Abstract
The heart has traditionally been considered a radio-resistant organ that would be unaffected by cardiac doses below about 30 Gray. During the last few years, however, evidence that radiation-related heart disease can occur following lower doses has emerged from several sources. These include studies of breast cancer patients, who received mean cardiac doses of 3–17 Gray when given radiotherapy following surgery, and studies of survivors of the atomic bombings of Japan who received doses of up to 4 Gray.
At doses above 30 Gray, radiation-related heart disease may occur within a year or two of exposure and risk increases with higher radiotherapy dose, younger age at irradiation, and the presence of conventional risk factors. At lower doses the typical latent period is much longer and is often more than a decade. However, the nature and magnitude of the risk following lower doses is not well characterized, and it is not yet clear whether there is a threshold dose below which there is no risk.
The evidence regarding radiation-related heart disease comes from several different disciplines. The present review brings together information from pathology, radiobiology, cardiology, radiation oncology and epidemiology. It summarises current knowledge, identifies gaps in that knowledge, and outlines some potential strategies for filling them. Further knowledge about the nature and magnitude of radiation-related heart disease would have immediate application in radiation oncology. It would also provide a basis for radiation protection policies for use in diagnostic radiology and occupational exposure.
Keywords: heart disease, radiotherapy, radiation, breast cancer, lymphoma
INTRODUCTION
It has been recognized since the 1960s that the heart may be damaged by substantial doses of radiation [>30 Gray (Gy)], such as used to occur during mantle radiotherapy for Hodgkin lymphoma. During the last few years, however, evidence that radiation-related heart disease (RRHD) can occur following doses below 20 Gy has emerged from several independent sources. Those sources include studies of breast cancer patients who received mean cardiac doses of 3 to 17 Gy when given radiotherapy following surgery and studies of survivors of the atomic bombings of Japan who received doses of up to 4 Gy.
At doses above 30 Gy, an increased risk of RRHD can becomes apparent within a year or two of exposure, and the risk increases with higher radiotherapy dose, younger age at irradiation, and the presence of conventional risk factors. At lower doses, the typical latency period is much longer and is often more than a decade. The nature and magnitude of the risk following lower doses is not well characterized, and it is not yet clear whether there is a threshold dose below which there is no risk.
The evidence regarding RRHD comes from several different disciplines. The present review brings together information from pathology, radiobiology, cardiology, radiation oncology, and epidemiology; it summarizes current knowledge, identifies gaps in that knowledge, and outlines some potential strategies for filling them.
CURRENT KNOWLEDGE
Pathology
The pathological expressions of RRHD documented following therapeutic irradiation can be broadly reduced to four conditions: pericarditis, pericardial fibrosis, diffuse myocardial fibrosis, and coronary artery disease (CAD) 1 and 2. Radiation may also cause valvular disease, although the evidence for this is not as strong. None of these conditions is specific to radiation.
Radiation-related pericarditis is characterized by an exudate of a variable amount of protein-rich fluid within the pericardial sac (pericardial effusion). Rapid accumulation of this fluid can, in rare cases, cause potentially fatal cardiac tamponade. Almost invariably, fibrin accumulates on the mesothelial lining of the epicardium or the parietal pericardium.
Pericardial fibrosis consists of collagen deposition, usually in the parietal pericardium, replacing the peripheral adipose layer and increasing the thickness of the fibrous layer (normally, < 0.5 mm) to as much as 8 mm. This results in a rigid pericardial sac (Fig. 1) that may produce constriction and is often accompanied by effusion. Fibrin also accumulates interstitially in the thickened pericardium (1). accompanied by effusion. Fibrin also accumulates interstitially in the thickened pericardium (1).
Fig. 1.
