Abstract
Objectives. We examined the impact of school water, sanitation, and hygiene (WASH) interventions on diarrhea-related outcomes among younger siblings of school-going children.
Methods. We conducted a cluster-randomized trial among 185 schools in Kenya from 2007 to 2009. We assigned schools to 1 of 2 study groups according to water availability. Multilevel logistic regression models, adjusted for baseline measures, assessed differences between intervention and control arms in 1-week period prevalence of diarrhea and 2-week period prevalence of clinic visits among children younger than 5 years with at least 1 sibling attending a program school.
Results. Among water-scarce schools, comprehensive WASH improvements were associated with decreased odds of diarrhea (odds ratio [OR] = 0.44; 95% confidence interval [CI] = 0.27, 0.73) and visiting a clinic (OR = 0.36; 95% CI = 0.19, 0.68), relative to control schools. In our separate study group of schools with greater water availability, school hygiene promotion and water treatment interventions and school sanitation improvements were not associated with differences in diarrhea prevalence between intervention and control schools.
Conclusions. In water-scarce areas, school WASH interventions that include robust water supply improvements can reduce diarrheal diseases among young children.
Diarrhea accounts for 700 000 deaths per year among children younger than 5 years,1 or 10.5% of total under-5 mortality.2 These deaths are largely preventable. An estimated 85% of diarrhea mortality is attributed to unsafe drinking water, inadequate sanitation, and substandard hygiene practices.3 A recent meta-analysis calculated reductions in diarrhea associated with hand-washing promotion, water quality improvements, and improvements in excreta disposal of 48%, 17%, and 36%, respectively.4 These estimates have been widely adopted in the international health community.5,6 Although some studies have found limited evidence of health impact associated with water supply improvements,7 emerging research suggests that relationships between water supply and diarrhea may be mediated by several factors, including collection time and distance to source.8,9
Estimates of the health impact of water, sanitation, and hygiene (WASH) interventions on children younger than 5 years are derived from interventions that promote or improve services and practices in domestic environments.4 The impact of WASH improvements at institutions—such as schools—on child diarrhea remains underexplored. WASH interventions in schools can influence diarrheal outcomes among children younger than 5 years who themselves are not attending school through 2 primary pathways. First, these interventions may result in the diffusion of improved practices and behaviors to domestic environments and the broader community. Studies have documented both the transfer of knowledge about proper hygiene and point-of-use water treatment practices from school-based10,11 and clinic-based12 interventions. Second, interventions may interrupt pathogen transmission within the public sphere, reducing transmission to and exposures in domestic environments.13 Water supply improvements in schools may also serve broader community needs, resulting in changes in both domestic and public settings. The potential for domestic or public WASH improvements to reduce disease burden depends on several factors, including background disease burden and baseline WASH access.14
School WASH interventions have been associated with improvements in educational outcomes11,15,16 and reductions in absence caused by illness17,18 and diarrhea19 among school-aged children. Because the majority of the WASH-attributable disease burden involves children younger than 5 years,20,21 it is important to understand the extent to which school interventions affect younger children. We analyzed data from a cluster-randomized trial in Kenya to quantify the impact of school WASH improvements on parent-reported diarrhea and clinic visits for gastrointestinal symptoms among children younger than 5 years living in households within the catchment areas of study schools.
METHODS
Our study was embedded within a larger trial that assessed the health and educational impacts of WASH improvements carried out in schools in Nyanza Province, Kenya. Following a rapid assessment of school conditions conducted by the Ministry of Education, we divided schools into 2 study groups according to water availability: water-available schools had a dry season water source within 1 kilometer; water-scarce schools had no improved water source within 2 kilometers and no dry season source of any kind within 1 kilometer. We excluded schools that met government-mandated pupil-to-latrine ratios (25 to 1 for girls, 30 to 1 for boys) from the study.22
From the 198 schools that met inclusion criteria for the water-available study group, we randomly selected 135 and allocated them to 1 of 3 study arms of 45 schools each: (1) hygiene promotion and water treatment (HP&WT), (2) HP&WT plus additional school latrines (Sanitation + HP&WT), and (3) control. From the 98 schools that met criteria for the water-scarce study group, we randomly selected 50 schools. We randomly allocated 25 to a treatment arm that received water supply improvements in addition to the Sanitation + HP&WT intervention (Water + Sanitation + HP&WT). We allocated the remaining 25 schools to the control arm of the water-scarce study group. We implemented 1 of 2 water supply interventions according to groundwater availability: a machine-dug borehole with a hand pump located either on school property or in an adjacent community with guaranteed access for the school (n = 12) or 60-cubic-meter-capacity rainwater-harvesting (RWH) tanks that primarily served the school population (n = 13). Water supply improvements were intended to serve schools and neighboring communities. Participant selection and randomization occurred in March 2007, and implementation was completed by June 2008 (Figure 1).
