454 | 227 | |
| ARIC | 6406 | 1.00 (Ref) | 0.61 (0.32, 1.18) | 3.68 (2.26, 6.00) | 3.48 (2.17, 5.58) |
| GPS | 1509 | 1.00 (Ref) | 2.76 (1.18, 6.48) | 3.35 (1.45, 7.75) | 1.68 (0.72, 3.96) |
| NHS (1980–1986) | 79 479 | 1.00 (Ref) | 1.53 (1.12, 2.09) | 2.92 (1.98, 4.30) | 5.42 (4.16, 7.05) |
| NHS (1986–1996) | 60 083 | 1.00 (Ref) | 1.32 (1.07, 1.63) | 4.20 (3.47, 5.08) | NA |
| VIP | 9758 | 1.00 (Ref) | 2.85 (0.68, 2.00) | 7.82 (2.18, 28.0) | NA |
| WHS | 34 832 | 1.00 (Ref) | 1.37 (0.89, 2.13) | 5.63 (3.67, 8.65) | NA |
| Pooled | 192 067 | 1.00 (Ref) | 1.30 (1.11, 1.51) | 4.12 (3.57, 4.76) | 4.85 (4.00, 5.88) |
| Men, No. or HR (95% CI) | |||||
| CHD cases | 567 | 672 | 375 | 1347 | |
| ARIC | 5166 | 1.00 (Ref) | 1.49 (1.05, 2.12) | 1.06 (0.54, 2.06) | 2.91 (2.01, 4.21) |
| GPS | 1294 | 1.00 (Ref) | 1.41 (0.39, 5.11) | 2.30 (0.91, 5.83) | 1.16 (0.34, 3.96) |
| HPFS | 38 654 | 1.00 (Ref) | 1.13 (0.99, 1.28) | 1.59 (1.16, 2.18) | 2.24 (1.83, 2.73) |
| VIP | 8486 | 1.00 (Ref) | 1.01 (0.63, 1.61) | 2.86 (1.80, 4.52) | NA |
| Pooled | 74 720 | 1.00 (Ref) | 1.16 (1.03, 1.30) | 1.95 (1.66, 2.28) | 2.35 (2.03, 2.71) |
Note. ARIC = Atherosclerosis Risk in Communities Study; CHD = coronary heart disease; CI = confidence interval; GPS = Glostrup Population Study; HPFS = Health Professionals Follow-up Study; HR = hazard ratio; NA = not applicable because of limited number of cases; NHS = Nurses’ Health Study; VIP = Västerbotten Intervention Program; WHS = Women’s Health Study. Multivariable hazard ratios were adjusted for age, year of baseline questionnaire, study origin, alcohol intake, body mass index, education, physical activity, and energy, polyunsaturated fat, monounsaturated fat, saturated fat, fiber, and cholesterol intake.
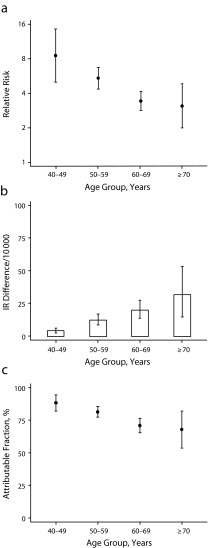
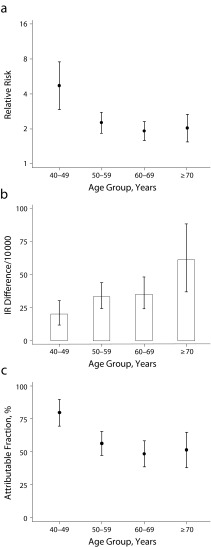
We estimated multivariate-adjusted HRs, incidence rate differences, and attributable fractions of CHD in 4 age groups (40–49, 50–59, 60–69, and ≥ 70 years; Figures 1 and 2). The HR of CHD in current smokers, relative to never smokers, was highest in the youngest and lowest in the oldest women and men. For example, the HR among female current smokers aged 40 to 49 years was 8.5 (95% CI = 5.0, 14) and 3.1 (95% CI = 2.0, 4.9) among those aged 70 years and older. By contrast, we observed higher incidence rate differences in the oldest women and men. For example, the incidence rate differences (per 10 000 person-years) between women who smoked and never smokers were 4.4 (95% CI = 2.8, 6.4) among participants aged 40 to 49 years and 32 (95% CI = 15, 54) among those aged 70 years and older. The CHD-attributable fractions among smokers were highest among the youngest and lowest among the oldest women and men. However, in all age groups and among both men and women, a substantial proportion of cases among smokers could be attributed to smoking. For example, attributable fractions among women who smoked were 88% (95% CI = 82%, 94%) for participants aged 40 to 49 years, 81% (95% CI = 77%, 85%) for those aged 50 to 59 years, 71% (95% CI = 65%, 76%) for those aged 60 to 69 years, and 68% (95% CI = 53%, 82%) for those aged 70 years and older.
