Abstract
BACKGROUND
Additional prostate cancer (PCa) risk-associated single nucleotide polymorphisms (SNPs) continue to be identified. It is unclear whether addition of newly identified SNPs improves the discriminative performance of biopsy outcomes over previously established SNPs.
METHODS
A total of 667 consecutive patients that underwent prostate biopsy for detection of PCa at Huashan Hospital and Changhai Hospital, Shanghai, China were recruited. Genetic scores were calculated for each patient using various combinations of 29 PCa risk-associated SNPs. Performance of these genetic scores for discriminating prostate biopsy outcomes were compared using the area under a receiver operating characteristic curve (AUC).
RESULTS
The discriminative performance of genetic score derived from a panel of all 29 SNPs (24 previous and 5 new) was similar to that derived from the 24 previously established SNPs, the AUC of which were 0.60 and 0.61, respectively (P = 0.72). When SNPs with the strongest effect on PCa risk (ranked based on contribution to the total genetic variance from an external study) were sequentially added to the models for calculating genetic score, the AUC gradually increased and peaked at 0.62 with the top 13 strongest SNPs. Under the 13-SNP model, the PCa detection rate was 21.52%, 36.74%, and 51.98%, respectively for men with low (<0.5), intermediate (0.5–1.5), and high (>1.5) genetic score, P-trend = 9.91 × 10−6.
CONCLUSION
Genetic score based on PCa risk-associated SNPs implicated to date is a significant predictor of biopsy outcome. Additional small-effect PCa risk-associated SNPs to be discovered in the future are unlikely to further improve predictive performance.
Keywords: prostate, SNPs, genetic score, ChinaPCa, biopsy, AUC
INTRODUCTION
Prostate cancer (PCa) is the most common cancer affecting men in western developed countries and its incidence has been gradually increasing in many other countries, such as in China [1]. The etiology of PCa and the different incidence rates among countries, races, and geographic regions are largely unknown. It is hypothesized that a combination of factors such as prevalence of PCa screening using prostate-specific antigen (PSA), life expectancy, dietary and environmental exposures, and genetic factors may contribute to differential risks to PCa.
Genetic susceptibility to PCa is well established [2]. Men with a positive family history of PCa have increased risk for the disease [3–5]. More importantly, about four dozen PCa risk-associated single nucleotide polymorphisms (SNPs) have been identified during the past 7 years with the use of genome-wide association studies (GWAS) among populations of European, African-American, Japanese, and Chinese descent [6–27]. PCa risk-associated variants of these SNPs are common in respective populations and typically confer modest to moderate risk, with estimated odd ratios (ORs) ranging from 1.04 to 1.82. However, they have a stronger cumulative effect on PCa risk; men whom inherited a greater number of PCa risk-associated variants have several-fold higher risk than those inheriting fewer risk-associated variants [28]. As a result, genetic scores derived from multiple PCa risk-associated SNPs are able to significantly discriminate an individual’s risk to PCa [29–36]. Several studies have further demonstrated the clinical utility of genetic scores in discriminating outcomes of initial and repeat prostate biopsies in populations of European descent [37,38] and Chinese descent [39].
With the increasing sample size utilized in GWAS through combined or meta-analysis, more PCa risk-associated SNPs are expected to be identified. For example, 23 novel PCa risk-associated SNPs were recently discovered after evaluating 211,155 SNPs across the genome among 25,074 PCa cases and 24,272 controls from the international PRACTICAL Consortium [40]. The effect of these SNPs on PCa risk was relatively smaller compared to most prior SNPs, with ORs in the range of 1.06–1.15. This observation could be expected, as stronger PCa risk-associated SNPs were more likely to have been detected in prior studies with smaller sample sizes. However, an outstanding question is whether these newly discovered and smaller-effect PCa risk-associated SNPs can improve the discriminative performance of genetic score of previously established PCa risk-associated SNPs.
In this study, we aimed to explore this question by comparing the discriminative performance of genetic scores derived from previously established PCa risk-associated SNPs versus genetic scores based on the addition of newly implicated PCa risk-associated SNPs in a biopsy cohort from two hospitals in Shanghai, China.
METHODS
Study Subjects
The subjects included in this study were 667 consecutive patients that underwent prostate biopsy for detection of PCa at Huashan Hospital and Changhai Hospital, both of which are tertiary care hospitals in Shanghai, China. Therefore, the subjects in this study are representative of prostate biopsy patients in metropolitan areas of Southeast China. The time period for recruitment was both between April 2011 and August 2012 at Huashan Hospital and Changhai Hospital. The typical indications for prostate biopsy at these two hospitals were: (1) total PSA level >4.0 ng/ml, (2) free-to-total PSA ratio <0.16, (3) PSA density (PSAD) >0.15; or (4) presence of prostate nodules detected by digital rectal examination (DRE) or ultrasound. Transrectal ultrasound (TRUS)-guided biopsy was both performed using a 10-core scheme at Huashan Hospital and Changhai Hospital. All biopsy specimens were reviewed at the Pathology Department of both hospital. Demographic and clinical variables prior to biopsy were collected for these patients, including age, total PSA levels, and free-to-total PSA ratio (% free PSA) (Table I). In addition, peripheral blood was collected for DNA isolation. Written informed consent was obtained from each patient. This study was approved by the Institutional Review Board of both hospitals.
TABLE I.
Key Demographic and Clinical Variables in Subjects of the Biopsy Cohort
| Biopsy outcomes | ||||
|---|---|---|---|---|
| Variables | All | PCa | Non-PCa | Univariate P-value |
| No. (%) of subjects | 667 (100%) | 260 (38.98%) | 407 (61.02%) | |
| Age (n = 649) | ||||
| Mean (SD), year | 68.24 (9.06) | 71.40 (7.92) | 66.19 (9.17) | 6.28E–14 |
| Total PSA level (n = 652) | ||||
| Median (Q1–Q3), ng/ml | 11.99 (7.56–23.53) | 22.3 (11.88–73.4) | 9.73 (6.43–13.99) | 7.90E–31 |
| Mean (SD), ng/ml | 46.47 (165.08) | 98.22 (254.26) | 12.80 (11.70) | 1.66E–07 |
| Free/total ratio (n = 565) | ||||
| Median (Q1–Q3) | 0.14 (0.09–0.19) | 0.11 (0.07–0.16) | 0.16 (0.11–0.21) | 3.16E–11 |
| Mean (SD) | 0.22 (0.80) | 0.14 (0.17) | 0.26 (1.00) | 2.26E–02 |
| Gleason score (n = 251) | ||||
| ≤6 | 70 (27.9%) | |||
| 7 | 104 (41.4%) | |||
| 8 | 35 (13.9%) | |||
| 9 | 32 (12.7%) | |||
| 10 | 10 (4.0%) | |||
PCa Risk-Associated SNPs in Han Chinese
In a previous study of 1,922 PCa cases and 2,175 controls selected from the Chinese Consortium for Prostate Cancer Genetics (ChinaPCa), Na et al. [41] evaluated 53 PCa risk-associated SNPs reported prior to the end of 2012 from PCa GWAS in populations of European, Japanese, and Chinese descent, leading to the confirmation of 24 SNPs in Han Chinese at P < 0.05. The estimated ORs for these 24 SNPs ranged from 1.10 to 1.49 in Han Chinese. When a similar analysis was extended to the 23 new PCa risk-associated SNPs recently reported from PCa GWAS of the international PRACTICAL Consortium [40], five more SNPs were confirmed in the ChinaPCa (unpublished data). The OR ranged from 1.14 to 1.21 for these five SNPs. These 29 SNPs are estimated to account for 18% of the genetic variance in the Chinese population. The association results for all 29 PCa risk-associated SNPs in the ChinaPCa study are presented in Supplementary Table SI.
Genotyping of SNPs
The 29 SNPs that are associated with PCa risk in Han Chinese were selected for genotyping in 667 patients that underwent prostate biopsy. SNP genotyping was performed using MassARRAY iPLEX (Sequenom, Inc., San Diego, CA) at the Fudan Center for Genetic Epidemiology at Fudan University. Duplicates from two subjects and two water samples (negative controls) were included in each 96-well plate for genotyping quality control. The call rate was >98% for each of these SNPs and the overall concordance rate was 99.9% among duplicates.