FIGURE 1—
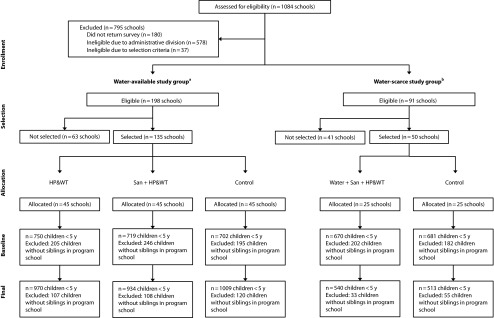
Design of study of impact of school sanitation improvements on health of younger siblings of school-going children: Kenya, 2007–2009.
Note. HP&WT = hygiene promotion and water treatment; san = sanitation; water = water supply.
aSchools having no water source within 1 kilometer and no improved source within 2 kilometers were classified as water scarce and all others were classified as water available.
bSelection was stratified across 3 geographic clusters covering contiguous administrative divisions in 4 districts: Nyando, Kisumu, Rachuonyo, and Suba.
Diarrheal disease among children younger than 5 years was a secondary outcome measure for the larger intervention trial, constraining sample size. We used data from the 2003 Kenya Demographic and Health Survey to calculate a baseline 1-week period prevalence of diarrhea of 15% and a design effect of 1.6.23 At α = 0.05 and power of 80%, an average of 20 children in each of 45 clusters per treatment arm in the water-available study group allowed us to detect a 40% difference in diarrhea prevalence between either intervention arm and the control arm. In the water-scarce study group, an average of 20 children in each of 25 clusters per arm allowed us to detect a 54% difference in diarrhea prevalence.
Data Collection
We completed surveys in a systematic sample of households in the catchment area of each participating school at baseline (February–March 2007) and approximately 1 year after interventions had been completed in schools (June–July 2009). We used the catchment area of participating schools, comprising 1 or more villages, as the cluster in all subsequent analyses. At both baseline and end line, we identified a sampling frame for each cluster from the estimated number of households and surveyed a systematic sample of 25 households. At baseline, we oversampled households in the water-scarce study group to capture a sufficient number of children younger than 5 years. We excluded sampled households without at least 1 child younger than 18 years from data collection.
Enumerators collected data on household demographics, water source availability, and hygiene and water treatment practices. Enumerators directly observed household sanitation facilities, household possessions, and structured hand-washing demonstrations in each household. The primary respondent, typically the female head of household, provided details on all school-aged children, including whether the child was enrolled in the local study school. For each child younger than 5 years, we collected data on age, gender, diarrhea episodes in the past week, clinic visits in the past 2 weeks, and, if applicable, reasons for visiting the clinic, such as diarrhea or vomiting. We used a 1-week recall period for diarrhea events to limit potential recall bias.24–27 For clinic visits, which are less prone to errors with longer recall periods,26 we used a 2-week recall to capture sufficient events for analysis.
We collected data on personal digital assistants with Syware Visual CE software (Cambridge, MA) and uploaded them to a Microsoft Access 2007 database (Microsoft Corp, Redmond, WA) weekly.
Analyses
We cleaned data with SAS 9.2 (SAS Institute, Cary, NC) and analyzed them with Stata/IC 11 (StataCorp, College Station, TX). We considered children younger than 5 years with a sibling attending a school participating in the larger cluster-randomized trial the primary population for this analysis. Outcome measures were period prevalence of diarrhea in the week before data collection and of clinic visits for gastrointestinal symptoms (vomiting, diarrhea, or both) in the past 2 weeks. We performed analysis at the level of each child. We developed multilevel logistic regression models with the XTMELOGIT procedure with cluster-specific random intercepts. We modeled intervention impact with a standard difference-in-differences model on the logit scale, which compares changes in treatment and control groups over time through the interaction between intervention group assignment and data collection round.28
Because of different school eligibility criteria and separate selection and randomization procedures, we ran models separately for children in the water-scarce and water-available study groups. We used an intention-to-treat approach to analysis in which we analyzed data according to study group and treatment arm assignment.29 Our first set of models assessed differences in outcomes without adjustment for any potential confounding variables. Our second set of models adjusted for several participant-specific covariates and aggregate cluster-specific characteristics at baseline, which we selected a priori as possible confounders. Child-specific covariates were age, gender, total number of children in the household, maternal education, and household wealth. We analyzed maternal education as a categorical variable corresponding to less than primary education, completion of primary education, and no education information available or mother had died. We estimated household wealth via principal components analysis of a list of household possessions,30,31 categorized as wealth quintiles.
