Abstract
Thyroid cancer risk following exposure to ionizing radiation in childhood and adolescence is a topic of public concern. To characterize the long-term temporal trend and age-at-exposure variation in the radiation-induced risk of thyroid cancer, we analyzed thyroid cancer incidence data for the period from 1958 through 2005 among 105,401 members of the Life Span Study cohort of Japanese atomic-bomb survivors. During the follow-up period, 371 thyroid cancer cases (excluding those with microcarcinoma with a diameter <10 mm) were identified as a first primary among the eligible subjects. Using a linear dose–response model, the excess relative risk of thyroid cancer at 1 Gy of radiation exposure was estimated as 1.28 (95% confidence interval: 0.59–2.70) at age 60 after acute exposure at age 10. The risk decreased sharply with increasing age-at-exposure and there was little evidence of increased thyroid cancer rates for those exposed after age 20. About 36% of the thyroid cancer cases among those exposed before age 20 were estimated to be attributable to radiation exposure. While the magnitude of the excess risk has decreased with increasing attained age or time since exposure, the excess thyroid cancer risk associated with childhood exposure has persisted for >50 years after exposure
Keywords: Thyroid cancer, radiation effects, epidemiological cohort study
INTRODUCTION
Thyroid cancer is not frequent but is a focus of special attention in populations exposed to ionizing radiation, particularly when children are exposed. This is because the thyroid is known to be highly susceptible to the carcinogenic effect of radiation exposure in childhood or adolescence, with increased risks apparent from about 5 years after exposure.1, 2 Studies of Japanese atomic-bomb survivors3, 4 and other populations5–9 have shown that the risk of thyroid cancer associated with radiation exposure tends to decrease with time since exposure or with increasing age-at-exposure. However, with little empirical data existing on the lifetime risk of radiation-related thyroid cancer, important questions remain about the duration and temporal pattern of the risk. Having extended the follow-up in the Life Span Study (LSS) cohort of atomic-bomb survivors through 2005, the current study analyzed the temporal patterns of thyroid cancer risk up to 60 years after exposure, which provides one of the longest thyroid cancer follow-ups of a general population following radiation exposure.
MATERIAL AND METHODS
The LSS cohort includes about 93,000 atomic-bomb survivors in Hiroshima and Nagasaki and about 27,000 people who were not in the cities at the time of bombings.10 The incident thyroid cancer cases in this cohort were identified from a special study, in which records from the Hiroshima and Nagasaki tumor registries and histological materials collected from area hospitals and pathology laboratories were reviewed by a panel of pathologists for diagnostic confirmation and classification. The Hiroshima and Nagasaki tumor registries, to which the LSS data were linked, are population-based registries that have been in operation since 1958. In the present analysis, we excluded cohort members who could not be traced, or were known to have had cancer or died before 1958 (8,399 people), or did not have radiation dose estimates (6,521 people), which resulted in the 105,401 eligible subjects (43% of whom were alive at the end of 2005). We analyzed first primary thyroid cancer diagnosed in the Hiroshima or Nagasaki tumor registry catchment area, with person-years of follow-up adjusted, as in other LSS cancer incidence analyses,4 for the probability of residence in the catchment area estimated using the information from the Adult Health Study (AHS) sub-cohort, a clinical subset of the LSS cohort. As described elsewhere,11 thyroid papillary microcarcinomas (with a diameter of <10 mm) in this cohort had primarily been identified at autopsy and were excluded from the current analyses. These analyses were based on thyroid doses estimated using the current survivor dosimetry system (DS02).12 As usual in analysis with the LSS, we used the weighted dose (the gamma dose plus 10 times the neutron dose, expressed in units of weighted dose in Gy) to reflect the relatively larger biologic effect assumed of neutron radiation and incorporated an adjustment to allow for effects of dose measurement error.13 The median, mean and maximum of the weighted thyroid doses among the members who were in the cities at the time of bombing were 0.009, 0.142 and 4.26 Gy weighted dose, respectively. The effect of radiation exposure on the thyroid cancer incidence was analyzed based on both excess relative risk (ERR) and excess absolute rate (EAR) models with adjustment for city, sex, attained age, age and location at exposure, time since exposure and participation in the AHS program offering biennial health examinations and affecting baseline thyroid cancer rates (see below) to a subset of the LSS.14 Parameter estimates and hypothesis testing were based on Poisson regression models fitted using maximum-likelihood methods with the Epicure software.15 Confidence intervals (CIs) for the parameters were calculated with the likelihood ratio method using Epicure, and CIs for attributable fractions were based on the Bayesian posterior distribution (with non-informative priors) using WinBUGS.16 While we considered the effect of exposure received at any age, our primary focus in this report concerned the long-term effects of childhood and adolescent exposures (i.e., exposures occurring before age 20).
