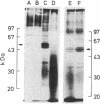
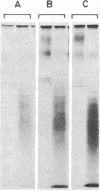
Abstract
Tyrosylprotein sulfotransferase, the enzyme catalyzing the sulfation of proteins on tyrosine residues, was characterized by using the acidic polymer containing tyrosine (Glu62, Ala30, Tyr8)n (referred to as Glu,Ala,Tyr) as exogenous "protein" substrate. After subcellular fractionation of a bovine adrenal medulla homogenate, tyrosylprotein sulfotransferase activity was found to be highest in fractions enriched in Golgi membrane vesicles. Tyrosylprotein sulfotransferase required the presence of a nonionic detergent for sulfation of exogenous Glu,Ala,Tyr, indicating an orientation of the catalytic site of the enzyme toward the Golgi lumen. Tyrosylprotein sulfotransferase was solubilized by Triton X-100, suggesting that the enzyme was tightly associated with the Golgi membrane, possibly as an integral membrane protein. The apparent Golgi localization of tyrosylprotein sulfotransferase was supported by the observation that tyrosine sulfation of proteins in intact cells was blocked by monensin and was in line with previous observations that all tyrosine-sulfated proteins known so far are secretory. Glu,Ala,Tyr was found to have a very high affinity for tyrosylprotein sulfotransferase (apparent Km, 300 nM), similar to that reported for certain tyrosylprotein kinases. While this may suggest some similarity between these enzymes, the Golgi localization of tyrosylprotein sulfotransferase segregates tyrosine sulfation from the sites of tyrosine phosphorylation of proteins in the intact cell. If, however, tyrosylprotein sulfotransferase was allowed to react with cytoplasmic proteins by using a nonionic detergent, tyrosine sulfation of tubulin was observed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Huttner W. B. Inhibition of N-glycosylation induces tyrosine sulphation of hybridoma immunoglobulin G. EMBO J. 1984 Oct;3(10):2209–2215. doi: 10.1002/j.1460-2075.1984.tb02118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A., Huttner W. B. Tyrosine sulfation of yolk proteins 1, 2, and 3 in Drosophila melanogaster. J Biol Chem. 1985 May 25;260(10):6434–6439. [PubMed] [Google Scholar]
- Baldwin G. S., Knesel J., Monckton J. M. Phosphorylation of gastrin-17 by epidermal growth factor-stimulated tyrosine kinase. Nature. 1983 Feb 3;301(5899):435–437. doi: 10.1038/301435a0. [DOI] [PubMed] [Google Scholar]
- Braun S., Raymond W. E., Racker E. Synthetic tyrosine polymers as substrates and inhibitors of tyrosine-specific protein kinases. J Biol Chem. 1984 Feb 25;259(4):2051–2054. [PubMed] [Google Scholar]
- Bretz R., Stäubli W. Detergent influence on rat-liver galactosyltransferase activities towards different acceptors. Eur J Biochem. 1977 Jul 1;77(1):181–192. doi: 10.1111/j.1432-1033.1977.tb11656.x. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGORY H., HARDY P. M., JONES D. S., KENNER G. W., SHEPPARD R. C. THE ANTRAL HORMONE GASTRIN. STRUCTURE OF GASTRIN. Nature. 1964 Dec 5;204:931–933. doi: 10.1038/204931a0. [DOI] [PubMed] [Google Scholar]
- Hille A., Rosa P., Huttner W. B. Tyrosine sulfation: a post-translational modification of proteins destined for secretion? FEBS Lett. 1984 Nov 5;177(1):129–134. doi: 10.1016/0014-5793(84)80996-5. [DOI] [PubMed] [Google Scholar]
- Huttner W. B. Determination and occurrence of tyrosine O-sulfate in proteins. Methods Enzymol. 1984;107:200–223. doi: 10.1016/0076-6879(84)07013-0. [DOI] [PubMed] [Google Scholar]
- Huttner W. B. Sulphation of tyrosine residues-a widespread modification of proteins. Nature. 1982 Sep 16;299(5880):273–276. doi: 10.1038/299273a0. [DOI] [PubMed] [Google Scholar]
- Kudryk B., Okada M., Redman C. M., Blombäck B. Biosynthesis of dog fibrinogen. Characterization of nascent fibrinogen in the rough endoplasmic reticulum. Eur J Biochem. 1982 Jul;125(3):673–682. doi: 10.1111/j.1432-1033.1982.tb06735.x. [DOI] [PubMed] [Google Scholar]
- LIPMANN F. Biological sulfate activation and transfer. Science. 1958 Sep 12;128(3324):575–580. doi: 10.1126/science.128.3324.575. [DOI] [PubMed] [Google Scholar]
- Lee R. W., Huttner W. B. Tyrosine-O-sulfated proteins of PC12 pheochromocytoma cells and their sulfation by a tyrosylprotein sulfotransferase. J Biol Chem. 1983 Sep 25;258(18):11326–11334. [PubMed] [Google Scholar]
- Lee R. W., Suchanek C., Huttner W. B. Direct photoaffinity labeling of proteins with adenosine 3'-[32P]phosphate 5'-phosphosulfate. Atractyloside inhibits labeling of a Mr = 34,000 protein in an adrenal medullary Golgi fraction. J Biol Chem. 1984 Sep 10;259(17):11153–11156. [PubMed] [Google Scholar]
- Liu M. C., Lipmann F. Isolation of tyrosine-O-sulfate by Pronase hydrolysis from fibronectin secreted by Fujinami sarcoma virus-infected rat fibroblasts. Proc Natl Acad Sci U S A. 1985 Jan;82(1):34–37. doi: 10.1073/pnas.82.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees-Jones R. W., Hendricks S. A., Quarum M., Roth J. The insulin receptor of rat brain is coupled to tyrosine kinase activity. J Biol Chem. 1984 Mar 25;259(6):3470–3474. [PubMed] [Google Scholar]
- Rosa P., Fumagalli G., Zanini A., Huttner W. B. The major tyrosine-sulfated protein of the bovine anterior pituitary is a secretory protein present in gonadotrophs, thyrotrophs, mammotrophs, and corticotrophs. J Cell Biol. 1985 Mar;100(3):928–937. doi: 10.1083/jcb.100.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. K., Capasso J. M., Hirschberg C. B. Translocation of adenosine 3'-phosphate 5'-phosphosulfate into rat liver Golgi vesicles. J Biol Chem. 1984 Mar 25;259(6):3554–3559. [PubMed] [Google Scholar]
- Sefton B. M., Hunter T. Tyrosine protein kinases. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:195–226. [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Duerr A. C. Isolation and characterization of a Golgi-rich fraction from the adrenal medulla. Biochim Biophys Acta. 1976 Jan 14;421(1):153–167. doi: 10.1016/0304-4165(76)90179-3. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]