Aggregate cluster-specific WASH covariates in our analysis were the percentage of households at baseline in each cluster with an observed latrine, detectable chlorine residue in drinking water, a primary water source that was protected from runoff or surface contamination, a less than 30-minute walk to the current primary water source, and a respondent who used soap during a structured hand-washing demonstration. Because these covariates represented possible intermediate impacts of our intervention, we incorporated only baseline values in our analysis. Household WASH characteristics were not available for some baseline records; however, because household WASH covariates were limited to aggregated cluster measures, we retained these records in our analysis and adjusted them by cluster means.
In line with the emergent hypothesis that school-based RWH interventions were fundamentally different than community borehole interventions, we assessed the proportion of children in households that reported using a protected water source for current drinking water and that reported traveling less than 30 minutes to the current drinking water source for each of the 2 water supply intervention types and the control group in the water-scarce study arm. We modeled diarrhea and clinic visits for each intervention type against the control group. Our analysis was below the unit of randomization, however, limiting interpretation.
RESULTS
We collected information on 3523 children younger than 5 years with an older sibling attending a program school in our study at baseline and on 3969 at project end line. At baseline, individual and household characteristics were generally similar across intervention arms in each study group (Table 1).
TABLE 1—
Baseline Characteristics by Intervention Group in Trial of School Sanitation Improvements and Impact on Diarrhea Among Younger Siblings of Pupils: Kenya, 2007
| Water-Available Group |
Water-Scarce Group |
||||
| Characteristic | HP&WT (n = 750), Mean (SD) or % | San + HP&WT (n = 719), Mean (SD) or % | Control (n = 702), Mean (SD) or % | Water Supply + San + HP&WT (n = 671), Mean (SD) or % | Control (n = 681), Mean (SD) or % |
| Individual | |||||
| Age, mo | 27.2 (16.5) | 27.4 (16.4) | 27.6 (16.0) | 28.2 (15.1) | 27.2 (15.8) |
| Male | 51 | 53 | 53 | 51 | 51 |
| Household | |||||
| Children in household | 4.3 (1.6) | 4.1 (1.5) | 4.1 (1.5) | 4.2 (1.5) | 4.2 (1.6) |
| Mothers with completed primary education | 47 | 41 | 43 | 44 | 47 |
| Lowest wealth quintile | 20 | 27 | 22 | 19 | 20 |
| Has latrine | 36 | 34 | 35 | 36 | 43 |
| Mother used soap during hand-washing demonstration | 79 | 70 | 71 | 71 | 68 |
| Detectable chlorine residual in household stored drinking water | 4 | 5 | 7 | 5 | 4 |
| Using protected water source | 60 | 57 | 59 | 60 | 65 |
| Has water source > 30 min away | 17 | 20 | 14 | 27 | 25 |
Note. HP&WT = hygiene promotion and water treatment; san = sanitation. Diarrhea outcomes were diarrhea in the past week and clinic visit for diarrhea or vomiting in the past 2 weeks among children younger than 5 years. Water-available schools had a dry season water source within 1 km; water-scarce schools had no improved water source within 2 km and no dry season source of any kind within 1 km. Not all household data were available for all children at baseline.
Water-Available Group
In the water-available study population, baseline 1-week period prevalence of diarrhea was 18% in the HP&WT group, 23% in the Sanitation + HP&WT group, and 22% in the control group (Table 2). At project end line, prevalence of diarrhea declined to 11% in the HP&WT arm, 10% in the Sanitation + HP&WT arm, and 11% in the control arm. Two-week period prevalence of clinic visits for gastrointestinal illness symptoms went from 13% to 7% (baseline to end line) in the HP&WT arm, 12% to 6% in the Sanitation + HP&WT arm, and 12% to 9% in the control arm.