Statistical Methods
A genetic score was calculated for each subject based on genotypes at these 29 SNPs and weighted by ORs of these SNPs derived from an external study using a method described by Pharoah et al. [42] Briefly, (1) the allelic OR for each SNP was obtained from an external study (ChinaPCa, Supplementary Table SI), (2) the genotypic OR of each SNP was estimated from the allelic OR assuming a multiplicative model, (3) the risk relative to the average risk in the population was calculated for each genotype based on genotypic OR and genotype frequency in the HapMap CHB population, and (4) genetic score was obtained by multiplying the risks relative to the population of all SNPs. Therefore, a genetic score of 1.0 indicates an average risk in the general population.
A genetic score was also calculated for each subject based on the 24 PCa risk-associated SNPs that were previously confirmed in ChinaPCa [41]. The performance of these two genetic scores in discriminating biopsy outcomes (PCa or non-PCa) was compared using the area under the receiver operating characteristic (ROC) curve (AUC). A nonparametric test was used to test different AUC’s of these two genetic scores [43].
To evaluate the model fitting and discriminative performance of genetic scores derived from various numbers of PCa risk-associated SNPs for biopsy outcomes, we first ranked these 29 SNPs based on their effect on PCa risk in the Chinese population, as measured by proportion of genetic variance explained by the SNP (Supplementary Table SII) [42]. We then calculated a series of genetic scores sequentially using the top n SNPs, where n is from 1 to 29. Finally, we fit a series of logistic regression models where in each model the dependent variable was biopsy outcome and independent variable was each genetic score. Other covariates known to be associated with biopsy outcomes such as age and total PSA levels were also included as independent variables. The Akaike information criterion (AIC) was used to compare the model fit for these genetic scores and AUC was used to compare the discriminative performance of these genetic scores.
The t-test was used to test the difference in mean of normally distributed variables between two groups (PCa and non-PCa). For variables that were not normally distributed (PSA and genetic score), two tests were performed; (1) a nonparametric method using the Wilcoxon rank sum test, and (2) t-tests for different means between two groups after log-transformation. For binary variables, a chi-square test of the proportion was performed.
RESULTS
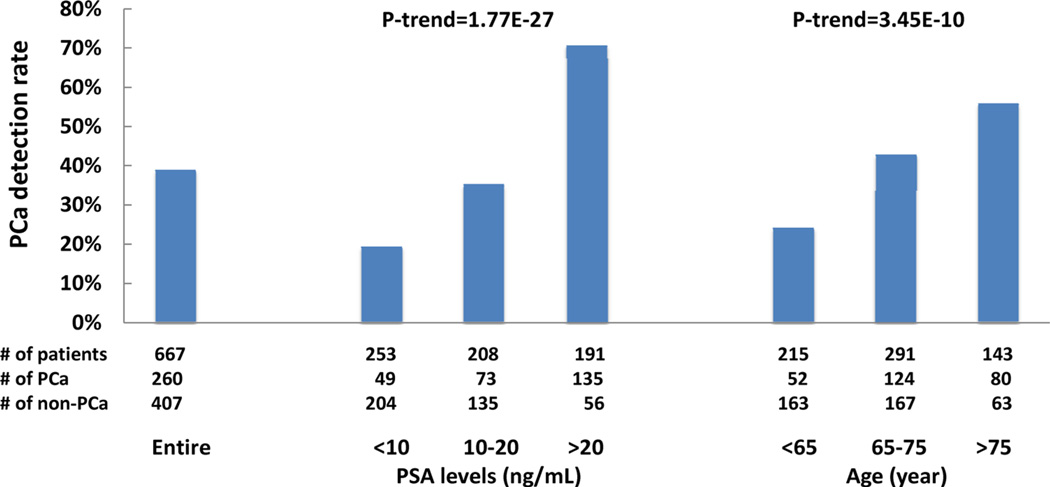
The detection rate of PCa was 38.98% overall in this biopsy cohort and was significantly higher among patients with higher total PSA levels or older ages (Fig. 1). The detection rate of PCa was 19.37%, 35.10%, and 70.68% among patients with total PSA levels <10, 10–20, and >20 ng/ml, respectively, P-trend = 1.77 × 10−27, and was 24.19%, 42.61%, and 55.94% among patients with age <65, 65–75, and >75, respectively, P-trend = 3.45 × 10−10.
Fig. 1.
Prostate cancer detection rate in the entire biopsy cohort as we as in subgroups based on PSA levels and age.
The median genetic score based on the 29 PCa risk-associated SNPs, including 24 previously implicated SNPs and 5 newly implicated SNPs, was significantly higher among patients diagnosed with PCa (1.09) than that among patients without PCa (0.88), P = 6.05 × 10−6 (Table II). Compared to men with a genetic score <1.0, men with a higher genetic score (≥1.0) had a significantly higher risk to be diagnosed with PCa, OR = 1.76, 95% confidence interval (CI): 1.39–2.23, P = 2.75 × 10−6. The performance of the genetic score to discriminate PCa cases from subjects without PCa, measured by AUC, was 0.60. The PCa detection rate increased with genetic score, and was 29.52%, 36.10%, and 50.85% in men with low (<0.5), intermediate (0.5–1.5), and high (>1.5) genetic score, respectively, P-trend = 0.0001.
TABLE II.
Association of Genetic Score and Prostate Biopsy Outcomes
| 29 SNPs | 24 SNPs | |
|---|---|---|
| Genetic score (median) | ||
| PCa | 1.09 | 1.19 |
| Non-PCa | 0.88 | 0.88 |
| P-Value | 6.05 × 10−6 | 5.61 × 10−7 |
| Association with PCa, OR (95% CI) | ||
| Genetic score ≤1.0 | 1 | 1 |
| Genetic score >1.0 | 1.76 (1.39–0.23) | 1.92 (1.49–0.45) |
| P-Value | 2.75 × 10−6 | 3.20 × 10−7 |
| Discrimination of PCa | ||
| AUC (95% CI) | 0.60 | 0.61 |
| Detection rate of PCa (%) | ||
| Genetic score <0.5 | 29.52 | 30.95 |
| Genetic score =0.5–1.49 | 36.10 | 34.96 |
| Genetic score ≥1.5 | 50.85 | 52.30 |
| P-Value | 0.0001 | 9.14 × 10−5 |
As a comparison, we also calculated genetic score based on only the 24 previously implicated SNPs. We found the association and discriminative performance of this genetic score was similar to that based on the 29 SNPs (Table II). The median genetic score based on the 24 SNPs was 1.19 and 0.88 for patients diagnosed with PCa and those without PCa, respectively, P = 5.61 × 10−7. The OR of the genetic score for a PCa diagnosis was 1.92 (95% CI: 1.49–2.45), P = 3.20 × 10−7, slightly higher than that of 29 SNPs. Similarly, the AUC of this genetic score to discriminate PCa from non-PCa was 0.61, also slightly higher than that of 29 SNPs (0.60), although the difference between these two AUCs was not statistically significant, P = 0.72. The detection rate of PCa increased with genetic score; 30.95%, 34.96%, and 52.32% in men with low (<0.5), intermediate (0.5–1.5), and high (>1.5) genetic score, respectively, P-trend = 9.14 × 10−5.