FIGURE 1—
Coronary heart disease (CHD) among women by (a) relative risk among current smokers relative to never smokers, (b) incidence rate (IR) differences among current smokers relative to never smokers, and (c) attributable fractions among current smokers.
Note. Vertical bars represent 95% confidence intervals. Analyses adjusted for year of baseline questionnaire, study origin, education, alcohol intake, body mass index, physical activity, total energy intake, and polyunsaturated fat, monounsaturated fat, saturated fat, fiber, and cholesterol intake.
FIGURE 2—
Coronary heart disease (CHD) among men by (a) relative risk among current smokers relative to never smokers, (b) incidence rate (IR) differences among current smokers relative to never smokers, and (c) attributable fractions among current smokers.
Note. Vertical bars represent 95% confidence intervals. Analyses adjusted for year of baseline questionnaire, study origin, education, alcohol intake, body mass index, physical activity, total energy intake, and polyunsaturated fat, monounsaturated fat, saturated fat, fiber, and cholesterol intake.
DISCUSSION
In our pooled data set of 192 067 women and 74 720 men, we found that a large proportion of CHD cases among smokers were attributable to smoking in all age groups. This proportion was highest among currently smoking women (88%) and men (79%) aged 40 to 49 years. We also found that although the HR attenuated with increasing age, the rate difference increased with age. As a measure of absolute risk, the rate difference is usually considered a more relevant effect measure to public health than a relative measure such as HR because it is a more direct indication of the number of individuals affected.
These results have important public health implications. First, smoking prevention should remain a high priority, and equally so in all age groups. CHD events caused by smoking also occur in young adults, in our study defined as age 40 to 49 years. This finding does not support the standard belief that smoking-related CHD does not occur until old age. The incidence of CHD may be low in this age group, and hence the burden of disease is much smaller than in the older age groups, but this finding constitutes important knowledge for public health.
Ours was one of only a few studies to focus on the effect of smoking on CHD according to age.26–28 We analyzed a large body of data with thorough assessments of relevant potential confounders, including diet. The size of the study population allowed us to perform subset analyses exploring the association between smoking and CHD in strata of younger men and women, which even large individual cohorts do not have the power to address. Our findings were strengthened by the prospective design, which provided information on the sequence of events, allowing for conclusions on causality, assuming proper confounding control.
Limitations
Only information on smoking status and amount of current smoking was available, and we had no information on smoking duration. Naturally, smoking duration varies widely, especially among older individuals, who may have started to smoke at very different ages (or have quit for a time and started again). It is plausible that measuring smoking in dimensions of both duration and amount (e.g., pack-years) will more accurately capture the risk associated with smoking than does a measure of current smoking only.
Information on smoking status was available at baseline only. Thus, participants who quit smoking during the follow-up period (≤ 10 years) were categorized as smokers and not, more correctly, as ex-smokers. This would lead to some underestimation of the risk among current smokers. Naturally, participants who were never smokers at baseline could have started to smoke during follow-up, but this is less likely because most smokers start early in life; more than 80% of adult smokers began smoking before age 18 years.29
Residual confounding from psychosocial factors such as stress cannot be excluded. Stress has been shown to be associated with both smoking and CHD, so it is possible that increased stress among the smokers was responsible for some of the effect.30 Furthermore, the majority of the cohorts in our data set were not representative of the general population, and participants may have had a more healthy distribution of lifestyle factors than did the general population; hence other risk factors may have contributed less and smoking more to disease. If so, we overestimated the fractions attributable to smoking.
Conclusions
Our results indicate that smoking causes the majority of CHD events among smokers in all age groups. This important public health finding adds to the voluminous data on the hazardous effects of smoking.
Acknowledgments
A part of these results were presented at the Fifth Conference of Epidemiological Longitudinal Studies (CELSE2010); October 13–15, 2010; Paphos, Cyprus.
We thank Eilis O’Reilly for help with the data set.
Human Participant Protection
The study protocol was approved by the review board of the Pooling Project on Diet and Coronary Heart Disease.