RESULTS
Among the eligible subjects, 371 thyroid cancer cases (299 papillary carcinomas, 15 follicular carcinomas, 12 anaplastic carcinomas, three medullary carcinomas, 42 carcinomas NOS or other) in the tumor registry catchment area were identified as a first primary during the follow-up period between 1958 and 2005. Crude thyroid cancer rates increased markedly with radiation dose among those exposed as children or adolescents and were higher for women than for men (Table 1). Fitted baseline rates generally increased with increasing birth year (p < 0.001) and were significantly higher for women than for men (p < 0.001). After allowing for radiation effects, AHS participants had higher baseline rates than non-participating counterparts (p < 0.001), with larger differences in younger birth-cohorts (p = 0.04). This is likely due to increased detection or awareness of cancer-related conditions among AHS subjects because the routine biennial health examinations do not include ultrasonography or other screening procedures to detect thyroid nodules.
Table 1.
Observed and fitted cases of thyroid cancer incidence in the LSS (1958–2005) by categories of dose and other variables
| Age at exposure < 20 years
|
Age at exposure >= 20 years
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Case | Rate1 | Fitted values
|
n | Case | Rate1 | Fitted values
|
|||||||
| Background | Fitted excess | AF2 (%) | 95%CI | Background | Fitted excess | AF2 (%) | 95%CI | |||||||
| Total | ||||||||||||||
|
| ||||||||||||||
| 45,738 | 191 | 12.2 | 153.5 | 41.1 | 36* | (22–46) | 59,663 | 180 | 12.5 | 173.2 | 3.3 | 4* | (1–17) | |
|
| ||||||||||||||
| Sex | ||||||||||||||
|
| ||||||||||||||
| Male | 21,571 | 40 | 5.6 | 33.7 | 5.6 | 14 | (0–27) | 21,319 | 21 | 5.0 | 21.6 | 0.2 | 1 | (0–6) |
|
| ||||||||||||||
| Female | 24,167 | 151 | 17.5 | 119.7 | 35.5 | 23 | (13–32) | 38,344 | 159 | 15.5 | 151.6 | 3.1 | 2 | (0–9) |
|
| ||||||||||||||
| Weighted thyroid dose (Gy) | ||||||||||||||
|
| ||||||||||||||
| NIC | 10,867 | 33 | 8.6 | 26.6 | 0.0 | 0 | (0–0) | 14,377 | 25 | 6.9 | 31.4 | 0.0 | 0 | (0–0) |
|
| ||||||||||||||
| 0–0.005 | 15,243 | 45 | 8.8 | 53.9 | 0.1 | 0 | (0–0) | 19,071 | 61 | 13.4 | 57.7 | 0.0 | 0 | (0–0) |
|
| ||||||||||||||
| 0.005–0.1 | 12,143 | 37 | 8.8 | 39.6 | 2.7 | 6 | (3–9) | 15,485 | 50 | 13.5 | 46.3 | 0.2 | 0 | (0–2) |
|
| ||||||||||||||
| 0.1–0.25 | 2,981 | 20 | 19.1 | 10.9 | 3.8 | 26 | (14–35) | 4,308 | 17 | 16.4 | 14.2 | 0.3 | 2 | (0–11) |
|
| ||||||||||||||
| 0.25–0.5 | 1,798 | 15 | 24.0 | 7.5 | 5.3 | 41 | (25–53) | 2,695 | 11 | 17.1 | 9.2 | 0.4 | 4 | (1–22) |
|
| ||||||||||||||
| 0.5–1 | 1,405 | 15 | 31.8 | 7.6 | 9.0 | 54 | (38–66) | 2,111 | 8 | 16.0 | 8.0 | 0.7 | 9 | (2–35) |
|
| ||||||||||||||
| 1+ | 1,301 | 26 | 63.5 | 7.3 | 20.1 | 73 | (59–83) | 1,616 | 8 | 21.8 | 6.4 | 1.7 | 21 | (6–59) |
|
| ||||||||||||||
| Attained age (year) | ||||||||||||||
|
| ||||||||||||||
| –39 | 38 | 5.9 | 24.9 | 13.4 | 35 | (13–52) | 3 | 7.8 | 2.6 | 0.3 | 9 | (2–31) | ||
|
| ||||||||||||||