TABLE 2—
Diarrhea Outcomes Among Younger Siblings of Pupils at Water-Available or Water-Scarce Sanitation Intervention Schools: Kenya, 2007–2009
| Baseline, No. (%) | End Line, No. (%) | Difference, % | OR (95% CI) | AORa (95% CI) | ICC | |
| Water-available study groupb | ||||||
| Diarrhea in past wk | 0.0701 | |||||
| HP&WT group | 138 (18) | 104 (11) | −8 | 1.23 (0.82, 1.83) | 1.21 (0.81, 1.80) | |
| Sanitation + HP&WT group | 165 (23) | 90 (10) | −13 | 0.77 (0.51, 1.14) | 0.76 (0.51, 1.13) | |
| Control group | 153 (22) | 109 (11) | −11 | 1.00 (Ref) | 1.00 (Ref) | |
| Clinic visit for diarrhea or vomiting in past 2 wk | 0.0737 | |||||
| HP&WT group | 97 (13) | 62 (6) | −7 | 0.65 (0.41, 1.04) | 0.64 (0.40, 1.03) | |
| Sanitation + HP&WT group | 83 (12) | 57 (6) | −6 | 0.64 (0.39, 1.05) | 0.65 (0.40, 1.06) | |
| Control group | 81 (12) | 85 (9) | −3 | 1.00 (Ref) | 1.00 (Ref) | |
| Water-scarce study groupc | ||||||
| Diarrhea in past wk | 0.0415 | |||||
| Water + Sanitation + HP&WT group | 177 (26) | 44 (8) | −18 | 0.45 (0.27, 0.73) | 0.44 (0.27, 0.72) | |
| Control group | 135 (20) | 61 (12) | −8 | 1.00 (Ref) | 1.00 (Ref) | |
| Clinic visit for diarrhea or vomiting in past 2 wk | 0.0626 | |||||
| Water + Sanitation + HP&WT group | 91 (14) | 42 (5) | −9 | 0.36 (0.19, 0.68) | 0.36 (0.19, 0.68) | |
| Control group | 67 (10) | 24 (8) | −1 | 1.00 (Ref) | 1.00 (Ref) | |
Note. AOR = adjusted odds ratio; CI = confidence interval; ICC = intraclass correlation coefficient; HP&WT = hygiene promotion and water treatment; water = water supply. Siblings were younger than 5 years. Water-available schools had a dry season water source within 1 km; water-scarce schools had no improved water source within 2 km and no dry season source of any kind within 1 km.
Adjusted for age, gender, maternal education, household size, wealth quintile and baseline cluster-specific proportion of households with detectable chlorine residuals, soap used during hand-washing demonstration, water source greater than 30-min round trip, water source protected, and latrine observed on compound.
Baseline was n = 2171; end line, n = 2910.
Baseline was n = 1351; end line, n = 1053.
We found no evidence of differences in the odds of diarrhea among children in the HP&WT (adjusted odds ratio [AOR] = 1.21; 95% confidence interval [CI] = 0.81, 1.80) or Sanitation + HP&WT (AOR = 0.76; CI = 0.51, 1.13) arm and children in the control arm. We noted larger and more consistent declines in the 2-week period prevalence of clinic visits for diarrhea or vomiting. In the HP&WT and Sanitation + HP&WT arms, odds of a child visiting a clinic declined by 36% (AOR = 0.64; CI = 0.40, 1.03) and 35% (AOR = 0.65 CI = 0.40, 1.06), respectively; however, these declines were not statistically significant.
Water-Scarce Group
In the water-scarce study group, baseline period prevalence of diarrhea was 26% in the intervention and 20% in the control arm (Table 2). At end line, period prevalence of diarrhea declined to 8% in the intervention and 12% in the control arm. The baseline 2-week period prevalence of clinic visits for gastrointestinal symptoms was 14% in the intervention and 10% in the control arm, with marked declines in the intervention arm (to 5%) and only a small decline (to 8%) in the control arm.
We found statistically significant differences in the odds of both recent diarrhea and recent clinic visits between water-scarce intervention and control groups. For a child in the Water + Sanitation + HP&WT treatment arm, we found 56% lower odds of a child having diarrhea than for a child in the control arm (AOR = 0.44; CI = 0.27, 0.72) and a 66% difference in the odds of a clinic visit in the past 2 weeks (AOR = 0.36; CI = 0.19, 0.68).
