Abstract
Altered bone structure and function contribute to the high rates of fractures in dialysis patients compared to the general population. Fracture events may increase the risk of subsequent adverse clinical outcomes. Here we assessed incidence of post-fracture morbidity and mortality in an international cohort of 34, 579 in-center hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). We estimated country-specific rates of fractures requiring a hospital admission and associated length of stay in the hospital. Incidence rates of death and of a composite event of death/re-hospitalization were estimated for the 1-year post-fracture. Overall, 3% of participants experienced a fracture. Fracture incidence varied across countries, from 12 events/1000 patient year (p-y) in Japan to 45/1000 p-y in Belgium. In all countries, fracture rates were higher in the hemodialysis group compared to those reported for the general population. Median length of stay ranged from 7 to 37 days in the United States and Japan, respectively. In most countries, post-fracture mortality rates exceeded 500/1000 p-y and death/re-hospitalization rates exceeded 1500/1000 p-y. Fracture patients had higher unadjusted rates of death (3.7- fold) and death/re-hospitalization (4.0-fold) compared to the overall DOPPS population. Mortality and hospitalization rates were highest in the first month after the fracture and declined thereafter. Thus, the high frequency of fractures and increased adverse outcomes following a fracture pose a significant health burden for dialysis patients. Fracture prevention strategies should be identified and applied broadly in nephrology practices.
INTRODUCTION
Mineral and endocrine disturbances in the course of chronic kidney disease (CKD) result in altered bone structure and function, including abnormalities in bone turnover, mineralization and volume1 which likely contribute to the elevated rates of bone fracture observed in dialysis compared to the general population2–5. The magnitude of this risk may vary by bone histology types depending on, for instance, the presence of osteomalacia, high turnover bone disease, or adynamic bone disease6, 7. In addition, the presence of certain risk factors including abnormal parathyroid hormone (PTH) levels and the use of narcotics and psycho-active medications may increase the likelihood of fracture2, 3, 8.
In the general population, patients experiencing a major bone fracture (e.g. hip fracture) have a marked increase in subsequent morbidity and mortality, especially among the elderly9–12. In the frail dialysis population, the immediate and long-term burden for patients who experience a fracture may be substantial, although this has not been thoroughly investigated, particularly in populations outside the US. One study of dialysis patients in the United States (US)2, 8, 13 reported nearly twofold higher mortality rates (1.99) among patients who experienced a hip fracture (774.9/1000 py) than those who did not (360.2/1000 py)13. For dialysis populations outside of the US, aside from a previous analysis using DOPPS data (1996–2001) describing fracture rates3, there is no contemporary data describing fractures or post-fracture clinical outcomes.
The aim of the current study was to assess health burden related to bone fractures in an international cohort of patients receiving in-center hemodialysis. Using data from the Dialysis Outcomes and Practice Patterns Study (DOPPS), we (a) estimated country-specific rates for allcause and hip-specific fractures requiring hospitalization; (b) estimated post-fracture rates of hospitalization and death, and (c) compared these rates of hospitalization and death post-fracture to the rates observed in the overall DOPPS population.
RESULTS
Study Sample
A total of 36,337 patients were enrolled over the three DOPPS phases across the 12 participating countries. Of these, 1,758 patients had missing or incorrect follow-up data and were excluded, leaving 34,579 patients for the final analysis. A total of 1,122 participants (3%) experienced a fracture requiring a hospitalization (491 hip fractures, 643 other fractures) during the follow-up period. The overall median length of follow-up was 1.6 years (interquartile range = 0.8 – 2.4); median follow-up following a fracture requiring hospitalization was 0.6 years (interquartile range = 0.2–1.2). Over the follow-up period, the majority of patients (59%) were administratively censored; 20% died or withdrew from hemodialysis; 8% switched modality; and 12% transferred to another facility. Table 1 shows patient characteristics for all DOPPS participants included in this analysis, as well as for those who did and did not experience a fracture-related hospitalization, stratified by DOPPS region. Patients who experienced a fracture requiring hospitalization during follow-up were older, more likely to be female and white (North America only), had lived more years on dialysis, had lower BMI, and higher PTH and Kt/V compared to those who did not. In addition, with the exception of Japan, they also had a higher comorbidity burden and were more likely to have had a prior hip fracture.
Table 1.