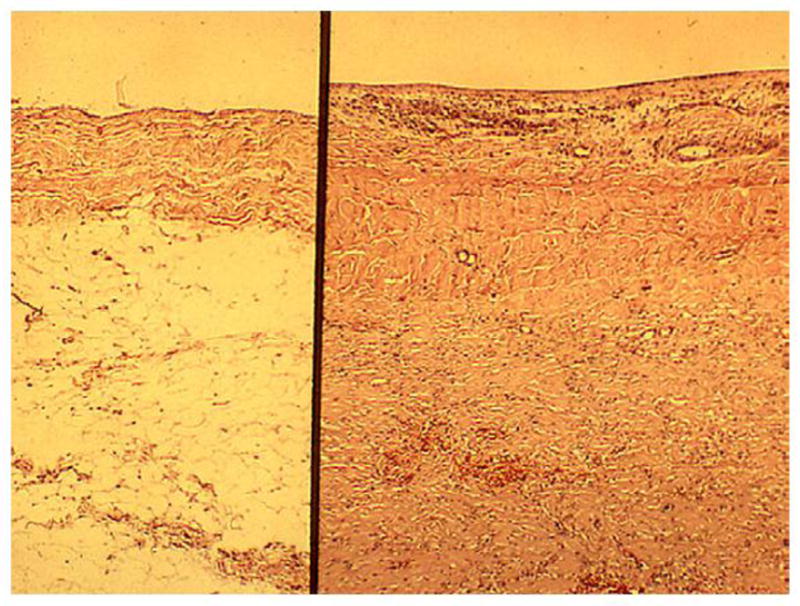
Perpendicular sections of human pericardium. The left panel illustrates the normal parietal pericardium with a thin, uniform fibrous layer that faces the heart (upper section) and an outer layer of adipose tissue (lower section). The right panel is a typical example of irradiated pericardium at 17 months after receiving 67 Gy. The adipose tissue has been replaced by dense fibrous tissue that actually extends well below the limits of this micrograph. Hematoxylin-eosin stain. (From Kajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Keynote lecture. 5th Nordic Conference on Radiation Oncology. Bergen. Norway. Acta Oncologica 2005;44:13–22. with permission.)
As the name implies, diffuse myocardial fibrosis consists of diffuse proliferation of bands of collagen separating and/or replacing myocytes (Fig. 2). It occurs in patches, often in the anterior wall of the left ventricle. Experimental studies have demonstrated that this condition results from damage to the endothelium of the myocardial blood capillaries and can lead to ischemia and ultimately fibrosis (3). If it is extensive, myocardial fibrosis may lead to congestive heart failure. The morphology of radiation-related CAD is essentially the same as CAD resulting from atherosclerosis from other causes: intimal proliferation of myofibroblasts, with lipid-containing macrophages forming plaques that may fissure causing thrombosis (4). This reduces the arterial lumen to various degrees (Fig. 3), resulting in the clinical manifestations of ischemic heart disease: stable angina pectoris, unstable angina, myocardial infarction (MI), and chronic ischemic heart disease. Endovascular irradiation (used until recently to prevent coronary arterial restenosis postangioplasty) often causes fibrosis of the adventitia in swine and probably also in humans. However, that feature is often absent in CAD resulting from external irradiation. Valvular lesions characterized by fibrosis and calcification have been described in irradiated patients (5).
Fig. 2.
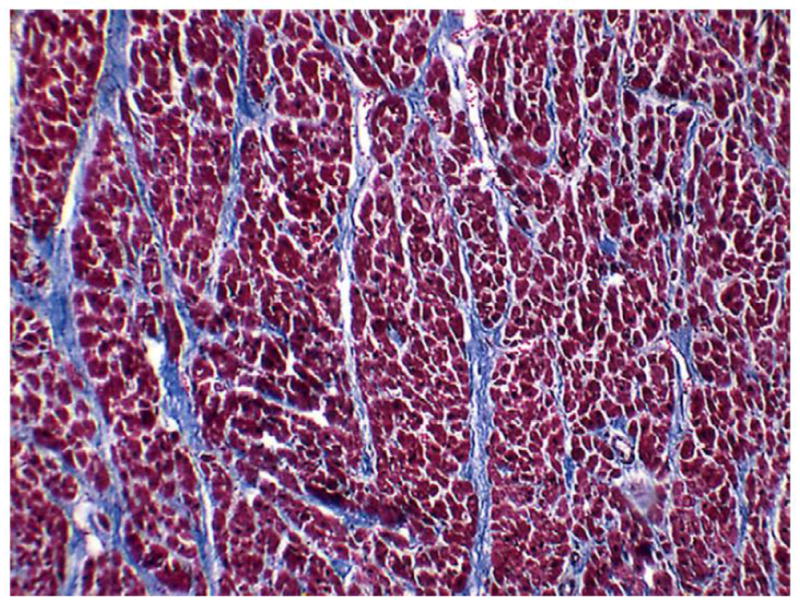
Fatal diffuse myocardial fibrosis several years after irradiation for Hodgkin’s disease. Whereas normally there should be very little collagen among the dark red myocytes, this heart muscle is criss-crossed by multiple bands of blue collagen. Gomori trichrome stain.