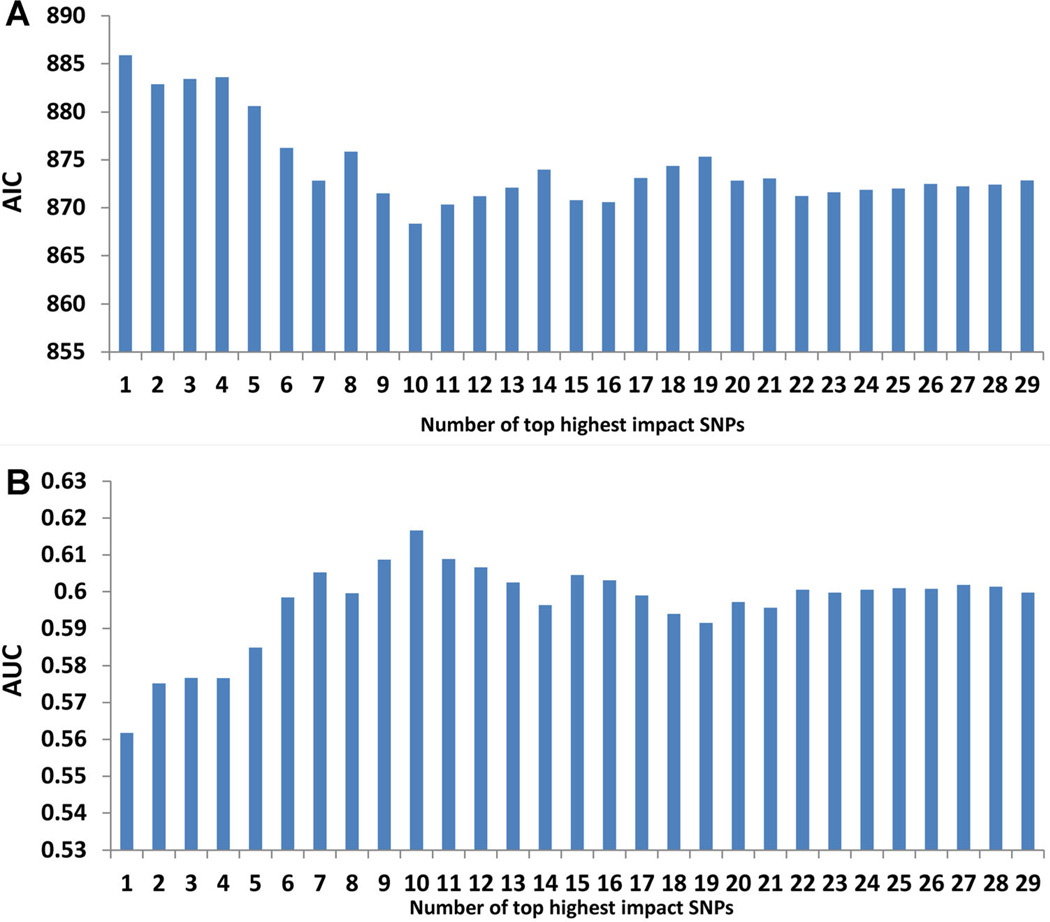
To further explore the impact of number of SNPs on the predictive performance of genetic score, we systematically evaluated model fitting (AIC) and discriminative performance (AUC) of genetic scores derived from the top n highest impact PCa risk-associated SNPs based on the contribution of SNPs to the total genetic variance, where n is from 1 to 29 SNPs (Supplementary Table SII). The AIC of these genetic scores decreased gradually first, reached a bottom for the genetic score derived from the top 13 highest impact SNPs (i.e., best fit), then increased slightly and finally stabilized when the remaining SNPs were included (Fig. 2A). Correspondingly, the AUC increased gradually and reached a peak of 0.62 for the genetic score derived from the top 13 highest impact SNPs, then decreased slightly and stabilized at ~0.60 when more SNPs were added (Fig. 2B).
Fig. 2.
Model fitting (A) and discriminative performance (B) for models derived from the top n highest impact PCa risk-associated SNPs based on contribution of SNPs to the total genetic variance, where n is from1to 29 SNPs. AIC, Akaike information criterion; AUC, area under the curve for the receiver operating characteristic.
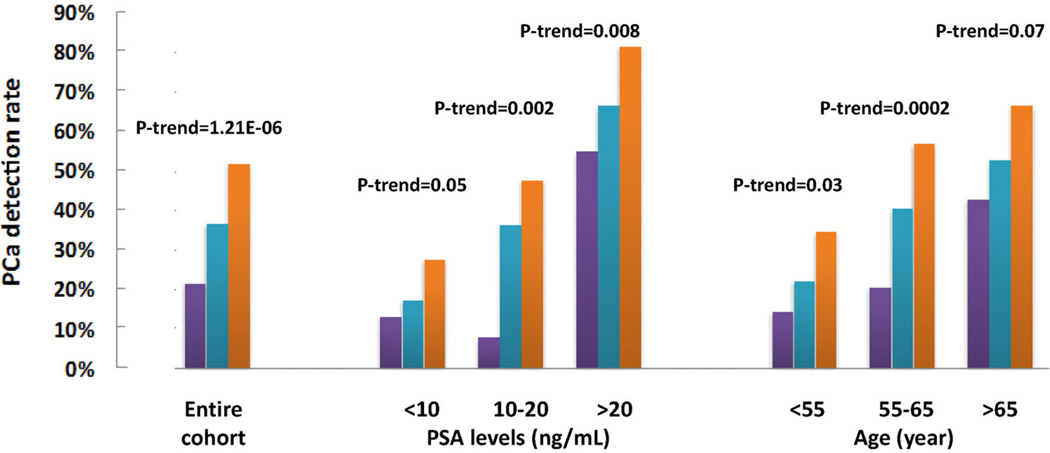
Considering that the genetic score derived from the top 13 highest impact SNPs was the best fitting and most discriminative model in this biopsy cohort, we evaluated the discriminative performance of this model in the entire cohort as well as in subsets of patients based on their PSA level and age. In the entire cohort, the detection rate of PCa increased significantly with increasing genetic score; 21.52%, 36.74%, and 51.98% for men with low (<0.5), intermediate (0.5–1.5), and high (>1.5) genetic score, respectively, P-trend = 1.21 × 10−6 (Fig. 3). The association of increasing PCa detection rate with higher genetic score was consistently observed in all subgroups based on tPSA levels or age (Fig. 3). Detailed results of PCa detection rates by genetic score in each of these subgroups, as well as AUC of the genetic score, are presented in Table III.
Fig. 3.
Prostate cancer detection rate in subjects with low (purple), intermediate (blue), and high (orange)genetic score in the entire biopsy cohort as we as in subgroups based on PSA levels and age.
TABLE III.
Discriminative Performance of Genetic Score Derived From the Top 13 Highest Impact SNPs
| Entire cohort | No. of PCa/No. of biopsed patients (PCa detection rate) at genetic score |
||||||
|---|---|---|---|---|---|---|---|
| No. of patients |
No. (%) of PCa |
AUC | <0.50 | 0.50–1.50 | ≥1.50 | P | |
| All subjects | 667 | 260 (38.98) | 0.62 | 17/79 (21.52%) | 151/411 (36.74%) | 92/177 (51.98%) | 1.21E–06 |
| Stratified by PSA levels | |||||||
| <10 ng/ml | 253 | 49 (19.37) | 0.57 | 4/31 (12.90%) | 27/157 (17.20%) | 18/65 (27.69%) | 4.97E–02 |
| 10–20 ng/ml | 208 | 73(35.10) | 0.63 | 2/26 (7.69%) | 51/140 (36.43%) | 20/42 (47.62%) | 1.50E–03 |
| >20 ng/ml | 191 | 135(70.68) | 0.63 | 11/20 (55.00%) | 70/105 (66.67%) | 54/66 (81.82%) | 7.80E–03 |
| Stratified by age | |||||||
| <65 years | 215 | 52 (24.19) | 0.59 | 4/28 (14.29%) | 30/135 (22.22%) | 18/52 (34.62%) | 2.95E–02 |
| 65–75 years | 291 | 124(42.61) | 0.64 | 7/34 (20.59%) | 72/178 (40.45%) | 45/79 (56.96%) | 2.00E–04 |
| >75 years | 143 | 80(55.94) | 0.58 | 6/14 (42.86%) | 46/87 (52.87%) | 28/42 (66.67%) | 7.26E–02 |
Finally, we assessed the performance of the genetic score derived from the top 13 SNPs in predicting high-grade PCa from prostate biopsy. Among the 667 patients that underwent prostate biopsy, 77 (11.54%) were diagnosed with high-grade PCa (Gleason score ≥8). Patients diagnosed with high-grade PCa had significantly higher genetic scores (median: 1.13) than other subjects, including patients with a negative biopsy and patients whose Gleason score <8 (median: 0.96), P = 0.04. The detection rate of PCa with Gleason score ≥8 was 6.49%, 11.60%, and 14.20%, for men in the low (<0.5), intermediate (0.5–1.5), and high (>1.5) genetic score groups, respectively. P-trend = 0.09. However, genetic score did not differentiate high-grade from low-grade disease among patients diagnosed with PCa; the median genetic score was 1.13 and 1.19 in PCa patients with Gleason ≥8 and ≤7, respectively, P = 0.67.
DISCUSSION
About 70 PCa risk-associated SNPs have been discovered throughout the genome since 2007, using GWAS in European, African American, Japanese, and Chinese populations [6–27,40]. Several studies have consistently demonstrated that genetic score calculated based on these risk-associated SNPs outperforms family history in measuring inherited risk for PCa [44] and is an independent predictor of biopsy outcome [37–39]. It is also expected that additional PCa risk-associated variants, including common SNPs and rare variants, will be identified with the use of larger sample sizes and sequencing and genotyping technologies that have a better genome coverage. An outstanding question, however, is whether these additional PCa risk-associated SNPs will further improve the overall performance in measuring genetic risk [45].