Demographics and clinical characteristics of all DOPPS participants, of those who experienced and those who did not experience a fracture requiring hospitalization, by geographic region
| Europe, Australia, New Zealand | Japan | North America | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Fracture | Non Fracture |
All | Fracture | Non Fracture |
All | Fracture | Non Fracture |
|
| Patients (#) | 18903 | 667 | 18236 | 6782 | 143 | 6639 | 9137 | 324 | 8813 |
| Prior hip fracture (%) | 293 | 293 | 0 | 36 | 36 | 0 | 162 | 162 | 0 |
| Patient-years | 25858 | 669 | 25189 | 11914 | 157 | 11757 | 10969 | 296 | 10673 |
| Mean (SD), median (IQR) or % | |||||||||
| Age (years) (median) | 67 (55–76) | 74 (65–79) | 67 (54–75) | 64 (55–72) | 68 (60–76) | 64 (55–72) | 64 (52–74) | 71 (59–80) | 63 (52–74) |
| Black (%) | 2 | 1 | 2 | 0 | 0 | 0 | 25 | 13 | 25 |
| Male (%) | 60 | 43 | 60 | 62 | 46 | 63 | 55 | 47 | 55 |
| Years on dialysis (median) | 1.7 (0.4–4.7) | 2.7 (0.8–5.8) | 1.7 (0.4–4.7) | 3.8 (0.7–9.4) | 5.4 (2.2–10.3) | 3.8 (0.7–9.3) | 1.5 (0.3–4.0) | 2.1 (0.6–4.3) | 1.5 (0.3–4.0) |
| Body mass index (kg/m2) | 25 (22–28) | 24 (21–28) | 25 (22–28) | 21 (19–23) | 20 (18–22) | 21 (19–23) | 26 (23–31) | 25 (22–31) | 26 (23–31) |
| Diabetes (%) | 34 | 39 | 33 | 36 | 36 | 36 | 54 | 60 | 54 |
| Hypertension (%) | 82 | 83 | 82 | 75 | 72 | 75 | 90 | 89 | 91 |
| Coronary artery disease (%) | 44 | 53 | 44 | 32 | 34 | 32 | 58 | 64 | 58 |
| Congestive heart failure (%) | 29 | 38 | 29 | 23 | 24 | 23 | 42 | 49 | 42 |
| Cerebrovascular disease (%) | 17 | 23 | 17 | 15 | 17 | 14 | 18 | 23 | 18 |
| Peripheral vascular disease (%) | 30 | 34 | 30 | 16 | 18 | 16 | 32 | 37 | 32 |
| Other cardiovascular (%) | 36 | 47 | 36 | 30 | 29 | 30 | 33 | 38 | 33 |
| Cancer (other than skin) (%) | 15 | 20 | 15 | 9 | 11 | 9 | 14 | 13 | 14 |
| GI bleeding (%) | 5 | 7 | 5 | 5 | 6 | 5 | 7 | 8 | 7 |
| Lung disease (%) | 13 | 15 | 13 | 3 | 4 | 3 | 18 | 24 | 17 |
| Recurr. cellulitis, gangrene (%) | 9 | 13 | 9 | 4 | 5 | 4 | 11 | 13 | 11 |
| Neurologic disease (%) | 11 | 15 | 11 | 9 | 10 | 9 | 13 | 15 | 13 |
| Psychiatric disorder (%) | 17 | 21 | 17 | 4 | 4 | 4 | 26 | 25 | 26 |
| Psychiatric disorder (%) | 3 | 12 | 3 | 2 | 8 | 2 | 3 | 9 | 3 |
| Calcium (total) (mg/dl) (mean) | 9.1 (0.9) | 9.2 (0.9) | 9.1 (0.9) | 8.9 (0.9) | 9 (0.9) | 8.9 (0.9) | 9 (0.8) | 9.1 (0.8) | 9 (0.8) |
| Phosphorus (mg/dl) (mean) | 5.2 (1.8) | 4.9 (1.6) | 5.3 (1.8) | 5.5 (1.5) | 5.4 (1.5) | 5.5 (1.5) | 5.4 (1.8) | 5.2 (1.7) | 5.4 (1.8) |
| PTH (intact) (pg/ml) (mean) | 299 (385) | 304 (551) | 299 (377) | 194 (235) | 208 (224) | 194 (235) | 348 (412) | 369 (593) | 348 (404) |
| Albumin (g/dl) (mean) | 3.7 (0.5) | 3.6 (0.5) | 3.7 (0.5) | 3.7 (0.5) | 3.7 (0.4) | 3.7 (0.5) | 3.6 (0.5) | 3.5 (0.5) | 3.6 (0.5) |
| Hemoglobin (g/dl) (mean) | 11.3 (1.6) | 11.5 (1.4) | 11.3 (1.6) | 10.1 (1.4) | 10 (1.3) | 10.1 (1.4) | 11.5 (1.5) | 11.5 (1.4) | 11.4 (1.5) |
| Single-Pool Kt/V (mean) | 1.5 (0.3) | 1.6 (0.3) | 1.4 (0.3) | 1.3 (0.3) | 1.4 (0.3) | 1.3 (0.3) | 1.5 (0.3) | 1.6 (0.3) | 1.5 (0.3) |
Parenthesized values following medians (indicated in 1st column label) are the inter-quartile ranges; those following means are the standard deviations.
“All” includes all study participants; “Fracture” includes only patients who experienced a fracture requiring hospitalization during follow-up.
North America = US + Canada; Europe, Australia, New Zealand = Australia, Belgium, France, Germany, Italy, New Zealand, Spain, and Sweden.