Fig. 3.
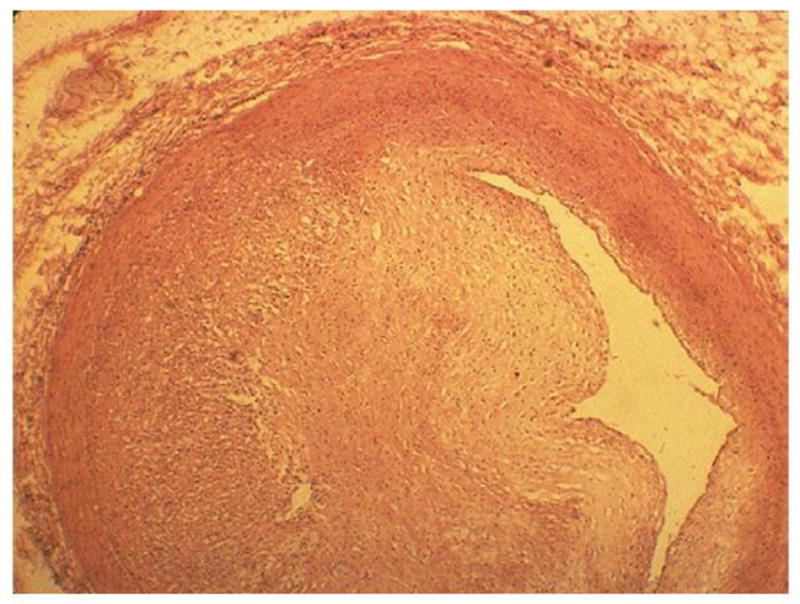
Left anterior descending coronary artery in a 16-year-old boy 1 year after receiving 40 Gy mantle radiotherapy for Hodgkin’s disease. Myointimal proliferation has considerably narrowed the lumen. Fatal cases like this in a patient who had no cardiac risk factors other than radiation illustrate that the morphology of arterial disease due to radiation is essentially no different from that of age-related atherosclerosis. Hematoxylin-eosin stain. (From Fajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Keynote lecture. 5th Nordic Conference on Radiation Oncology, Bergen. Norway. Acta Oncologica 2005;44:13–22; with permission.)
Experimental Models
Pericardial, myocardial, and coronary artery lesions can all be reproduced by irradiating appropriate animal models 1, 2 and 3, but direct experimental proof regarding radiation-induced valvular disease is lacking at present. Experimental models commonly used to study RRHD include rat, rabbit, and dog. Myocardial fibrosis and loss of cardiac function develop several months after irradiation in all three species, although interstrain and interspecies differences in expression 3, 6 and 7 mean that caution is required in extrapolating to humans. Nevertheless, these animal models have provided insight into possible mechanisms of RRHD.
Evidence from rodent models suggests that radiation can cause both microvascular and macrovascular cardiac pathology. The microvascular pathology is characterized by a decrease in capillary density, causing chronic myocardial ischemia and fibrosis. Macrovascular disease occurs through an accelerated development of age-related atherosclerosis.
Experiments investigating the effects of high-precision X-ray irradiation of the whole heart in rats demonstrate that observable congestive heart failure develops in 100% of irradiated animals within their normal lifespan following single doses of >15 Gy 2 and 8. At 10 Gy, reduced capillary density has also been observed, but damage progression was slow, and latency to clinical symptoms exceeded the normal life span of rats. Analysis of the effect of varying dose fractionations on median latency to heart failure in experiments with up to 10 fractions yields alpha/beta ratios of <4 Gy (9). The low ratio suggests that the myocardium behaves as a classical late-reacting tissue.
Studies of radiation-induced microvascular pathology in rats demonstrate focal loss of alkaline phosphatase activity within a few weeks of heart irradiation. Loss of capillaries, preceded by increased endothelial proliferation, occurs in enzyme-negative areas only (10). Those foci start small and gradually increase in size, with a progressive decrease in local capillary density. Histopathology at the time of heart failure demonstrates foci of ischemic necrosis and reparative fibrosis unrelated to the distribution of major blood vessels. Myocytes are terminally differentiated cells and are therefore relatively resistant to the direct cytotoxic effects of irradiation. Experimental evidence suggests that radiation-induced heart failure is an effect of indirect myocyte toxicity secondary to microvascular damage and ischemia. This is in contrast to anthracycline-induced heart failure, where anthracyclines are known to be directly toxic to myocytes.