In the current study, we performed two analyses in a prostate biopsy cohort from China to explore this question. In the first analysis, we directly compared the performance of two genetic scores in predicting biopsy outcomes; one was based on 24 previously implicated PCa risk-associated SNPs, and the other was based on the 24 SNPs and 5 recently implicated PCa risk-associated SNPs. Results from this analysis revealed that the performance was similar between these two genetic scores. In fact, the performance was slightly worse, although not statistically significant, for the genetic score based on 29 SNPs. This comparison was relevant because it reflected the reality of these sequentially discovered PCa risk-associated SNPs, where each of the five new PCa risk-associated SNPs generally confers lower risk (OR from 1.14 to 1.21) than each of the 24 previously implicated SNPs (OR from 1.10 to 1.49). In the second analysis, we firstly ranked each of these discovered PCa risk-associated SNPs by its contribution to the total genetic variance and then systematically evaluated the predictive performance of genetic scores derived from the top highest impact SNPs. Interestingly, the analysis revealed that the predictive performance reached a peak when the top 13 highest impact PCa risk-associated SNPs were included in calculating the genetic score. Considering that PCa risk-associated variants with higher impact (stronger OR and higher frequency) have likely been discovered, results from these two analyses suggest that lower impact PCa risk-associated SNPs to be discovered in the future are unlikely to further improve the performance of genetic score in measuring inherited risk to PCa.
A similar result was reported previously in a population-based case–control study in Sweden, including 2,899 PCa cases and 1,722 controls [32]. When a set of genetic scores were calculated based on 28 ordered PCa risk-associated SNPs, from highest to lowest contribution to the total genetic variance, their positive predictive value of PCa increased gradually and reached a plateau when the top 11 SNPs were included in calculating genetic score. In addition, we observed the similar finding from the REduction by DUtasteride of Prostate Cancer Events (REDUCE) trial, a randomized chemoprevention trial of PCa using dutasteride (unpublished data). Genotype data of 64 known PCa risk-associated SNPs were available among 1,654 Caucasian men in the placebo arm of the REDUCE. We performed a similar analysis as the current study. When SNPs with the strongest effect on PCa risk (ranked based on contribution to the total genetic variance from an external study) were sequentially added to the models for calculating genetic score, the AUC gradually increased, peaked at 0.62 with the top 43 strongest SNPs, and then gradually decreased to 0.61. These additional data suggest the reported phenomenon of the current study is not limited to this Chinese population and maybe a general finding.
The plateau effect is also reported in simulated data and other diseases. In a simulation study evaluating factors affecting the predictive performance (AUC) of risk-associated SNPs discovered from GWAS, including the number of SNPs (20, 50, 100, 200, 300, and 400 most significant SNPs), sample size (500, 1,000, 2,000, 5,000, and 10,000), and classification algorithms (logistic regression, risk-score, and support vector machine), Kang et al. [46] found that the risk-score logistic regression model with 20–50 SNPs provided the best performance when the ORs of these SNPs were moderate (median OR was 1.31). These ORs were similar to that discovered for PCa. In that same paper, Kang et al. also evaluated the predictive performance of risk-associated SNPs for Crohn’s disease in a GWAS study of 547 cases and 549 controls. They evaluated the predictive performance of the top 2, 10, 20, 50, and 100 most significant SNPs using a risk-score and found the best performance was observed when the top 20 SNPs were included in the model.
Results from this study provide evidence that genetic score calculated from PCa risk-associated SNPs may help to supplement PSA levels to better determine the need for prostate biopsy which is used to diagnose PCa. Currently, the primary indication for prostate biopsy is elevated tPSA levels. However, because tPSA is not PCa specific, moderately elevated PSA levels have limited specificity. As shown in this study, the overall PCa detection rate from this biopsy cohort was only 38.98%, typical for tertiary hospitals in major cities of China. In other words, more than 50% of patients that currently undergo prostate biopsy for the purpose of diagnosing PCa may be having an unnecessary invasive procedure. However, if we add genetic score information to the information available to make decisions for prostate biopsy, this may reduce the number of unnecessary biopsies while increasing the likelihood of detecting PCa among biopsied patients. For example, the PCa detection rates are below 18% for patients with tPSA <10 ng/ml if they have low or intermediate genetic score and for patients with tPSA at 10–20 ng/ml if they have low genetic score. This rate of <18% for PCa may be accepted by most patients and their treating urologists because it is equivalent to the detection rate of tPSA <2 ng/ml [47]. About 33% of patients in this biopsy cohort belong to these groups based on their tPSA levels and genetic score. On the other hand, the expected PCa detection rate was 47.62% among patients with tPSA of 10–20 ng/ml if they have a high genetic score, considerably higher than the average PCa detection rate of 35.10% in this tPSA subgroup. It is also noted that the added value of genetic score to PSA is most prominent in patients with tPSA <20 ng/ml. For patients with tPSA >20 ng/ml, although higher genetic score was significantly associated with higher PCa detection, the PCa detection rates in all genetic score groups were high enough (>55%) to warrant a biopsy.
Genetic score may have another important clinical application, in determining the need for PSA screening for PCa. The goal of PSA screening is the identification of PCa at an earlier and more treatable stage in order to reduce mortality. However, conflicting results regarding its impact on mortality were reported from two large randomized trials; the European Randomized Study for the Screening of Prostate Cancer (ERSPC) and The Prostate, Lung, Colorectal and Ovarian (PLCO) [48,49]. A updated analysis of the ERSPC in 2012 suggested that PSA screening results in modest reductions in PCa-specific mortality [50]. By weighing the benefit versus potential harms downstream of PSA screening such as prostate biopsy and treatment, the U.S. Preventive Services Task Force (USPSTF) issued new draft recommendations against PSA screening for PCa in all men [51]. In responding to the USPSTF’s recommendation, the American Urology Association (AUA) issued a new recommendation for PSA screening in 2013, emphasizing targeted PSA screening based on an individual’s risk for PCa. Specifically, the AUA does not recommend a routine screening among men between 40 and 54 years old at “average” risk, and strongly recommends shared decision making for men aging 55–69 years old who need to consider PSA screening [52]. A central question of the new guideline is to understand an individual’s risk for PCa prior to a PSA test. Unfortunately, currently approach to define risk, which primarily relies on family history, is limited. Considering that genetic score is a more objective and accurate measurement of inherited risk than family history [43], it is rational to suggest that genetic score should be included to supplement family history in defining PCa risk. This approach is particularly useful for ~80% men in the general population who do not have a positive family history because a subset of these men are also at a higher risk. The clinical utility and cost-effectiveness of this genomic-targeted PSA screening approach, however, needs to be directly assessed in evidence-based studies.
Genetic score is particularly important in countries such as China where family history is uninformative due to historically low incidence of PCa. The percentage of men with a positive family history of PCa is extremely low in China because the disease was rarely diagnosed in this country in prior decades. The historically low incidence is likely attributable to the low adoption of PSA screening and low life expectancy in China, rather than low genetic susceptibility in Chinese. This assumption is supported by the fact that 29 PCa risk-associated SNPs have been implicated in Chinese and that genetic score derived from these SNPs is associated with PCa detection rate. This difference between genetic score and family history in China highlights key distinctions between these two measurements of inherited risk. The former is a direct measurement of genetic material of individuals self while the latter is an indirect measurement through relatives. Therefore, unlike family history that is influenced by historical disease incidence, family size, age and survival status of male relatives, genetic score is an objective measurement and does not change during a lifetime. As such, genetic score has great potential to be widely used in China to measure inherited risk of PCa for targeted PSA screening, prevention, and early diagnosis.
There are several limitations in this study. First, the sample size of this study was relatively small and all patients were from two tertiary hospitals in Shanghai, China. Although a highly significant association between genetic score and PCa detection rate was observed even with this small study, these limitations may affect the estimate of its association and the ability to generalize the results. Larger and multi-center studies are needed to establish more reliable estimates of PCa detection rates at different cutoffs of genetic score. Second, many important clinical variables and novel biomarkers such as prostate volume, serum p2PSA, and urine PCA3 and fusion genes were not collected in this study. It is expected that a combination of these variables may further improve prediction of PCa detection rates and help to determine the need for biopsy. Finally, although we found that increasing genetic score was significantly associated with a diagnosis of high-grade PCa (Gleason score ≥8), the genetic score did not significantly distinguish risk between high-grade and low-grade PCa. It is noted that none of these 29 PCa risk associated SNPs was significantly associated with Gleason score of PCa in Chinese [41], a similar finding to that of Caucasians [53]. More effort should be devoted to the identification of SNPs that are associated with aggressive but not indolent PCa. Such SNPs would be helpful to identify patients at high risk for aggressive PCa, and thus in guiding biopsy decisions.