Fracture Hospitalization Rates and Length of Stay
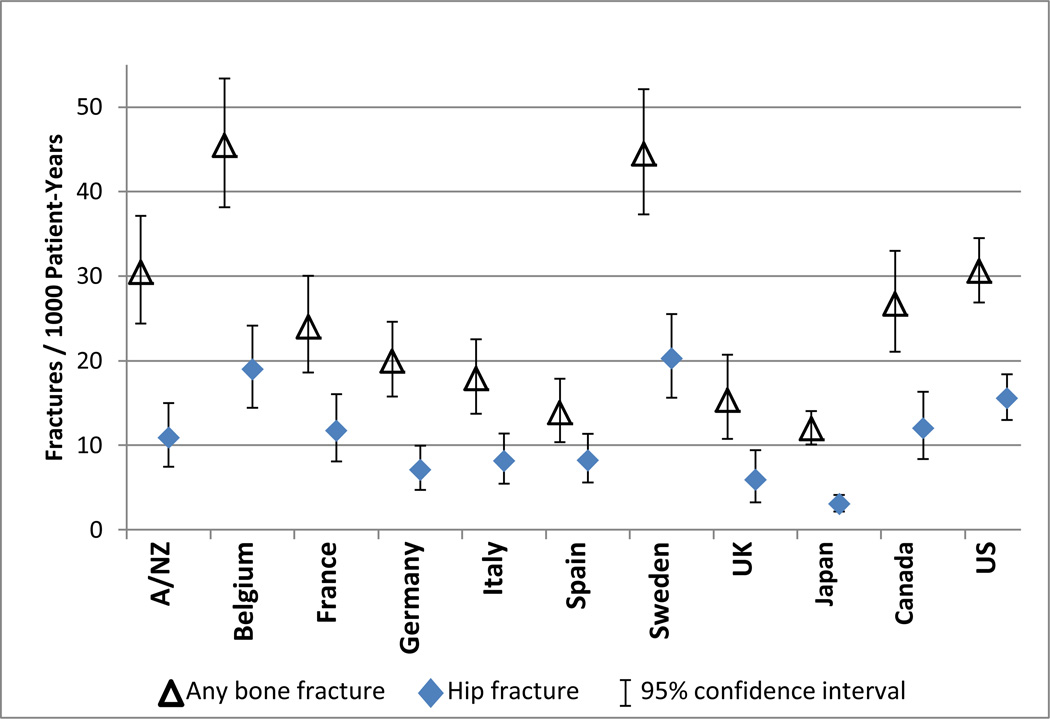
Rates of fractures requiring hospitalization in the overall DOPPS population did not vary substantively over time (21 per 1000 patient-years for DOPPS 2 [2002–2004], 25 per 1000 p-y for DOPPS 3 [2005–2008], and 24 per 1000 p-y for DOPPS 4 [2009–2011]; p-value for trend=0.19), and were therefore combined. The incidence of any fracture requiring hospitalization varied across countries ranging from 12 per 1000 p-y in Japan to 45 per 1000 p-y in Belgium. Hip-specific fracture rates ranged from 3 per 1000 p-y (Japan) to 20 per 1000 p-y (Sweden) (Figure 1). This burden of fractures in the dialysis population is contrasted with the burden of fractures reported for the general non-dialysis population (Figure 2). The rates across all countries included in the DOPPS were substantially higher for patients receiving dialysis as compared to the general population.
Figure 1. Incidence of fractures resulting in a hospital admission among DOPPS participants, by country.
Only the first fracture event for each patient was included in the calculation of these rates.
A/NZ = Australia and New Zealand
Figure 2. Hip fracture rates among DOPPS participants and in the general non-dialysis population, within each DOPPS country.
Rates among DOPPS participants refer to hip fractures requiring a hospital admission; rates in the general population were derived from a review by Kanis et al14 and may include hip fractures that did not require hospitalization
The median hospital length of stay varied widely across countries (Figure 3), ranging from 7 days (US) (interquartile range = 4–14 days) to 37 days (Japan) (interquartile range = 21–61 days). In Australia/New Zealand, Belgium, Germany, Japan, the UK, and the US, overall length of hospital stay was longer for hip fractures than for other fractures (p < 0.05).
Figure 3. Length of stay in the hospital for fracture-related admissions, by country.
*The numbers of fracture-related hospitalizations with a reported length of stay > 120 days were: Canada - 6 any bone fractures, 4 hip fractures; France - 4 any, 3 hip; Japan - 8 any, 3 hip; Spain - 1 any, 1 hip; UK - 2 any, 2 hip.