| 40–49 | 34 | 10.2 | 26.3 | 8.6 | 25 | (14–33) | 15 | 9.2 | 13.2 | 0.6 | 5 | (1–18) | ||
|
| ||||||||||||||
| 50–59 | 53 | 16.8 | 41.3 | 10.4 | 20 | (10–28) | 29 | 9.5 | 29.6 | 0.7 | 2 | (0–12) | ||
|
| ||||||||||||||
| 60–69 | 45 | 21.0 | 42.2 | 6.9 | 14 | (6–22) | 44 | 11.2 | 45.0 | 0.7 | 2 | (0–8) | ||
|
| ||||||||||||||
| 70– | 21 | 30.3 | 18.8 | 1.8 | 9 | (3–17) | 89 | 16.4 | 82.7 | 0.9 | 1 | (0–6) | ||
|
| ||||||||||||||
| Calendar year | ||||||||||||||
|
| ||||||||||||||
| 1958–1965 | 45,7383 | 22 | 7.1 | 11.2 | 5.7 | 34 | (8–58) | 59,6633 | 45 | 10.8 | 41.9 | 0.7 | 2 | (0–10) |
|
| ||||||||||||||
| 1966–1975 | 45,042 | 17 | 4.8 | 17.0 | 6.6 | 28 | (12–42) | 50,421 | 40 | 9.5 | 40.7 | 0.7 | 2 | (0–9) |
|
| ||||||||||||||
| 1976–1985 | 43,890 | 31 | 9.3 | 26.0 | 7.9 | 23 | (13–32) | 38,379 | 30 | 9.9 | 36.1 | 0.7 | 2 | (0–8) |
|
| ||||||||||||||
| 1986–1995 | 41,971 | 57 | 18.4 | 40.0 | 9.6 | 19 | (10–27) | 26,396 | 44 | 22.6 | 31.1 | 0.7 | 2 | (0–8) |
|
| ||||||||||||||
| 1996–2005 | 38,181 | 64 | 23.6 | 59.3 | 11.1 | 16 | (6–24) | 15,416 | 21 | 20.3 | 23.4 | 0.5 | 2 | (0–8) |
Cases per 100,000 person-years.
Attributable faction (*among people with dose > 0.005 Gy weighted thyroid dose).
The number of subjects at risk at the beginning of the period.
NIC: subjects who were not in the cities at the time of bombings.
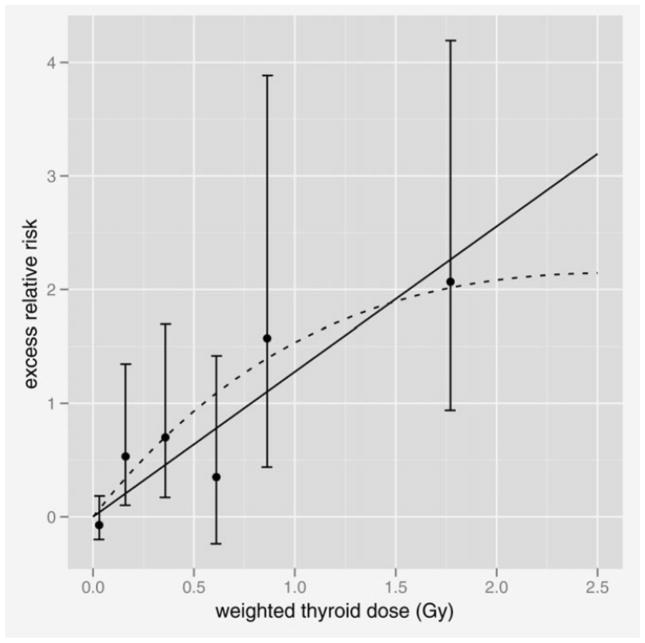
Based on a linear dose–response model with effect modification by age-at-exposure and attained age, the gender-averaged ERR and EAR at age 60 after exposure at age 10 were estimated as 1.28 (95% CI: 0.59–2.70) per 1 Gy weighted dose and 29.5 (13.8–49.6) cases per 100,000 person-year-Gy weighted dose, respectively. Figure 1 shows the fitted linear dose response (solid line) with non-parametric estimates in dose categories with 95% CIs under the ERR model. The fitted linear EAR dose response and points were similar in shape to those of ERR. Both the ERR and EAR significantly and rapidly decreased with increasing age-at-exposure by 53% (p = 0.03) and 70% (p = 0.002), respectively, per decade increase in exposure age. Allowing for the modifying effect of age-at-exposure, the ERR tended to decrease and the EAR to increase with increasing attained age, in proportion to age to the power −1.27 (p = 0.15) and 1.03 (p = 0.11), respectively. The EAR for women was significantly higher than that for men, with a female:male ratio of 6.3 (p = 0.001), while the ERR sex ratio was smaller and not statistically significant (2.0; p = 0.30). There was no significant dependence of the ERR on AHS participation (p = 0.23). There was a suggestion of a flattening of the dose–response at higher doses based on a model with a linear-exponential dose response (p = 0.07, the dashed curve in Fig. 1) but no evidence against linearity when the analysis was restricted to those with dose <2 Gy (p = 0.44). The estimated lowest dose range with a significant linear ERR was 0–0.2 Gy weighted dose (p = 0.02) with no evidence of a threshold level (p > 0.5).