In conclusion, genetic score based on PCa risk-associated SNPs implicated to date is a significant predictor of biopsy outcome. Additional small-effect PCa risk-associated SNPs to be discovered in the future are unlikely to further improve predictive performance.
Supplementary Material
ACKNOWLEDGMENTS
We thank all of the subjects included in this study. This work was partially funded by The National Science Foundation of China (no. 81101946) to S.R., the Prostate Cancer Foundation Young Investigator Award to S.R., Shanghai Pujiang Program (12PJD008) to S.R., the National Key Basic Research Program Grant 973 (2012CB518301) to J.X., the Key Project of the National Natural Science Foundation of China (81130047) to J.X., intramural grants from Fudan University “Thousand Talents Program” and Huashan Hospital to J.X., the National Institutes of Health (NCI CA129684) to J.X., the grants from Science and Technology Commission of Shanghai Municipality (11410708200) to Q.D.
Grant sponsor: National Science Foundation of China; Grant number: 81101946; Grant sponsor: Prostate Cancer Foundation Young Investigator Award; Grant sponsor: Shanghai Pujiang Program; Grant number: 12PJD008; Grant sponsor: National Key Basic Research Program Grant 973; Grant number: 2012CB518301; Grant sponsor: Key Project of the National Natural Science Foundation of China; Grant number: 81130047; Grant sponsor: Fudan University “Thousand Talents Program”; Grant sponsor: Huashan Hospital; Grant sponsor: National Institutes of Health; Grant number: NCI CA129684; Grant sponsor: Science and Technology Commission of Shanghai Municipality; Grant number: 11410708200; Grant sponsor: The National Basic Research Program to Y.S; Grant number: 2012CB518300.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s website.
TABLE SI Prostate Cancer Risk-Associated SNPs Confirmed in the Chinese Population
TABLE SII Contribution of SNPs to Genetic Variance
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Sun J, Zheng SL. Prostate cancer risk-associated genetic markers and their potential clinical utility. Asian J Androl. 2013;15(3):314–322. doi: 10.1038/aja.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johns LE, Houlston RS. A systematic review and meta-analysis of familial prostate cancer risk. BJU Int. 2003;91:789–794. doi: 10.1046/j.1464-410x.2003.04232.x. [DOI] [PubMed] [Google Scholar]
- 4.Bruner DB, Moore D, Parlanti A, Dorgan J, Engstrom P. Relative risk of prostate cancer for men with affected relatives: Systematic review and meta-analysis. Int J Cancer. 2003;107:797–803. doi: 10.1002/ijc.11466. [DOI] [PubMed] [Google Scholar]
- 5.Zeegers MPA, Jellema A, Ostrer H. Empiric risk of prostate carcinoma for relatives of patients with prostate carcinoma. A meta-analysis. Cancer. 2003;97:1894–1903. doi: 10.1002/cncr.11262. [DOI] [PubMed] [Google Scholar]
- 6.Amundadottir LT, Sulem P, Gudmundsson J, Helgason A, Baker A, Agnarsson BA, Sigurdsson A, Benediktsdottir KR, Cazier JB, Sainz J, Jakobsdottir M, Kostic J, Magnusdottir DN, Ghosh S, Agnarsson K, Birgisdottir B, Le Roux L, Olafsdottir A, Blondal T, Andresdottir M, Gretarsdottir OS, Bergthorsson JT, Gudbjartsson D, Gylfason A, Thorleifsson G, Manolescu A, Kristjansson K, Geirsson G, Isaksson H, Douglas J, Johansson JE, Bälter K, Wiklund F, Montie JE, Yu X, Suarez BK, Ober C, Cooney KA, Gronberg H, Catalona WJ, Einarsson GV, Barkardottir RB, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. A common variant associated with prostate cancer in European and African populations. Nat Genet. 2006;38(6):652–867. doi: 10.1038/ng1808. [DOI] [PubMed] [Google Scholar]
- 7.Yeager M, Orr N, Hayes RB, Jacobs KB, Kraft P, Wacholder S, Minichiello MJ, Fearnhead P, Yu K, Chatterjee N, Wang Z, Welch R, Staats BJ, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cancel-Tassin G, Cussenot O, Valeri A, Andriole GL, Gelmann EP, Tucker M, Gerhard DS, Fraumeni JF, Jr, Hoover R, Hunter DJ, Chanock SJ, Thomas G. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. 2007;39:645–649. doi: 10.1038/ng2022. [DOI] [PubMed] [Google Scholar]
- 8.Gudmundsson J, Sulem P, Manolescu A, Amundadottir LT, Gudbjartsson D, Helgason A, Rafnar T, Bergthorsson JT, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Xu J, Blondal T, Kostic J, Sun J, Ghosh S, Stacey SN, Mouy M, Saemundsdottir J, Backman VM, Kristjansson K, Tres A, Partin AW, Albers-Akkers MT, Godino-Ivan Marcos J, Walsh PC, Swinkels DW, Navarrete S, Isaacs SD, Aben KK, Graif T, Cashy J, Ruiz-Echarri M, Wiley KE, Suarez BK, Witjes JA, Frigge M, Ober C, Jonsson E, Einarsson GV, Mayordomo JI, Kiemeney LA, Isaacs WB, Catalona WJ, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet. 2007;39:631–637. doi: 10.1038/ng1999. [DOI] [PubMed] [Google Scholar]
- 9.Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Blondal T, Stacey SN, Helgason A, Gunnarsdottir S, Olafsdottir A, Kristinsson KT, Birgisdottir B, Ghosh S, Thorlacius S, Magnusdottir D, Stefansdottir G, Kristjansson K, Bagger Y, Wilensky RL, Reilly MP, Morris AD, Kimber CH, Adeyemo A, Chen Y, Zhou J, So WY, Tong PC, Ng MC, Hansen T, Andersen G, Borch-Johnsen K, Jorgensen T, Tres A, Fuertes F, Ruiz-Echarri M, Asin L, Saez B, van Boven E, Klaver S, Swinkels DW, Aben KK, Graif T, Cashy J, Suarez BK, van Vierssen Trip O, Frigge ML, Ober C, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Palmer CN, Rotimi C, Chan JC, Pedersen O, Sigurdsson G, Benediktsson R, Jonsson E, Einarsson GV, Mayordomo JI, Catalona WJ, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39:977–983. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 10.Duggan D, Zheng SL, Knowlton M, Benitez D, Dimitrov L, Wiklund F, Robbins C, Isaacs SD, Cheng Y, Li G, Sun J, Chang BL, Marovich L, Wiley KE, Bälter K, Stattin P, Adami HO, Gielzak M, Yan G, Sauvageot J, Liu W, Kim JW, Bleecker ER, Meyers DA, Trock BJ, Partin AW, Walsh PC, Isaacs WB, Grönberg H, Xu J, Carpten JD. Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene DAB2IP. J Natl Cancer Inst. 2007;99:1836–1844. doi: 10.1093/jnci/djm250. [DOI] [PubMed] [Google Scholar]
- 11.Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, Yu K, Chatterjee N, Welch R, Hutchinson A, Crenshaw A, Cancel-Tassin G, Staats BJ, Wang Z, Gonzalez-Bosquet J, Fang J, Deng X, Berndt SI, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cussenot O, Valeri A, Andriole GL, Crawford ED, Tucker M, Gerhard DS, Fraumeni JF, Jr, Hoover R, Hayes RB, Hunter DJ, Chanock SJ. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40:310–315. doi: 10.1038/ng.91. [DOI] [PubMed] [Google Scholar]