Post-Fracture Clinical Outcomes
The incidence of post-fracture clinical outcomes varied across countries (Figure 4). In most countries mortality rates exceeded 500 per 1000 p-y, and when subsequent hospitalizations were also included (composite event), rates exceed 1500 per 1000 p-y. As in the general DOPPS sample, the most common cause of death among patients who experienced a fracture requiring hospitalization was cardiovascular events (45%) and infections (21%). In most countries, unadjusted rates of post-fracture clinical outcomes were substantively higher for patients experiencing any type of fracture compared to patients in the general DOPPS hemodialysis sample. Adverse clinical outcomes were also more common for patients experiencing a hip vs. other type of fracture, but no statistically significant differences in post-fracture outcomes were observed between patients who had a prior history of hip fracture and those who did not (533 v. 490 deaths per 1000 PY and 1556 v. 1578 composite death/hospitalization events per 1000 PY). While females were more likely to experience a fracture, males had a slightly higher mortality rate in the follow-up period. One potential explanation for this is that females are more likely to die during the fracture hospitalization than males, thereby removing women (who may have more severe complications as a result of the fracture) from the cohort that is then followed for post-fracture outcomes.
Figure 4. Death and hospitalization rates in the year following a fracture event requiring hospitalization, by country.
Rates are restricted to events happening within 1-year of the fracture (fracture admission for death, fracture discharge for hospitalization/death). The background rate is the rate among all DOPPS participants, including patients who experienced a fracture and those who did not.
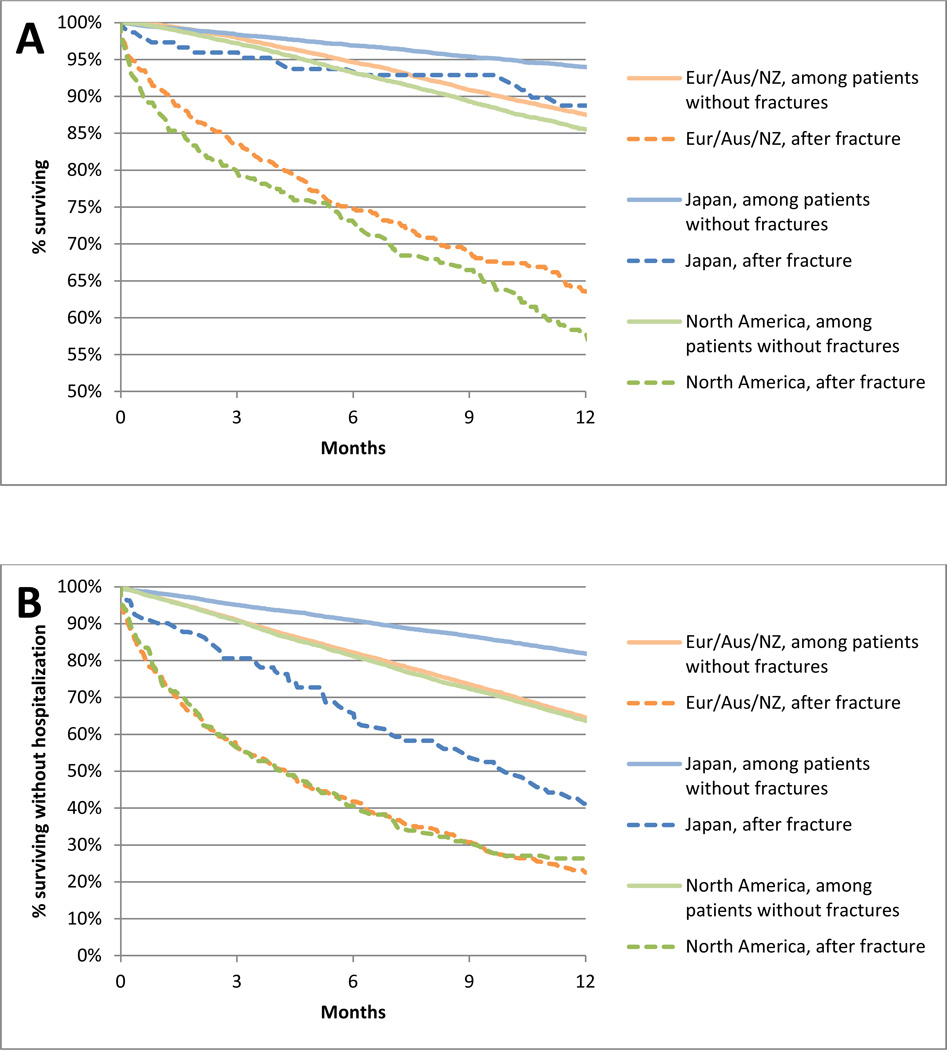
Survival curves for study participants who experienced and those who did not experience a fracture requiring hospitalization within each DOPPS region are shown in Figure 5A. In each region, the curves separate immediately showing a marked difference between the death rate during the year following the fracture event and the death rate in non-fracture patients. In the fracture group, mortality was highest in the month immediately following the fracture event (Europe/Aus/NZ: 87, Japan: 25, and North America: 115 deaths/1000 patient-months), with rates in individual countries 3.8 to 11.6 fold higher in fracture patients vs. the overall DOPPS sample. Mortality rates began to decline in months 1–6 (Europe/Aus/NZ: 34, Japan: 6, and North America: 32 deaths/1000 patient-months) and dropped further between months 6–12 (Europe/Aus/NZ: 18, Japan: 7, and North America: 34 deaths/1000 patient-months). In most countries, mortality rates still remained elevated at 1-year post-fracture compared to the overall DOPPS sample. Similar patterns were observed for the composite event of death or first hospitalization (Figure 5B).