Water Supply Improvement Types
To assess changes in domestic water access associated with each of the water supply intervention arms, we compared the proportion of children living in households at project end line reporting (1) a travel time to a water source of less than 30 minutes and (2) use of a protected water source. At the end of the study, we found that more households in both the borehole intervention group (52%) and the RWH intervention group (40%) than in the control group (35%) used a protected water source. However, fewer households in the borehole (26%) and RWH (31%) groups than in the control group (35%) were closer than a 30-minute walk to a water source. Changes in measures of domestic water access for either water supply intervention did not differ statistically significantly from the control group in the water-scarce study arm.
Both water supply interventions were associated with statistically significant differences from the control group in the odds of diarrhea (Table 3). Odds of diarrhea were 44% lower (AOR = 0.54; CI = 0.29, 0.99) in the borehole group than in the control arm. In the RWH arm, odds were 65% lower than in the control arm (AOR = 0.35; CI = 0.18, 0.66). Differences from the control arm in the odds of a clinic visit for diarrhea or vomiting in the past 2 weeks were significant for the RWH treatment arm (AOR = 0.25; CI = 0.10, 0.55) but not for the borehole treatment arm (AOR = 0.54; CI = 0.25, 1.18).
TABLE 3—
Logistic Regression Models of Diarrhea Outcomes Among Younger Siblings of Pupils Attending Water-Scarce Intervention and Control Schools: Kenya, 2007–2009
| Variable | OR (95% CI) | AORa (95% CI) | ICC |
| Diarrhea in past wk | 0.0401 | ||
| Borehole intervention | 0.55 (0.31, 0.99) | 0.54 (0.29, 0.99) | |
| RWH intervention | 0.35 (0.19, 0.66) | 0.35 (0.18, 0.66) | |
| Clinic visit for diarrhea or vomiting in past 2 wk | 0.0626 | ||
| Borehole intervention | 0.49 (0.23, 1.02) | 0.54 (0.25, 1.18) | |
| RWH intervention | 0.26 (0.12, 0.59) | 0.25 (0.10, 0.54) |
Note. AOR = adjusted odds ratio; CI = confidence interval; ICC = intraclass correlation coefficient; OR = odds ratio; RWH = rainwater harvesting. Scarce-water intervention schools received guaranteed access to machine-dug boreholes as part of the water supply intervention or 60-cubic-meter-capacity RWH tanks that primarily served the school population.
Adjusted for age, gender, maternal education, household size, wealth quintile and baseline cluster-specific proportion of households with: detectable chlorine residuals, soap used during handwashing demonstration, water source greater than 30 min round trip, water source protected, latrine observed on compound.
We found no evidence of changes in diarrhea or recent clinic visits among children younger than 5 years without a sibling attending a program school in any treatment arm in both study groups (data not shown).
DISCUSSION
Our results suggest that interventions that promote improved hand washing and water treatment in schools, improve the quantity of available latrines, and improve the availability of safe drinking water at school can result in measureable improvements in diarrheal diseases among children younger than 5 years whose siblings attend intervention schools. By contrast, interventions that only improve hygiene and water treatment interventions at the school and the quantity of sanitation facilities may not.
Our findings are consistent with other results from this trial. Freeman et al. found a 66% reduction in the period prevalence of diarrhea among pupils in the Water + Sanitation + HP&WT schools relative to control participants, but sanitation and hygiene interventions alone had no measureable effect on pupil-reported diarrhea.19 Greene et al. reported that pupils in HP&WT schools were as likely as pupils in control schools to have detectable Escherichia coli bacteria on hands, and risk of contaminated hands was more than twice as high among girls in the Sanitation + HP&WT group as among girls in control schools, suggesting that our HP&WT and Sanitation + HP&WT interventions may have done little to reduce the presence, and potentially the transmission, of enteric pathogens in study schools and subsequently in homes.32
Studies suggest that the combined effect of multiple WASH interventions is no greater than the effect of the individual components.7,33 Water supply improvements in the water-scarce study group may have been a necessary precursor for improved hygiene practices at both school and home; thus the large measured reduction in diarrhea may have resulted from a cumulative effect among intervention components. Studies have documented reduced impact of hygiene and hand-washing improvements when water availability or water consumption is low.29,34,35 Water supply improvements, which can result in improvements in both water quality and water quantity, may have had an independent effect on health outcomes.7,8 Because we used water scarcity criteria to define separate study arms, each with a unique control group, direct comparisons between study groups should be made with caution.