- 12.Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, Mulholland S, Leongamornlert DA, Edwards SM, Morrison J, Field HI, Southey MC, Severi G, Donovan JL, Hamdy FC, Dearnaley DP, Muir KR, Smith C, Bagnato M, Ardern-Jones AT, Hall AL, O’Brien LT, Gehr-Swain BN, Wilkinson RA, Cox A, Lewis S, Brown PM, Jhavar SG, Tymrakiewicz M, Lophatananon A, Bryant SL, Huddart RA, Khoo VS, Parker CC, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Fisher C, Jamieson C, Cooper CS, English DR, Hopper JL, Neal DE, Easton DF UK Genetic Prostate Cancer Study Collaborators, British Association of Urological Surgeons’ Section of Oncology, UK ProtecT Study Collaborators. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet. 2008;40:316–321. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 13.Gudmundsson J, Sulem P, Rafnar T, Bergthorsson JT, Manolescu A, Gudbjartsson D, Agnarsson BA, Sigurdsson A, Benediktsdottir KR, Blondal T, Jakobsdottir M, Stacey SN, Kostic J, Kristinsson KT, Birgisdottir B, Ghosh S, Magnusdottir DN, Thorlacius S, Thorleifsson G, Zheng SL, Sun J, Chang BL, Elmore JB, Breyer JP, McReynolds KM, Bradley KM, Yaspan BL, Wiklund F, Stattin P, Lindström S, Adami HO, McDonnell SK, Schaid DJ, Cunningham JM, Wang L, Cerhan JR, St Sauver JL, Isaacs SD, Wiley KE, Partin AW, Walsh PC, Polo S, Ruiz-Echarri M, Navarrete S, Fuertes F, Saez B, Godino J, Weijerman PC, Swinkels DW, Aben KK, Witjes JA, Suarez BK, Helfand BT, Frigge ML, Kristjansson K, Ober C, Jonsson E, Einarsson GV, Xu J, Gronberg H, Smith JR, Thibodeau SN, Isaacs WB, Catalona WJ, Mayordomo JI, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Common sequence variants on 2p15 and Xp11.22 confer susceptibility to prostate cancer. Nat Genet. 2008;40:281–283. doi: 10.1038/ng.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Olama AA, Kote-Jarai Z, Giles GG, Guy M, Morrison J, Severi G, Leongamornlert DA, Tymrakiewicz M, Jhavar S, Saunders E, Hopper JL, Southey MC, Muir KR, English DR, Dearnaley DP, Ardern-Jones AT, Hall AL, O’Brien LT, Wilkinson RA, Sawyer E, Lophatananon A, Horwich A, Huddart RA, Khoo VS, Parker CC, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Cooper C, Donovan JL, Hamdy FC, Neal DE, Eeles RA, Easton DF UK Genetic Prostate Cancer Study Collaborators/British Association of Urological Surgeons’ Section of Oncology, UK Prostate Testing for Cancer and Treatment Study (ProtecT Study) Collaborators. Multiple loci on 8q24 associated with prostate cancer susceptibility. Nat Genet. 2009;41:1058–1060. doi: 10.1038/ng.452. [DOI] [PubMed] [Google Scholar]
- 15.Yeager M, Chatterjee N, Ciampa J, Jacobs KB, Gonzalez-Bosquet J, Hayes RB, Kraft P, Wacholder S, Orr N, Berndt S, Yu K, Hutchinson A, Wang Z, Amundadottir L, Feigelson HS, Thun MJ, Diver WR, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Cancel-Tassin G, Cussenot O, Valeri A, Andriole GL, Crawford ED, Haiman CA, Henderson B, Kolonel L, Le Marchand L, Siddiq A, Riboli E, Key TJ, Kaaks R, Isaacs W, Isaacs S, Wiley KE, Gronberg H, Wiklund F, Stattin P, Xu J, Zheng SL, Sun J, Vatten LJ, Hveem K, Kumle M, Tucker M, Gerhard DS, Hoover RN, Fraumeni JF, Jr, Hunter DJ, Thomas G, Chanock SJ. Identification of a new prostate cancer susceptibility locus on chromosome 8q24. Nat Genet. 2009;41(10):1055–1057. doi: 10.1038/ng.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eeles RA, Kote-Jarai Z, Al Olama AA, Giles GG, Guy M, Severi G, Muir K, Hopper JL, Henderson BE, Haiman CA, Schleutker J, Hamdy FC, Neal DE, Donovan JL, Stanford JL, Ostrander EA, Ingles SA, John EM, Thibodeau SN, Schaid D, Park JY, Spurdle A, Clements J, Dickinson JL, Maier C, Vogel W, Dörk T, Rebbeck TR, Cooney KA, Cannon-Albright L, Chappuis PO, Hutter P, Zeegers M, Kaneva R, Zhang HW, Lu YJ, Foulkes WD, English DR, Leongamornlert DA, Tymrakiewicz M, Morrison J, Ardern-Jones AT, Hall AL, O’Brien LT, Wilkinson RA, Saunders EJ, Page EC, Sawyer EJ, Edwards SM, Dearnaley DP, Horwich A, Huddart RA, Khoo VS, Parker CC, Van As N, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Cooper CS, Southey MC, Lophatananon A, Liu JF, Kolonel LN, Le Marchand L, Wahlfors T, Tammela TL, Auvinen A, Lewis SJ, Cox A, FitzGerald LM, Koopmeiners JS, Karyadi DM, Kwon EM, Stern MC, Corral R, Joshi AD, Shahabi A, McDonnell SK, Sellers TA, Pow-Sang J, Chambers S, Aitken J, Gardiner RA, Batra J, Kedda MA, Lose F, Polanowski A, Patterson B, Serth J, Meyer A, Luedeke M, Stefflova K, Ray AM, Lange EM, Farnham J, Khan H, Slavov C, Mitkova A, Cao G, Cao G, Easton DF UK Genetic Prostate Cancer Study Collaborators/British Association of Urological Surgeons’ Section of Oncology, UK ProtecT Study Collaborators, PRACTICAL Consortium. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet. 2009;41:1116–1121. doi: 10.1038/ng.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gudmundsson J, Sulem P, Gudbjartsson DF, Blondal T, Gylfason A, Agnarsson BA, Benediktsdottir KR, Magnusdottir DN, Orlygsdottir G, Jakobsdottir M, Stacey SN, Sigurdsson A, Wahlfors T, Tammela T, Breyer JP, McReynolds KM, Bradley KM, Saez B, Godino J, Navarrete S, Fuertes F, Murillo L, Polo E, Aben KK, van Oort IM, Suarez BK, Helfand BT, Kan D, Zanon C, Frigge ML, Kristjansson K, Gulcher JR, Einarsson GV, Jonsson E, Catalona WJ, Mayordomo JI, Kiemeney LA, Smith JR, Schleutker J, Barkardottir RB, Kong A, Thorsteinsdottir U, Rafnar T, Stefansson K. Genome-wide association and replication studies identify four variants associated with prostate cancer susceptibility. Nat Genet. 2009;41:1122–1126. doi: 10.1038/ng.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumacher FR, Berndt SI, Siddiq A, Jacobs KB, Wang Z, Lindstrom S, Stevens VL, Chen C, Mondul AM, Travis RC, Stram DO, Eeles RA, Easton DF, Giles G, Hopper JL, Neal DE, Hamdy FC, Donovan JL, Muir K, Al Olama AA, Kote-Jarai Z, Guy M, Severi G, Grönberg H, Isaacs WB, Karlsson R, Wiklund F, Xu J, Allen NE, Andriole GL, Barricarte A, Boeing H, Buenode-Mesquita HB, Crawford ED, Diver WR, Gonzalez CA, Gaziano JM, Giovannucci EL, Johansson M, Le Marchand L, Ma J, Sieri S, Stattin P, Stampfer MJ, Tjonneland A, Vineis P, Virtamo J, Vogel U, Weinstein SJ, Yeager M, Thun MJ, Kolonel LN, Henderson BE, Albanes D, Hayes RB, Feigelson HS, Riboli E, Hunter DJ, Chanock SJ, Haiman CA, Kraft P. Genome-wide association study identifies new prostate cancer susceptibility loci. Hum Mol Genet. 2011;20:3867–3875. doi: 10.1093/hmg/ddr295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kote-Jarai Z, Amin Al Olama A, Leongamornlert D, Tymrakiewicz M, Saunders E, Guy M, Giles GG, Severi G, Southey M, Hopper JL, Sit KC, Harris JM, Batra J, Spurdle AB, Clements JA, Hamdy F, Neal D, Donovan J, Muir K, Pharoah PD, Chanock SJ, Brown N, Benlloch S, Castro E, Mahmud N, O’Brien L, Hall A, Sawyer E, Wilkinson R, Easton DF, Eeles RA. Identification of a novel prostate cancer susceptibility variant in the KLK3 gene transcript. Hum Genet. 2011;129:687–694. doi: 10.1007/s00439-011-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haiman CA, Chen GK, Blot WJ, Strom SS, Berndt SI, Kittles RA, Rybicki BA, Isaacs WB, Ingles SA, Stanford JL, Diver WR, Witte JS, Hsing AW, Nemesure B, Rebbeck TR, Cooney KA, Xu J, Kibel AS, Hu JJ, John EM, Gueye SM, Watya S, Signorello LB, Hayes RB, Wang Z, Yeboah E, Tettey Y, Cai Q, Kolb S, Ostrander EA, Zeigler-Johnson C, Yamamura Y, Neslund-Dudas C, Haslag-Minoff J, Wu W, Thomas V, Allen GO, Murphy A, Chang BL, Zheng SL, Leske MC, Wu SY, Ray AM, Hennis AJ, Thun MJ, Carpten J, Casey G, Carter EN, Duarte ER, Xia LY, Sheng X, Wan P, Pooler LC, Cheng I, Monroe KR, Schumacher F, Le Marchand L, Kolonel LN, Chanock SJ, Van Den Berg D, Stram DO, Henderson BE. Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nat Genet. 2011;43:570–573. doi: 10.1038/ng.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takata R, Akamatsu S, Kubo M, Takahashi A, Hosono N, Kawaguchi T, Tsunoda T, Inazawa J, Kamatani N, Ogawa O, Fujioka T, Nakamura Y, Nakagawa H. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat Genet. 2010;42:751–754. doi: 10.1038/ng.635. [DOI] [PubMed] [Google Scholar]