Figure 5A & 5B. Time to death and hospitalization among DOPPS participants who experienced and those who did not experience a fracture requiring hospitalization, by DOPPS region.
Panel A: Unadjusted survival (time to death) by DOPPS region. Panel B: Unadjusted survival without any hospitalizations (time to first hospitalization or death) by DOPPS region.
There were important case-mix differences between patients who experienced a fracture and the general DOPPS population (Table 1) which may also affect the underlying risk of death. We addressed these demographic differences between the two groups using model-based standardization, and despite this, death rates remained substantially elevated among patients who experienced a fracture; this finding was consistent across different patient sub-groups (Figure 6). Additional adjustment for comorbidity differences did not materially affect these results (not shown).
Figure 6. Mortality rates for fracture vs. background patients, by strata of patient characteristics.
“Cardiovasc dis” = cardiovascular disease defined as having any of the following conditions: coronary artery disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, or other cardiovascular disease
“Adj” = adjusted models; account for country, age, race (Black v. non-Black), sex, and years on dialysis
Due to the large sample size of many of the strata, confidence intervals for the overall death rates are very small and therefore not visible in the figure.
DISCUSSION
Our study of over 34,000 patients across 12 countries is the first to quantify health burden related to fractures in an international cohort of patients on chronic hemodialysis. In agreement with an earlier report3 we found a rate of fracture much higher than that in the general population, with significant variability in fracture rates between countries that could not be explained by case-mix differences. We also report for the first time a markedly elevated rate of subsequent mortality and hospitalization post-fracture relative to dialysis patients who did not experience a fracture requiring hospitalization.
Using data spanning 10 years in 12 countries, our study shows considerable variation in the incidence of fractures and post-fracture consequences across countries. Overall, country-specific fracture rates were consistent with those reported in a previous study using DOPPS data from 1996–20013. The fracture rates across countries observed in this study are consistent with previous studies in dialysis patients2,4,5 highlighting fracture as an important clinical outcome in this population. In comparison to the general population, dialysis patients are at significantly elevated risk of fracture, and this excess risk ranges from 1.5 to 8-fold depending on the country4,14. It is important to acknowledge that the comparison of rates from the DOPPS population with the general non-dialysis population in this study relied on published fracture rate estimates for the general population in each country14 precluding any ability to adjust for case-mix differences (e.g., age, gender, race, etc.). These comparisons should be considered in light of this limitation; nonetheless, these data provide evidence describing the potential excess risk of fracture among dialysis patients,2,3–5 which is likely attributable to the high prevalence of mineral and bone disorders and other risk factors in the dialysis population. The role of low bone mineral density in the pathogenesis of fractures is not clear1,15,16, however, abnormal PTH levels2,3,8 and the use of psycho-active drugs that may increase incidence of falls can contribute to an elevated risk of fracture.
Across nearly all countries, fracture events led to long hospital admissions. At one extreme, patients hospitalized in Japan had a median length of stay around 37 days; at the other extreme, patients hospitalized in the US had a median length of stay of 7 days. It is quite possible that these differences are largely explained by differences in health care systems and the delivery of care. In Japan, for example, physical rehabilitation takes place in the hospital as opposed to outpatient care centers (oral communication, Dr. Takashi Akiba, May 26, 2012) and so prolonged lengths of stay are reasonable. This may also explain why rate of post-fracture clinical outcomes were the lowest in Japan. In a prior DOPPS analysis, dialysis facilities with shorter median hospital length of stay had higher odds of readmission17, supporting the hypothesis that hospital admissions that are too short may have a negative effect on subsequent clinical events by precluding the delivery of optimal care.
The clinical burden following a fracture (including mortality and hospitalizations) was substantial and observed across nearly all countries. In the early months following discharge, mortality and hospitalization rates were 2–9 times higher compared to the general dialysis population within each country, standardized on basic demographic characteristics. This finding is consistent with the estimate of a 2.7-fold higher risk reported by Danese et al., using data from the US Renal Data System8, and other studies that showed higher mortality rates for patients experiencing a hip fracture2, 8, 13, 18. In this analysis we were unable to distinguish between idiopathic fractures and those potentially attributable to other clinical events that may have occurred concurrently (e.g. a stroke resulting in a fall and fracture). Thus, we cannot exclude the possibility that some of the observed excess risk post-fracture may be attributable to other events. Since patients who experience a fracture tend to have a different clinical profile than those who do not (e.g., are older), we compared the risk of mortality and hospitalization after adjusting for demographic differences and found that the rates remained substantially elevated (~3 times higher).