Water supply interventions were intended to provide a source of improved water to all members of the community, not just pupils or their households. Reductions in diarrhea among the Water + Sanitation + HP&WT group may, in fact, have reflected improvements in water quantity and water quality in the domestic setting alone, independent of school improvements. We found a larger proportion of households associated with schools where boreholes were implemented than of control households with access to an improved water source at end line. However, the proportion of households reporting a travel time to the current water source greater than 30 minutes also increased. Greater time or distance to a water source is associated with increased water contamination and risk of diarrhea.8,9,36 Improvements in domestic water access associated with borehole interventions were also far from universal. At end line, almost half of children in our borehole treatment arm lived in households that were using unimproved water sources. Domestic water access in the RHW treatment group was similar to that of control groups. Improvements in water access among the RWH schools were primarily limited to the school.
Our data also provide evidence on the potential mechanisms through which school interventions may affect diarrheal outcomes among children younger than 5 years: either diffusion of practices to the domestic environment or improvements in the school environment itself. Community behavior change components in the larger school program were minimal: community mobilization regarding the management and operation of new water points and suggestions to teachers during training that they encourage children to share WASH knowledge and messages with their families. The extent of household behavior change and adoption of improved hygiene, sanitation, and water treatment practices associated with our intervention was very limited.37
Our 2 water supply improvements may have benefited different populations, with borehole interventions benefiting both the domestic and school environments and the RWH interventions primarily benefiting the school. That our RWH intervention, which provided only minimal improvements in domestic water access, resulted in the largest and most robust reductions in diarrhea at home suggests that improvements in water quantity or quality in the school environment may have accounted for a large portion of disease reduction in our study. Results from our additional analysis of water supply intervention types must be interpreted with caution. We also found no difference in health outcomes among children younger than 5 years without a sibling attending a program school, suggesting that any measureable improvements in health outcomes in our study were attributable to changes at the school itself.
Limitations
It was not possible to blind community members to the intervention status of their local schools. This, combined with our subjective self-reported diarrhea and clinic visit outcomes, has been shown to lead to overestimation of intervention effects.38 The level of blinding across study populations and within study groups may have varied according to the extent to which programs provided visible infrastructure improvements in schools or expanded services to associated communities. Machine-dug boreholes serving both the community and the school were likely the most visible intervention component, contributing to the large reductions in reported diarrhea among this group. However, RWH interventions, which were limited to school grounds, were no more visible to community members than were other school infrastructure improvements, and this intervention was associated with significant health gains, whereas sanitation improvements were not. The effect of recall bias was minimized by the selection of a 1-week recall period for diarrhea and a 2-week recall for clinic visits.24
We found intervention compliance to be low in some schools, which may have reduced impact.13 Our classification of schools into water scarce and water available was limited to governmental recommendations and may have resulted in misallocation of schools and communities into inappropriate study groups. Therefore, direct comparisons of impacts between study populations should be made with caution.
Conclusions
Our results provide evidence that school WASH interventions may reduce diarrhea and gastrointestinal-related clinic visits among children younger than 5 years. Most school WASH interventions focus on effects among school-aged children; ours was the first study to assess the impact on those most vulnerable to diarrhea-related morbidity and mortality: children younger than 5 years. Our data suggest that impact is limited to specific circumstances, and the potential influence of reporting bias should be considered. The strongest reductions in diarrhea and clinic visits were found in clusters in which the water supply was improved in addition to school sanitation, hygiene promotion, and water treatment improvements.
Our study adds to the growing body of literature highlighting the importance of water supply improvements—in both schools and communities—either as an individual factor or as a means of achieving the full benefit of other WASH interventions. School interventions may serve as important barriers to public transmission of diarrheal disease pathogens among school-aged children, resulting in reduced health burden among their siblings. Understanding the impact of school WASH interventions on both school-going children and children younger than 5 years will inform cost–benefit analyses and policy considerations for school interventions.
Acknowledgments
This study was supported by the Bill and Melinda Gates Foundation and the Global Water Challenge.
We thank the study participants and acknowledge the hard work of our colleagues at the Great Lakes University of Kisumu, Kenya; CARE; Water.org; and Sustainable Aid in Africa International: Patrick Alubbe, Dan Kaseje, Brooks Keene, Peter Lochery, Alfred Luoba, John Migele, Alex Mwaki, Imelda Akinyi Ochari, Emily Awino Ogutu, Betty Ojeny, Ben Okech, Caroline Teti, Peter Waka, and Elizabeth Were. We thank Caitlin Kennedy and Rachel P. Chase for their comments and critical review of the article.