References
- 1.Ward BW, Barnes PM, Freeman G, Schiller JS. National Center for Health Statistics; 2010. Early release of selected estimates based on data from the January–June 2010 National Health Interview Survey. Available at: http://www.cdc.gov/nchs/nhis.htm. Accessed August 1, 2012. [Google Scholar]
- 2.Global Health Risks. Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 3.Ezzati M, Hoorn SV, Lopez AD . Comparative quantification of mortality and burden of disease attributable to selected risk factors. In: Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL, editors. Global Burden of Disease and Risk Factors. Washington, DC: World Bank; 2006. pp. 241–396. [PubMed] [Google Scholar]
- 4.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330(15):1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 5.Zdravkovic S, Wienke A, Pedersen NL, Marenberg ME, Yashin AI, de Faire U. Heritability of death from coronary heart disease: a 36-year follow-up of 20 966 Swedish twins. J Intern Med. 2002;252(3):247–254. doi: 10.1046/j.1365-2796.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- 6.Thun MJ, Myers DG, Day-Lally C . Age and the exposure-response relationships between cigarette smoking and premature death in Cancer Prevention Study II. In: Burns DM, Garfinkel L, Samet JM, editors. Changes in Cigarette-Related Disease Risks and Their Implications for Prevention and Control. Smoking and Tobacco Control Monograph 8. Bethesda, MD: National Cancer Institute; 1997. pp. 383–413. [Google Scholar]
- 7.Stamler J. Established major risk factors: historical overview. In: Marmot M, Elliott P, editors. Coronary Heart Disease Epidemiology: From Aetiologgy to Public Health. New York, NY: Oxford University Press; 2005. pp. 18–31. [Google Scholar]
- 8.Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med. 1992;152(7):1416–1424. [PubMed] [Google Scholar]
- 9.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 10.ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4(1):1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 11.Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol. 1994;139(12):1180–1189. doi: 10.1093/oxfordjournals.aje.a116964. [DOI] [PubMed] [Google Scholar]
- 12.Høyer AP, Engholm G. Serum lipids and breast cancer risk: a cohort study of 5,207 Danish women. Cancer Causes Control. 1992;3(5):403–408. doi: 10.1007/BF00051352. [DOI] [PubMed] [Google Scholar]
- 13.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 14.Balogh M, Medalie JH, Smith H, Groen JJ. The development of a dietary questionnaire for an ischemic heart disease survey. Isr J Med Sci. 1968;4(2):195–203. [PubMed] [Google Scholar]
- 15.Munger RG, Folsom AR, Kushi LH, Kaye SA, Sellers TA. Dietary assessment of older Iowa women with a food frequency questionnaire: nutrient intake, reproducibility, and comparison with 24-hour dietary recall interviews. Am J Epidemiol. 1992;136(2):192–200. doi: 10.1093/oxfordjournals.aje.a116485. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Sampson L, Stampfer MJ et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 17.Hallmans G, Agren A, Johansson G et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort—evaluation of risk factors and their interactions. Scand J Public Health. 2003;31(61 suppl):18–24. doi: 10.1080/14034950310001432. [DOI] [PubMed] [Google Scholar]
- 18.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women’s Health Study. J Womens Health Gend Based Med. 2000;9(1):19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 19.Devore EE, Kang JH, Okereke O, Grodstein F. Physical activity levels and cognition in women with type 2 diabetes. Am J Epidemiol. 2009;170(8):1040–1047. doi: 10.1093/aje/kwp224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation. 2003;107(19):2435–2439. doi: 10.1161/01.CIR.0000066906.11109.1F. [DOI] [PubMed] [Google Scholar]
- 21.Hemilä H, Kaprio J, Albanes D, Virtamo J. Physical activity and the risk of pneumonia in male smokers administered vitamin E and beta-carotene. Int J Sports Med. 2006;27(4):336–341. doi: 10.1055/s-2005-865670. [DOI] [PubMed] [Google Scholar]
- 22.Pereira MA, O’Reilly E, Augustsson K et al. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med. 2004;164(4):370–376. doi: 10.1001/archinte.164.4.370. [DOI] [PubMed] [Google Scholar]
- 23.Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: a meta-analysis. Addiction. 2000;95(10):1505–1523. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith-Warner SA, Spiegelman D, Ritz J et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163(11):1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 25.Wacholder S, Benichou J, Heineman EF, Hartge P, Hoover RN. Attributable risk: advantages of a broad definition of exposure. Am J Epidemiol. 1994;140(4):303–309. doi: 10.1093/oxfordjournals.aje.a117252. [DOI] [PubMed] [Google Scholar]
- 26.Oei HHS, Vliegenthart R, Hofman A, Oudkerk M, Witteman JC. Risk factors for coronary calcification in older subjects: the Rotterdam Coronary Calcification Study. Eur Heart J. 2004;25(1):48–55. doi: 10.1016/j.ehj.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan GA, Haan MN, Wallace RB. Understanding changing risk factor associations with increasing age in adults. Annu Rev Public Health. 1999;20:89–108. doi: 10.1146/annurev.publhealth.20.1.89. [DOI] [PubMed] [Google Scholar]
- 28.Menotti A, Lanti M, Nedeljkovic S, Nissinen A, Kafatos A, Kromhout D. The relationship of age, blood pressure, serum cholesterol and smoking habits with the risk of typical and atypical coronary heart disease death in the European cohorts of the Seven Countries Study. Int J Cardiol. 2006;106(2):157–163. doi: 10.1016/j.ijcard.2004.12.092. [DOI] [PubMed] [Google Scholar]
- 29.Smoking and tobacco use. Centers for Disease Control and Prevention, Office on Smoking and Health. 2012 Available at: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/youth_data/tobacco_use/index.htm. Accessed August 1. [Google Scholar]
- 30.Proietti R, Mapelli D, Volpe B et al. Mental stress and ischemic heart disease: evolving awareness of a complex association. Future Cardiol. 2011;7(3):425–437. doi: 10.2217/fca.11.13. [DOI] [PubMed] [Google Scholar]