- 22.Akamatsu S, Takata R, Haiman CA, Takahashi A, Inoue T, Kubo M, Furihata M, Kamatani N, Inazawa J, Chen GK, Le Marchand L, Kolonel LN, Katoh T, Yamano Y, Yamakado M, Takahashi H, Yamada H, Egawa S, Fujioka T, Henderson BE, Habuchi T, Ogawa O, Nakamura Y, Nakagawa H. Common variants at 11q12, 10q26 and 3p11.2 are associated with prostate cancer susceptibility in Japanese. Nat Genet. 2012;44:426–429. S1. doi: 10.1038/ng.1104. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Mo Z, Ye D, Wang M, Liu F, Jin G, Xu C, Wang X, Shao Q, Chen Z, Tao Z, Qi J, Zhou F, Wang Z, Fu Y, He D, Wei Q, Guo J, Wu D, Gao X, Yuan J, Wang G, Xu Y, Wang G, Yao H, Dong P, Jiao Y, Shen M, Yang J, Ou-Yang J, Jiang H, Zhu Y, Ren S, Zhang Z, Yin C, Gao X, Dai B, Hu Z, Yang Y, Wu Q, Chen H, Peng P, Zheng Y, Zheng X, Xiang Y, Long J, Gong J, Na R, Lin X, Yu H, Wang Z, Tao S, Feng J, Sun J, Liu W, Hsing A, Rao J, Ding Q, Wiklund F, Gronberg H, Shu XO, Zheng W, Shen H, Jin L, Shi R, Lu D, Zhang X, Sun J, Zheng SL, Sun Y. Genome-wide association study in Chinese men identifies two new prostate cancer risk loci at 9q31.2 and 19q13.4. Nat Genet. 2012;44:1231–1235. doi: 10.1038/ng.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng SL, Stevens VL, Wiklund F, Isaacs SD, Sun J, Smith S, Pruett K, Wiley KE, Kim ST, Zhu Y, Zhang Z, Hsu FC, Turner AR, Johansson JE, Liu W, Kim JW, Chang BL, Duggan D, Carpten J, Rodriguez C, Isaacs W, Grönberg H, Xu J. Two independent prostate cancer risk-associated Loci at 11q13. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1815–1820. doi: 10.1158/1055-9965.EPI-08-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun J, Zheng SL, Wiklund F, Isaacs SD, Purcell LD, Gao Z, Hsu FC, Kim ST, Liu W, Zhu Y, Stattin P, Adami HO, Wiley KE, Dimitrov L, Sun J, Li T, Turner AR, Adams TS, Adolfsson J, Johansson JE, Lowey J, Trock BJ, Partin AW, Walsh PC, Trent JM, Duggan D, Carpten J, Chang BL, Grönberg H, Isaacs WB, Xu J. Evidence for two independent prostate cancer risk-associated loci in the HNF1B gene at 17q12. Nat Genet. 2008;40(10):1153–1155. doi: 10.1038/ng.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu FC, Sun J, Wiklund F, Isaacs SD, Wiley KE, Purcell LD, Gao Z, Stattin P, Zhu Y, Kim ST, Zhang Z, Liu W, Chang BL, Walsh PC, Duggan D, Carpten JD, Isaacs WB, Grönberg H, Xu J, Zheng SL. A novel prostate cancer susceptibility locus at 19q13. Cancer Res. 2009;69(7):2720–2723. doi: 10.1158/0008-5472.CAN-08-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J, Zheng SL, Wiklund F, Isaacs SD, Li G, Wiley KE, Kim ST, Zhu Y, Zhang Z, Hsu FC, Turner AR, Stattin P, Liu W, Kim JW, Duggan D, Carpten J, Isaacs W, Grönberg H, Xu J, Chang BL. Sequence variants at 22q13 are associated with prostate cancer risk. Cancer Res. 2009;69(1):10–15. doi: 10.1158/0008-5472.CAN-08-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng SL, Sun J, Wiklund F, Smith S, Stattin P, Li G, Adami HO, Hsu FC, Zhu Y, Bälter K, Kader AK, Turner AR, Liu W, Bleecker ER, Meyers DA, Duggan D, Carpten JD, Chang BL, Isaacs WB, Xu J, Grönberg H. Cumulative association of five genetic variants with prostate cancer. N Engl J Med. 2008;358:910–919. doi: 10.1056/NEJMoa075819. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Sun J, Kader AK, Lindström S, Wiklund F, Hsu FC, Johansson JE, Zheng SL, Thomas G, Hayes RB, Kraft P, Hunter DJ, Chanock SJ, Isaacs WB, Grönberg H. Estimation of absolute risk for prostate cancer using genetic markers and family history. Prostate. 2009;69:1565–1572. doi: 10.1002/pros.21002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salinas CA, Koopmeiners JS, Kwon EM, FitzGerald L, Lin DW, Ostrander EA, Feng Z, Stanford JL. Clinical utility of five genetic variants for predicting prostate cancer risk and mortality. Prostate. 2009;69(4):363–372. doi: 10.1002/pros.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kote-Jarai Z, Easton DF, Stanford JL, Ostrander EA, Schleutker J, Ingles SA, Schaid D, Thibodeau S, Dörk T, Neal D, Donovan J, Hamdy F, Cox A, Maier C, Vogel W, Guy M, Muir K, Lophatananon A, Kedda MA, Spurdle A, Steginga S, John EM, Giles G, Hopper J, Chappuis PO, Hutter P, Foulkes WD, Hamel N, Salinas CA, Koopmeiners JS, Karyadi DM, Johanneson B, Wahlfors T, Tammela TL, Stern MC, Corral R, McDonnell SK, Schürmann P, Meyer A, Kuefer R, Leongamornlert DA, Tymrakiewicz M, Liu JF, O’Mara T, Gardiner RA, Aitken J, Joshi AD, Severi G, English DR, Southey M, Edwards SM, Al Olama AA, Eeles RA PRACTICAL Consortium. Multiple novel prostate cancer predisposition loci confirmed by an international study: The PRACTICAL Consortium. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2052–2061. doi: 10.1158/1055-9965.EPI-08-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J, Kader AK, Hsu FC, Kim ST, Zhu Y, Turner AR, Jin T, Zhang Z, Adolfsson J, Wiklund F, Zheng SL, Isaacs WB, Grönberg H, Xu J. Inherited genetic markers discovered to date are able to identify a significant number of men at considerably elevated risk for prostate cancer. Prostate. 2011;71(4):421–430. doi: 10.1002/pros.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindström S, Schumacher FR, Cox D, Travis RC, Albanes D, Allen NE, Andriole G, Berndt SI, Boeing H, Bueno-de-Mesquita HB, Crawford ED, Diver WR, Gaziano JM, Giles GG, Giovannucci E, Gonzalez CA, Henderson B, Hunter DJ, Johansson M, Kolonel LN, Ma J, Le Marchand L, Pala V, Stampfer M, Stram DO, Thun MJ, Tjonneland A, Trichopoulos D, Virtamo J, Weinstein SJ, Willett WC, Yeager M, Hayes RB, Severi G, Haiman CA, Chanock SJ, Kraft P. Common genetic variants in prostate cancer risk prediction—Results from the NCI Breast and Prostate Cancer Cohort Consortium (BPC3) Cancer Epidemiol Biomarkers Prev. 2012;21(3):437–444. doi: 10.1158/1055-9965.EPI-11-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein RJ, Hallden C, Gupta A, Savage CJ, Dahlin A, Bjartell A, Manjer J, Scardino PT, Ulmert D, Wallström P, Vickers AJ, Lilja H. Evaluation of multiple risk-associated single nucleotide polymorphisms versus prostate-specific antigen at baseline to predict prostate cancer in unscreened men. Eur Urol. 2012;61(3):471–477. doi: 10.1016/j.eururo.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akamatsu S, Takahashi A, Takata R, Kubo M, Inoue T, Morizono T, Tsunoda T, Kamatani N, Haiman CA, Wan P, Chen GK, Le Marchand L, Kolonel LN, Henderson BE, Fujioka T, Habuchi T, Nakamura Y, Ogawa O, Nakagawa H. Reproducibility, performance, and clinical utility of a genetic risk prediction model for prostate cancer in Japanese. PLoS ONE. 2012;7(10):e 46454. doi: 10.1371/journal.pone.0046454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng J, Liu F, Lin X, Wang X, Ding Q, Jiang H, Chen H, Lu D, Jin G, Hsing AW, Shao Q, Qi J, Ye Y, Wang Z, Gao X, Wang G, Chu LW, Ouyang J, Huang Y, Chen Y, Gao Y, Shi R, Wu Q, Wang M, Zhang Z, Hu Y, Sun J, Zheng SL, Gao X, Xu C, Mo Z, Sun Y, Xu J. Predictive performance of prostate cancer risk in Chinese men using 33 reported prostate cancer risk-associated SNPs. Prostate. 