Figure 1.
Fitted dose–response functions for thyroid cancer incidence in the LSS cohort. The solid line is the fitted linear ERR dose response, and the dashed curve is the fitted ERR based on linear-exponential dose–response model. The points are non-parametric estimates of the ERR in dose categories with 95% CIs. The line and points are all gender-averaged estimates at age 60 after exposure at age 10.
Under the fitted linear ERR model, about 36% (95% CI: 22–46) of the 191 cases among those exposed as children or adolescents were estimated to be attributable to radiation exposure, which was considerably higher than that of 4% (1–17) for those exposed as adults (Table 1). The majority (93%) of the 44 attributable cases in this cohort were from the younger group. Among those exposed as children or adolescents, the attributable fraction decreased with attained age, but remained elevated (16%, 95% CI: 6–24) during the latest follow-up period of 1996–2005.
DISCUSSION
With long-term follow-up of a large cohort of men and women of all ages, tumor registry-based case ascertainment supplemented with a pathology review, and well-characterized dose estimates, the LSS cohort is an excellent resource to obtain the full picture of the long-term trend of radiation-associated health effects. Based on the present findings, we conclude that the radiation-related increase in thyroid cancer in atomic-bomb survivors is mostly derived from the increase among those exposed as children or adolescents and that radiation-related thyroid cancer risk decreases with time but has persisted for at least 5 decades after exposure, and will likely continue throughout life. Children and adolescents internally exposed to radioactive iodine from Chernobyl fallout have also shown a high risk of thyroid cancer, similar in magnitude to the risk expected from atomic-bomb survivors.17, 18
Little or no evidence of increases in the thyroid cancer risks among atomic-bomb survivors exposed as adults is consistent with results from some previous studies on thyroid cancer rates following radiation exposures received as adults.2, 6, 19 Pooling thyroid cancer data from medically exposed populations and the atomic-bomb survivors, Ron et al. reported that there were limited data related to adult exposure, which provided insufficient evidence of a radiation effect.5 This should not be interpreted as the lack of a radiation effect, but is likely due to the lack of statistical power for detecting a small effect. While no significantly increased risk of thyroid cancer was seen in the adults exposed to relatively high doses to I-131 in medical circumstances,7 a considerably high risk of thyroid cancer has been attributed to adult exposure to I-131 among Chernobyl clean-up workers in Belarus, Russia and Baltic countries.20 Analyzing the tumor registry-based LSS cancer incidence data, Richardson reported a significant dose response for thyroid cancer among women, but not among men, aged 20 years or older at exposure.21 Thyroid cancer cases analyzed by Richardson included a sizable number (>100) of papillary microcarcinoma cases (diameter <10 mm) mostly detected at autopsy that were excluded from our analysis. A large proportion (95%) of these microcarcinoma cases in the LSS were seen in those exposed as adults with a significant radiation effect, especially among women.11 In our analysis, the ERR estimates for those exposed as adults were not significant for both women and men. The inclusion of microcarcinoma cases is likely to explain the difference between Richardson’s findings and ours.
Because the tumor registries in Hiroshima and Nagasaki were not in operation before 1958, cancer incidence in the LSS could not be ascertained for the first 13 years after the bombings. The high ERR for thyroid cancer seen for the youngest age-at-exposure group at the beginning of the follow-up period does involve primarily those occurring at adolescent ages and young adulthood, but not those that may have occurred shortly after exposure, i.e., in infancy or early childhood. The present data suggest, however, that the risk for thyroid cancer at pediatric ages may have been even higher; there were two cases diagnosed before age 20 years, both of which were highly exposed (1.3 and 1.9 Gy weighted dose).