The elevated morbidity and mortality in the early period following a fracture suggests that a bone fracture acts as an “acute” risk factor for adverse outcomes. Possible contributing mechanisms may include bleeding, prolonged immobilization, malnutrition, and high rates of infections19, all of which may precipitate pre-existing conditions. These potential risk factors may be directly related to the fracture event or more generally to being hospitalized; it is likely that immobilization and other functional limitations play an important role in increasing frailty and infection risk, and contribute to the high incidence of adverse clinical outcomes.
The fact that morbidity and mortality of fracture patients tended to be higher in the following months up to 1-year after a fracture suggests that additional mechanisms also contribute to adverse outcomes. Several studies have shown higher rates of vascular calcification in the presence of low bone turnover in patients with kidney disease20, 21. In a cohort of hemodialysis patients, vertebral fractures were associated with vascular calcifications in medium caliber arteries22. In the general population, decline in bone mineral density was associated with progression of aortic calcifications23, 24. All together these findings indicate that disregulation in bone metabolism is likely to impact calcium deposition in arteries and may contribute to the pathogenesis of cardiovascular disease in this population. However, detailed pathogenic mechanisms remain to be clarified.
In the general population, serious fractures are associated with high health care costs, with the estimated cost for hip fracture in the US exceeding $20 billion per year25. High costs related to fractures have also been reported among US dialysis patients8. While data available within the DOPPS preclude formal cost studies, the long length of hospital stay and high re-admission following fracture events indicate that fractures in hemodialysis patients are associated with high resource utilization. Future studies should investigate the post-fracture health care resource utilization to more fully quantify their burden.
Given the high health and economic burden related to bone fractures, strategies for fracture prevention should be identified and implemented26. Interventions will most likely target both abnormalities in bone structure and other modifiable risk factors peculiar to the hemodialysis population, with particular attention paid to subgroups of patients who are at higher risk of fractures (e.g. elderly, women, longer duration of end-stage renal disease). It is possible that high PTH levels may further increase the risk of fracture in this population3,8. Given the association between high PTH and cardiovascular outcomes18, most clinicians prescribe a therapeutic regimen aimed at lowering PTH levels. However, both high and low PTH levels have been associated with fracture rates2, 3, 8. Whether maintaining PTH levels within guideline targets will impact fracture risk remains to be demonstrated. This is particularly important given the recent trends towards higher PTH levels reported in most DOPPS countries, with ~ 18% of US participants in December 2011 having PTH>600 pg/ml27,28. Other strategies aimed at fracture prevention will include identification of frail patients who may have an increased risk of falls26, physical therapy as well as avoidance of hypotensive episodes, and careful prescription of psychoactive medications that may contribute to reducing fall-related fractures3, 26. Vitamin D supplementation resulting in normalization of serum 25 OH vitamin D levels may reduce falls risk, as demonstrated in older individuals without known kidney disease29. Finally, given the strong association between malnutrition, low body weight and bone health, nutritional interventions aimed at improving patient nutritional status and maintaining healthy body weights may impact the risk of bone fractures.
The extensive DOPPS database and detailed data collection allowed us to report rates of other major bone fractures and not only limited to hip fractures, as done by most prior studies4, 5, 13; however details on other fracture types were not available. Additional limitations of the current analysis are related to the observational nature of the DOPPS. First, since we only studied fractures resulting in hospitalizations, our findings likely underestimate the total incidence of fractures in hemodialysis patients. Second – while unlikely- we cannot exclude that differences in reporting hospitalization data may have contributed to the observed variability in fracture rates across DOPPS countries. Third, once patients are transferred out of the DOPPS facility, information on patient care is no longer captured. In some countries, discharge to long-term care or rehabilitation facilities is the standard of care and may represent additional burden and significant resource utilization not characterized in our analysis. Finally, data on surgical and/or rehabilitation approaches to management of serious fractures were not collected, and therefore, effect could not be examined in this investigation.
In conclusion, using the international DOPPS cohort we demonstrate that bone fractures are relatively common among hemodialysis patients in many countries and pose a significant health burden. Additional studies are needed to further quantify the overall burden related to fractures and to identify modifiable practices that may help minimize the fracture risk in this frail population.
METHODS
Data Source
The DOPPS is a prospective cohort study of in-center hemodialysis patients ≥ 18 years in 12 countries (Australia, Belgium, Canada, France, Germany, Italy, Japan, New Zealand, Sweden, Spain, the UK, and the US). The DOPPS study design has been described previously30, 31. Briefly, the study population is comprised of randomly selected patients from a random sample of dialysis facilities within each country. Detailed demographics, comorbidity and laboratory data were collected at study entry and updated throughout follow-up. Information on hospitalization and mortality (with primary and secondary causes) and reasons for loss-to-follow-up were also collected. In each DOPPS phase, patients lost-to-follow-up were replaced in order to maintain a representative cohort within each country. While many DOPPS facilities and some study participants in a given phase continued participation into a subsequent study phase, there was no overlap of the follow-up periods analytically. Informed patient consent was obtained in accordance with local requirements.
Study Population
The current study included all study participants in DOPPS phase 2 (2002–2004), phase 3 (2005–2008), and phase 4 (2009–2011).