2012;72(5):577–583. doi: 10.1002/pros.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aly M, Wiklund F, Xu J, Isaacs WB, Eklund M, D’Amato M, Adolfsson J, Grönberg H. Polygenic risk score improves prostate cancer risk prediction: Results from the Stockholm-1 cohort study. Eur Urol. 2011;60(1):21–28. doi: 10.1016/j.eururo.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kader AK, Sun J, Reck BH, Newcombe PJ, Kim ST, Hsu FC, D’Agostino RB, Jr, Tao S, Zhang Z, Turner AR, Platek GT, Spraggs CF, Whittaker JC, Lane BR, Isaacs WB, Meyers DA, Bleecker ER, Torti FM, Trent JM, McConnell JD, Zheng SL, Condreay LD, Rittmaster RS, Xu J. Potential impact of adding genetic markers to clinical parameters in predicting prostate biopsy outcomes in men following an initial negative biopsy: Findings from the REDUCE trial. Eur Urol. 2012;62(6):953–961. doi: 10.1016/j.eururo.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang H, Liu F, Wang Z, Na R, Zhang L, Wu Y, et al. Prediction of prostate cancer from prostate biopsy in Chinese men using a genetic score derived from 24 prostate cancer risk-associated SNPs. Prostate. 2013 doi: 10.1002/pros.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Ghoussaini M, Luccarini C, Dennis J, Jugurnauth-Little S, Dadaev T, Neal DE, Hamdy FC, Donovan JL, Muir K, Giles GG, Severi G, Wiklund F, Gronberg H, Haiman CA, Schumacher F, Henderson BE, Le Marchand L, Lindstrom S, Kraft P, Hunter DJ, Gapstur S, Chanock SJ, Berndt SI, Albanes D, Andriole G, Schleutker J, Weischer M, Canzian F, Riboli E, Key TJ, Travis RC, Campa D, Ingles SA, John EM, Hayes RB, Pharoah PD, Pashayan N, Khaw KT, Stanford JL, Ostrander EA, Signorello LB, Thibodeau SN, Schaid D, Maier C, Vogel W, Kibel AS, Cybulski C, Lubinski J, Cannon-Albright L, Brenner H, Park JY, Kaneva R, Batra J, Spurdle AB, Clements JA, Teixeira MR, Dicks E, Lee A, Dunning AM, Baynes C, Conroy D, Maranian MJ, Ahmed S, Govindasami K, Guy M, Wilkinson RA, Sawyer EJ, Morgan A, Dearnaley DP, Horwich A, Huddart RA, Khoo VS, Parker CC, Van As NJ, Woodhouse CJ, Thompson A, Dudderidge T, Ogden C, Cooper CS, Lophatananon A, Cox A, Southey MC, Hopper JL, English DR, Aly M, Adolfsson J, Xu J, Zheng SL, Yeager M, Kaaks R, Diver WR, Gaudet MM, Stern MC, Corral R, Joshi AD, Shahabi A, Wahlfors T, Tammela TL, Auvinen A, Virtamo J, Klarskov P, Nordestgaard BG, Røder MA, Nielsen SF, Bojesen SE, Siddiq A, Fitzgerald LM, Kolb S, Kwon EM, Karyadi DM, Blot WJ, Zheng W, Cai Q, McDonnell SK, Rinckleb AE, Drake B, Colditz G, Wokolorczyk D, Stephenson RA, Teerlink C, Muller H, Rothenbacher D, Sellers TA, Lin HY, Slavov C, Mitev V, Lose F, Srinivasan S, Maia S, Paulo P, Lange E, Cooney KA, Antoniou AC, Vincent D, Bacot F, Tessier DC, Kote-Jarai Z, Easton DF COGS-Cancer Research UK GWAS-ELLIPSE (Part of GAMEON) Initiative, Australian Prostate Cancer Bioresource, UK Genetic Prostate Cancer Study Collaborators/British Association of Urological Surgeons’ Section of Oncology, UK ProtecT (Prostate Testing for Cancer and Treatment) Study Collaborators, PRACTICAL (Prostate Cancer Association Group to Investigate Cancer-Associated Alterations in the Genome) Consortium. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet. 2013;45(4):385–391. doi: 10.1038/ng.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Na R, Liu F, Zhang P, Ye D, Xu C, Shao Q, Qi J, Wang X, Chen Z, Wang M, He D, Wang Z, Zhou F, Yuan J, Gao X, Wei Q, Yang J, Jiao Y, Ou-Yang J, Zhu Y, Wu Q, Chen H, Lu D, Shi R, Lin X, Jiang H, Wang Z, Jiang D, Sun J, Zheng S, Ding Q, Mo Z, Sun Y, Xu J. Evaluation of reported prostate cancer risk-associated SNPs from genome-wide association studies of various racial populations in Chinese men. Prostate. 2012 doi: 10.1002/pros.22629. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pharoah PDP, Antoniou AC, Easton DF, Ponder BAJ. Polygenes, risk prediction, and targeted prevention of breast cancer. N Engl J Med. 2008;358:2796–2803. doi: 10.1056/NEJMsa0708739. [DOI] [PubMed] [Google Scholar]
- 43.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 44.Sun J, Na R, Hsu FC, Zheng SL, Wiklund F, Condreay LD, Trent JM, Xu J. Genetic score is an objective and better measurement of inherited risk of prostate cancer than family history. Eur Urol. 2013;63(3):585–587. doi: 10.1016/j.eururo.2012.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kraft P, Hunter DJ. Genetic risk prediction—Are we there yet? N Engl J Med. 2009;360(17):1701–1703. doi: 10.1056/NEJMp0810107. [DOI] [PubMed] [Google Scholar]
- 46.Kang J, Cho J, Zhao H. Practical issues in building risk-predicting models for complex diseases. J Biopharm Stat. 2010;20(2):415–440. doi: 10.1080/10543400903572829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, Feng Z, Parnes HL, Coltman CA., Jr Assessing prostate cancer risk: Results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98(8):529–534. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 48.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Määttänen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 49.Andriole GL, Crawford ED, Grubb RL, III, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O’Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD PLCO Project Team. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Páez A, Määttänen L, Bangma CH, Aus G, Carlsson S, Villers A, Rebillard X, van der Kwast T, Kujala PM, Blijenberg BG, Stenman UH, Huber A, Taari K, Hakama M, Moss SM, de Koning HJ, Auvinen A ERSPC Investigators. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366(11):981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.U.S. Preventive Services Task Force. Screening for prostate cancer: A review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:185–191. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 52.Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, Holmberg L, Kantoff P, Konety BR, Murad MH, Penson DF, Zietman AL. Early detection of prostate cancer: AUA guideline. doi: 10.1016/j.juro.2013.04.119. http://www.auanet.org/education/guidelines/prostate-cancer-detection.cfm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macinnis RJ, Antoniou AC, Eeles RA, Severi G, Al Olama AA, McGuffog L, Kote-Jarai Z, Guy M, O’Brien LT, Hall AL, Wilkinson RA, Sawyer E, Ardern-Jones AT, Dearnaley DP, Horwich A, Khoo VS, Parker CC, Huddart RA, Van As N, McCredie MR, English DR, Giles GG, Hopper JL, Easton DF. A risk prediction algorithm based on family history and common genetic variants: Application to prostate cancer with potential clinical impact. Genet Epidemiol. 2011;35(6):549–556. doi: 10.1002/gepi.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.