Many people have, or will have their thyroid exposed to radiation from medical, environmental or accidental sources, a large proportion of whom are likely to be children. There has recently been a marked increase in collective dose received from medical exposure in the general population, mostly due to increased utilization of various medical imaging modalities, particularly CT.22 It is important to understand the lifetime risk of radiation-related thyroid cancer among those exposed as a child or adolescent, as they have a long life. Further follow-up of the LSS and other populations exposed to radiation at young ages will be essential in obtaining the full picture of the long-term thyroid cancer risk, which is important in both clinical practice and the organization of public health measures.
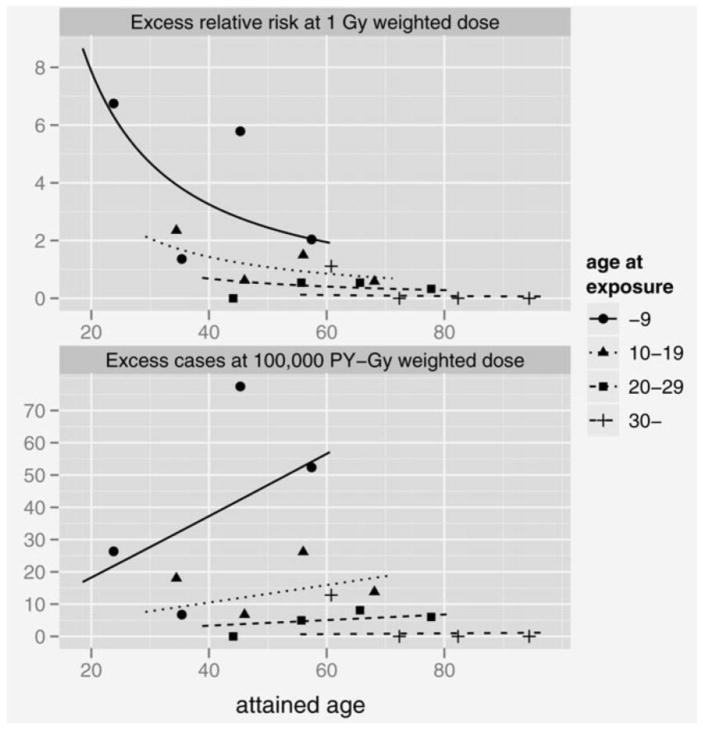
Figure 2.
Fitted temporal patterns and age-at-exposure variation in the radiation-associated risk for thyroid cancer in the LSS cohort. The upper panel presents the ERR at 1 Gy and the lower panel the fitted EAR at 100,000 person-years-Gy, with attained age for ages at exposure of 5 (“0–9” for categorical estimates), 15 (“10–19”), 25 (“20–29”) and 41 (“30–”) years. All curves and points are gender-averaged estimates.
Acknowledgments
Grant sponsor: US National Cancer Institute Intramural Research Program; Grant number: N01CP31012 N; Grant sponsors: RERF Research Protocols 6-91 and 5-11, Japanese Ministry of Health, Labour and Welfare (MHLW) and the U.S. Department of Energy (DOE); Grant number: DE-HS0000031
The authors thank Dr. Yuzo Hayashi, General Health Service Facility Hidamari, Hiroshima and Dr. Nobuo Tsuda, Nagasaki Health Promotion Corporation, for their pathological review of the cases in this study. They are especially grateful to the diligent work of tracing and abstracting hospital records by the staff of the Hiroshima and Nagasaki tumor registries. The Radiation Effects Research Foundation, Hiroshima and Nagasaki, Japan is a private, non-profit foundation funded by the Japanese Ministry of Health, Labour and Welfare and the U.S. Department of Energy. The views of the authors do not necessarily reflect those of the two governments.