Human Participant Protection
Ethical approval was provided by the institutional review board of Emory University and the ethical review committee of the Great Lakes University of Kisumu. Written authorization for the study was provided by the Kenyan ministries of Health, Education, and Water.
References
- 1.Walker CLF, Rudan I, Liu L et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S et al. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 3.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global Burden of Disease and Risk Factors. Washington, DC: World Bank and Oxford University Press; 2006. [PubMed] [Google Scholar]
- 4.Cairncross S, Hunt C, Boisson S et al. Water, sanitation and hygiene for the prevention of diarrhoea. Int J Epidemiol. 2010;39(suppl 1):i193–i205. doi: 10.1093/ije/dyq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer Walker CL, Friberg IK, Binkin N et al. Scaling up diarrhea prevention and treatment interventions: a Lives Saved Tool analysis. PLoS Med. 2011;8(3):e1000428. doi: 10.1371/journal.pmed.1000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutta ZA, Das JK, Walker N et al. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet. 2013;381(9875):1417–1729. doi: 10.1016/S0140-6736(13)60648-0. [DOI] [PubMed] [Google Scholar]
- 7.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM., Jr Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5(1):42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 8.Pickering AJ, Davis J. Freshwater availability and water fetching distance affect child health in sub-Saharan Africa. Environ Sci Technol. 2012;46(4):2391–2397. doi: 10.1021/es203177v. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Hunter PR. A systematic review and meta-analysis of the association between self-reported diarrheal disease and distance from home to water source. Am J Trop Med Hyg. 2010;83(3):582–584. doi: 10.4269/ajtmh.2010.10-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanton E, Ombeki S, Oluoch GO, Mwaki A, Wannemuehler K, Quick R. Evaluation of the role of school children in the promotion of point-of-use water treatment and handwashing in schools and households—Nyanza Province, Western Kenya, 2007. Am J Trop Med Hyg. 2010;82(4):664–671. doi: 10.4269/ajtmh.2010.09-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Reilly CE, Freeman MC, Ravani M et al. The impact of a school-based safe water and hygiene programme on knowledge and practices of students and their parents: Nyanza Province, Western Kenya, 2006. Epidemiol Infect. 2008;136(1):80–91. doi: 10.1017/S0950268807008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker AA, Stephenson R, Riley PL et al. Sustained high levels of stored drinking water treatment and retention of hand-washing knowledge in rural Kenyan households following a clinic-based intervention. Epidemiol Infect. 2006;134(5):1029–1036. doi: 10.1017/S0950268806005954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cairncross S, Blumenthal U, Kolsky P, Moraes L, Tayeh A. The public and domestic domains in the transmission of disease. Trop Med Int Health. 1996;1(1):27–34. doi: 10.1046/j.1365-3156.1996.d01-9.x. [DOI] [PubMed] [Google Scholar]
- 14.Eisenberg JN, Scott JC, Porco T. Integrating disease control strategies: balancing water sanitation and hygiene interventions to reduce diarrheal disease burden. Am J Public Health. 2007;97(5):846–852. doi: 10.2105/AJPH.2006.086207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowen A, Ma H, Ou J et al. A cluster-randomized controlled trial evaluating the effect of a handwashing-promotion program in Chinese primary schools. Am J Trop Med Hyg. 2007;76(6):1166–1173. [PubMed] [Google Scholar]
- 16.Freeman MC, Greene LE, Dreibelbis R et al. Assessing the impact of a school-based water treatment, hygiene and sanitation programme on pupil absence in Nyanza Province, Kenya: a cluster-randomized trial. Trop Med Int Health. 2012;17(3):380–391. doi: 10.1111/j.1365-3156.2011.02927.x. [DOI] [PubMed] [Google Scholar]