Outcomes
We identified all first hospitalizations (defined by an overnight hospital stay) with an associated fracture diagnosis code. In the DOPPS dataset, fractures were coded as either “hip” or “other”; information on other types of fractures (e.g. vertebral fractures) were not available. Therefore, we characterized fracture-related hospitalization as either “any fracture” or “hip fracture”. We defined the length of stay in the hospital as the time between admission and either date of discharge or, if the patient died in the hospital, date of death. Length of stay was combined for overlapping hospitalizations or for hospitalizations occurring within five days of each other, where subsequent hospitalization(s) appeared to be a continuation of the first admission (e.g. physical therapy following the fracture event). For fracture event rates, follow-up started at study enrollment and continued until the first of death, fracture hospitalization, transplantation, renal replacement therapy modality switch, recovery of renal function, departure from the facility, or end of follow-up.
For the post-fracture outcomes, we identified all hospitalizations and deaths during the 1-year following discharge from the fracture hospitalization. For analyses of post-fracture mortality alone, follow-up started on the fracture admission date. For analyses of the composite event, follow-up started upon discharge from the hospital and continued until the first of death, subsequent hospitalization, transplantation, renal replacement therapy modality switch, recovery of renal function, departure from the facility, or 365 days.
Statistical Analysis
Standard descriptive statistics for categorical variables (count [n], percentage [%]) and continuous variables (mean, standard deviation [sd], median, 25th/75th percentile) were used to characterize patients at study enrollment, by DOPPS region (North America = US + Canada; Eur/ANZ = European countries + Australia and New Zealand; and Japan) and for patients who experienced a fracture-related hospitalization during follow-up.
Fracture rates
Incidence rates and 95% confidence intervals [CI] were estimated by country for fractures of any type and fractures of the hip requiring a hospitalization by country. Rates were calculated as the total number of events divided by the patient-time accumulated prior to censoring. 95% CI for rates were estimated using Byar’s approximation to the Poisson distribution32. The median, 5th, 25th, 75th, and 95th percentile estimates for the fracture hospitalization length of stay were also calculated.
Post-fracture Events
All-cause mortality rates and associated 95% CI were estimated for the 1-year following discharge from the fracture hospitalization. Rates were also calculated for a composite event of death or first re-hospitalization in an effort to minimize the effect of death as a competing event.
Comparison of the Post-fracture Events to a Reference Population
In order to provide some context to gauge the clinical significance of fracture consequences, rates of death, starting at the initial admission, and the composite of death and first hospitalization, starting at discharge, were estimated for the overall DOPPS population within each country. Then, the post-fracture population was standardized to the age, sex, race, and length of time on dialysis distribution in the overall population within each country, so that standardized events could be estimated.
Footnotes
Disclosures
The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Sanofi Renal (since 2009), AbbVie (since 2009), Baxter (since 2011), and Vifor Fresenius Renal Pharma (since 2011), without restrictions on publications.
Dr. Tentori is supported in part by Award Number K01DK087762 from the National Institute Of Diabetes And Digestive And Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health. Dr. Tentori has received honoraria from Amgen, Dialysis Clinic Inc and Renal Research Institute.
Dr. Robinson has received speaker fees for Kyowa Hakko Kirin.
Dr. Pisoni has received speaker fees from Amgen, Kyowa Hakko Kirin, and Vifor; has served as a consultant for Pursuit Vascular; and has served on an advisory panel for Merck.
Drs. Bradbury and Kilpatrick work in the Center for Observational Research at Amgen, Inc.