References
- 1.Shore RE, Woodard ED, Pasternack BS, et al. Radiation and host factors in human thyroid tumors following thymus irradiation. Health Phys. 1980;38:451–65. doi: 10.1097/00004032-198004000-00001. [DOI] [PubMed] [Google Scholar]
- 2.United Nations Scientific Committee on the Effects of Atomic Radiation, Effects of Ionizing Radiation. UNSCEAR 2006 Report. Vol. 1. United Nations; 2008. [Google Scholar]
- 3.Imaizumi M, Usa T, Tominaga T, et al. Radiation dose-response relationships for thyroid nodules and autoimmune thyroid diseases in Hiroshima and Nagasaki atomic bomb survivors 55–58 years after radiation exposure. JAMA. 2006;295:1011–22. doi: 10.1001/jama.295.9.1011. [DOI] [PubMed] [Google Scholar]
- 4.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 5.Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141:259–77. [PubMed] [Google Scholar]
- 6.Ron E, Schneider AB. Thyroid cancer. In: Schottenfeld D, Fraumeni JF, editors. Cancer epidemiology and prevention. 3. New York: Oxford University Press; 2006. [Google Scholar]
- 7.Dickman PW, Holm LE, Lundell G, et al. Thyroid cancer risk after thyroid examination with 131I: a population-based cohort study in Sweden. Int J Cancer. 2003;106:580–7. doi: 10.1002/ijc.11258. [DOI] [PubMed] [Google Scholar]
- 8.Sadetzki S, Chetrit A, Lubina A, et al. Risk of thyroid cancer after childhood exposure to ionizing radiation for tinea capitis. J Clin Endocrinol Metab. 2006;91:4798–804. doi: 10.1210/jc.2006-0743. [DOI] [PubMed] [Google Scholar]
- 9.Adams MJ, Shore RE, Dozier A, et al. Thyroid cancer risk 40+ years after irradiation for an enlarged thymus: an update of the Hempelmann cohort. Radiat Res. 2010;174:753–62. doi: 10.1667/RR2181.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozasa K, Shimizu Y, Suyama A, et al. Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: An overview of cancer and noncancer diseases. Radiat Res. 2012;177:229–43. doi: 10.1667/rr2629.1. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi Y, Lagarde F, Tsuda N, et al. Papillary microcarcinoma of the thyroid among atomic bomb survivors: tumor characteristics and radiation risk. Cancer. 2010;116:1646–55. doi: 10.1002/cncr.24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cullings HM, Fujita S, Funamoto S, et al. Dose estimation for atomic bomb survivor studies: its evolution and present status. Radiat Res. 2006;166:219–54. doi: 10.1667/RR3546.1. [DOI] [PubMed] [Google Scholar]
- 13.Pierce DA, Stram DO, Vaeth M. Allowing for random errors in radiation dose estimates for the atomic bomb survivor data. Radiat Res. 1990;123:275–84. [PubMed] [Google Scholar]
- 14.Yamada M, Wong FL, Fujiwara S, et al. Noncancer disease incidence in atomic bomb survivors, 1958–1998. Radiat Res. 2004;161:622–32. doi: 10.1667/rr3183. [DOI] [PubMed] [Google Scholar]
- 15.Preston DL, Lubin JH, Pierce DA, et al. Epicure users guide. Seattle, WA: Hirosoft International Corporation; 1993. [Google Scholar]
- 16.Lunn DJ, Thomas A, Best N, et al. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–37. [Google Scholar]
- 17.Brenner AV, Tronko MD, Hatch M, et al. I-131 dose response for incident thyroid cancers in Ukraine related to the Chornobyl accident. Environ Health Perspect. 2011;119:933–9. doi: 10.1289/ehp.1002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zablotska LB, Ron E, Rozhko AV, et al. Thyroid cancer risk in Belarus among children and adolescents exposed to radioiodine after the Chornobyl accident. Br J Cancer. 2011;104:181–7. doi: 10.1038/sj.bjc.6605967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boice JD., Jr Thyroid disease 60 years after Hiroshima and 20 years after Chernobyl. JAMA. 2006;295:1060–2. doi: 10.1001/jama.295.9.1060. [DOI] [PubMed] [Google Scholar]
- 20.Kesminiene A, Evrard A, Ivanov V, et al. Risk of thyroid cancer among Chernobyl liquidators. Third European IRPA Congress; 2010. pp. S01–9. [Google Scholar]
- 21.Richardson DB. Exposure to ionizing radiation in adulthood and thyroid cancer incidence. Epidemiology. 2009;20:181–7. doi: 10.1097/EDE.0b013e318196ac1c. [DOI] [PubMed] [Google Scholar]
- 22.National Council on Radiation Protection and Measurements. NCRP Report No 160. Bethesda, MD: 2009. Ionizing Radiation Exposure of the Population of the United States. [Google Scholar]