- 17.Nandrup-Bus I. Mandatory handwashing in elementary schools reduces absenteeism due to infectious illness among pupils: a pilot intervention study. Am J Infect Control. 2009;37(10):820–826. doi: 10.1016/j.ajic.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Talaat M, Afifi S, Dueger E et al. Effects of hand hygiene campaigns on incidence of laboratory-confirmed influenza and absenteeism in schoolchildren, Cairo, Egypt. Emerg Infect Dis. 2011;17(4):619–625. doi: 10.3201/eid1704.101353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman MC, Clasen T, Dreibelbis R, Saboori S, Greene LE, Rheingans R. The impact of a school-based water supply and treatment, hygiene, and sanitation programme on pupil diarrhea: a cluster-randomized trial. Epidemiol Infect. 2013;Epub ahead of print. May 24, 2013 doi: 10.1017/S0950268813001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black RE, Lanata CF. Diarrheal diseases. In: Nelson KE, Williams CM, editors. Infectious Disease Epidemiology: Theory and Practice. 2nd ed. Sudbury, MA: Jones and Bartlett; 2007. [Google Scholar]
- 21.Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 22.National School Water, Sanitation and Hygiene Promotion Strategy 2008–2015. Nairobi, Kenya: Republic of Kenya Ministry of Education; 2008. [Google Scholar]
- 23.Central Bureau of Statistics. Kenya Demographic and Health Survey 2003. Calverton, MD: Kenya Ministry of Health; 2004. [Google Scholar]
- 24.Alam N, Henry FJ, Rahaman MM. Reporting errors in one-week diarrhoea recall surveys: experience from a prospective study in rural Bangladesh. Int J Epidemiol. 1989;18(3):697–700. doi: 10.1093/ije/18.3.697. [DOI] [PubMed] [Google Scholar]
- 25.Boerma JT, Black RE, Sommerfelt AE, Rutstein SO, Bicego GT. Accuracy and completeness of mothers’ recall of diarrhoea occurrence in pre-school children in demographic and health surveys. Int J Epidemiol. 1991;20(4):1073–1080. doi: 10.1093/ije/20.4.1073. [DOI] [PubMed] [Google Scholar]
- 26.Feikin DR, Audi A, Olack B et al. Evaluation of the optimal recall period for disease symptoms in home-based morbidity surveillance in rural and urban Kenya. Int J Epidemiol. 2010;39(2):450–458. doi: 10.1093/ije/dyp374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt WP, Arnold BF, Boisson S et al. Epidemiological methods in diarrhoea studies—an update. Int J Epidemiol. 2011;40(6):1678–1692. doi: 10.1093/ije/dyr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes RJ, Moulton LH. Cluster Randomized Trials. Boca Raton, FL: Taylor & Francis Group; 2009. [Google Scholar]
- 29.Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ. 1999;319(7211):670–674. doi: 10.1136/bmj.319.7211.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filmer D, Pritchett L. Estimating Wealth Effects Without Expenditure Data or Tears: An Application to Educational Enrollments in States of India. Washington, DC: World Bank; 1988. [DOI] [PubMed] [Google Scholar]
- 31.Vyas S, Kumaranayake L. Constructing socio-economic indices: how to use principal components analysis. Health Policy Plan. 2006;21(6):459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 32.Greene LE, Freeman MC, Akoko D, Saboori S, Moe C, Rheingans R. Impact of school-based hygiene promotion and sanitation interventions on pupil hand contamination in Western Kenya: a cluster randomized trial. Am J Trop Med Hyg. 2012;87(3):385–393. doi: 10.4269/ajtmh.2012.11-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luby SP, Agboatwalla M, Painter J et al. Combining drinking water treatment and hand washing for diarrhoea prevention, a cluster randomised controlled trial. Trop Med Int Health. 2006;11(4):479–489. doi: 10.1111/j.1365-3156.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 34.Peterson EA, Roberts L, Toole MJ, Peterson DE. The effect of soap distribution on diarrhoea: Nyamithuthu Refugee Camp. Int J Epidemiol. 1998;27(3):520–524. doi: 10.1093/ije/27.3.520. [DOI] [PubMed] [Google Scholar]
- 35.Yeager BA, Lanata CF, Lazo F, Verastegui H, Black RE. Transmission factors and socioeconomic status as determinants of diarrhoeal incidence in Lima, Peru. J Diarrhoeal Dis Res. 1991;9(3):186–193. [PubMed] [Google Scholar]
- 36.Wright J, Gundry S, Conroy R. Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Trop Med Int Health. 2004;9(1):106–117. doi: 10.1046/j.1365-3156.2003.01160.x. [DOI] [PubMed] [Google Scholar]
- 37.Rheingans R, Freeman MC, Greene LE, Dreibelbis R, Saboori S. Can a school-based WASH intervention catalyze changes in household behaviors and environment? Evidence from a randomized trial in Western Kenya. Paper presented at: American Public Health Association Annual Meeting and Exposition; November 7–11, 2009; Philadelphia, PA.
- 38.Wood L, Egger M, Gluud LL et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601–605. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]