References
- 1.Eckardt KU, Kasiske B. Kidney Disease Improving Global Outcomes (KDIGO): clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int. 2009;76:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 2.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36:1115–1121. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 3.Jadoul M, Albert JM, Akiba T, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70:1358–1366. doi: 10.1038/sj.ki.5001754. [DOI] [PubMed] [Google Scholar]
- 4.Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58:396–399. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 5.Ball AM, Gillen DL, Sherrard D, et al. Risk of hip fracture among dialysis and renal transplant recipients. JAMA. 2002;288:3014–3018. doi: 10.1001/jama.288.23.3014. [DOI] [PubMed] [Google Scholar]
- 6.Araujo SM, Ambrosoni P, Lobao RR, et al. The renal osteodystrophy pattern in Brazil and Uruguay: an overview. Kidney Int Suppl. 2003:S54–S56. doi: 10.1046/j.1523-1755.63.s85.13.x. [DOI] [PubMed] [Google Scholar]
- 7.Gerakis A, Hadjidakis D, Kokkinakis E, et al. Correlation of bone mineral density with the histological findings of renal osteodystrophy in patients on hemodialysis. J Nephrol. 2000;13:437–443. [PubMed] [Google Scholar]
- 8.Danese MD, Kim J, Doan QV, et al. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis. 2006;47:149–156. doi: 10.1053/j.ajkd.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 9.White BL, Fisher WD, Laurin CA. Rate of mortality for elderly patients after fracture of the hip in the 1980's. J Bone Joint Surg Am. 1987;69:1335–1340. [PubMed] [Google Scholar]
- 10.Riggs BL, Melton LJ., 3rd The prevention and treatment of osteoporosis. N Engl J Med. 1992;327:620–627. doi: 10.1056/NEJM199208273270908. [DOI] [PubMed] [Google Scholar]
- 11.Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307:1248–1250. doi: 10.1136/bmj.307.6914.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browner WS, Pressman AR, Nevitt MC, et al. Mortality following fractures in older women. The study of osteoporotic fractures. Arch Intern Med. 1996;156:1521–1525. [PubMed] [Google Scholar]
- 13.Mittalhenkle A, Gillen DL, Stehman-Breen CO. Increased risk of mortality associated with hip fracture in the dialysis population. Am J Kidney Dis. 2004;44:672–679. [PubMed] [Google Scholar]
- 14.Kanis JA, Oden A, McCloskey EV, et al. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012 Sep;23(9):2239–2256. doi: 10.1007/s00198-012-1964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inaba M, Okuno S, Kumeda Y, Yamakawa T, Ishimura E, Nishizawa Y. Increased incidence of vertebral fracture in older female hemodialyzed patients with type 2 diabetes mellitus. Calcif Tissue Int. 2005 Apr;76(4):256–260. doi: 10.1007/s00223-004-0094-0. [DOI] [PubMed] [Google Scholar]
- 16.Jamal SA, Gilbert J, Gordon C, Bauer DC. Cortical pQCT measures are associated with fractures in dialysis patients. J Bone Miner Res. 2006 Apr;21(4):543–548. doi: 10.1359/jbmr.060105. [DOI] [PubMed] [Google Scholar]
- 17.Lopes AA, Leavey SF, McCullough K, et al. Early readmission and length of hospitalization practices in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Hemodial Int. 2004;8:287–294. doi: 10.1111/j.1492-7535.2004.01107.x. [DOI] [PubMed] [Google Scholar]
- 18.Naves M, Díaz-López JB, Gómez C, Rodríguez-Rebollar A, Rodríguez-García M, Cannata-Andía JB. The effect of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish population. Osteoporos Int. 2003 Jul;14(6):520–524. doi: 10.1007/s00198-003-1405-4. [DOI] [PubMed] [Google Scholar]
- 19.Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001;120:1883–1887. doi: 10.1378/chest.120.6.1883. [DOI] [PubMed] [Google Scholar]
- 20.Giachelli CM, Jono S, Shioi A, Nishizawa Y, Mori K, Morii H. Vascular calcification and inorganic phosphate. Kidney Dis. 2001 Oct;38(4 Suppl 1):S34–S37. doi: 10.1053/ajkd.2001.27394. [DOI] [PubMed] [Google Scholar]
- 21.Qunibi WY, Nolan CA, Ayus JC. Cardiovascular calcification in patients with end-stage renal disease: a century-old phenomenon. Kidney Int Suppl. 2002 Dec;(82):S73–S80. doi: 10.1046/j.1523-1755.62.s82.15.x. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-García M, Gómez-Alonso C, Naves-Díaz M, Diaz-Lopez JB, Diaz-Corte C, Cannata-Andía JB Asturias Study Group. Vascular calcifications, vertebral fractures and mortality in haemodialysis patients. Nephrol Dial Transplant. 2009 Jan;24(1):239–246. doi: 10.1093/ndt/gfn466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004 Sep;89(9):4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 24.Naves M, Rodríguez-García M, Díaz-López JB, Gómez-Alonso C, Cannata-Andía JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008 Aug;19(8):1161–1166. doi: 10.1007/s00198-007-0539-1. [DOI] [PubMed] [Google Scholar]
- 25.Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51:364–370. doi: 10.1046/j.1532-5415.2003.51110.x. [DOI] [PubMed] [Google Scholar]
- 26.Jadoul M. Towards the prevention of bone fractures in dialysed patients? Nephrol Dial Transplant. 2007;22:3377–3380. doi: 10.1093/ndt/gfm508. [DOI] [PubMed] [Google Scholar]
- 27.DOPPS Practice Monitor Reporting contemporary trends in US dialysis practice. [Accessed June 25, 2012]; Available at http://www.dopps.org/DPM/ [Google Scholar]
- 28.Fuller DS, Pisoni RL, Bieber BA, Gillespie BW, Robinson BM. The DOPPS Practice Monitor for US Dialysis Care: Trends Through December 2011. Am J Kidney Dis. 2012 Nov 2; doi: 10.1053/j.ajkd.2012.10.002. pii: S0272-6386(12)01255-3. [DOI] [PubMed] [Google Scholar]
- 29.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009 Oct 1;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int. 2000;57:S74–S81. doi: 10.1046/j.1523-1755.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 31.Pisoni RL, Gillespie BW, Dickinson DM, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44:7–15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Rothman KJ. Epidemiology: An Introduction: Oxford University Press, 2002. 2002 [Google Scholar]