The pathogenesis of radiation-related atherosclerosis in large arteries has been studied in ApoE−/− mice. These rodents spontaneously develop atherosclerotic plaques within 6 to 12 months, but radiation of vessels speeds this process due to local effects on the vascular endothelium. The phenotype of the plaques is also altered by doses of ≥14 Gy, with an increased frequency of intraplaque hemorrhage and inflammatory cells, so that they are more vulnerable to rupture, causing thrombosis (11).
Based on these experimental findings, it is reasonable to formulate two hypotheses for the biological mechanisms that lead to increased morbidity and mortality from coronary artery disease after radiation exposure in humans. The first hypothesis is that radiation increases the frequency of MI by interacting with the pathological pathway of age-related coronary artery atherosclerosis, resulting in accelerated atherosclerosis in which disease is seen at a younger age than would normally occur. The second hypothesis is that radiation increases the lethality of age-related MI by reducing the heart’s tolerance to acute infarctions as a result of microvascular damage to the myocardium. As shown in Fig. 4, these hypotheses are not mutually exclusive, and the two mechanisms may act together to produce clinical heart disease.
Fig. 4.
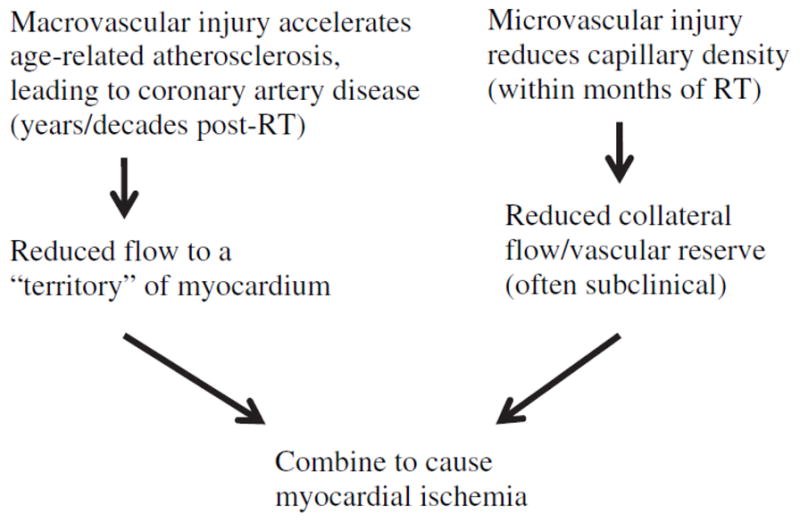
An outline of how microvascular and macrovascular radiation-related cardiac injury could theoretically combine to cause myocardial ischemia after RT.
Animal models have also been used to test potential intervention strategies both before and after local heart irradiation. Those strategies include use of methylprednisolone or ibuprofen (12), captopril (13), amifostine (14), and a combination of pentoxifylline and alpha-tocopherol (15). All agents showed some potential benefit in preventing morphologic or functional deterioration experimentally, but their clinical usefulness has not yet been demonstrated.
Clinical Manifestations and Management
A wide range of clinical cardiovascular problems can arise from radiation therapy (RT) for thoracic malignancy. Radiation-related pericardial and myocardial diseases are less common today than in the past, due to modifications in RT techniques, particularly for lymphomas (16), resulting in lower radiation doses to the heart. The predominant clinical manifestation of RRHD today is probably CAD, but the frequency with which it occurs is unknown, as it does not differ clinically from CAD from other causes, and many radiation-related cases may not be recognized as such.
Acute pericarditis is still occasionally seen within weeks after cardiac irradiation. Patients present with pleuritic chest pain, fever, tachycardia, a pericardial rub, and characteristic electrocardiographic abnormalities. Those signs and symptoms usually resolve quickly and without consequence with nonsteroidal anti-inflammatory drug therapy. A proportion of patients, however, develop chronic pericarditis up to 10 years later. The incidence of disease is related to the volume of pericardium irradiated, the dose received, and possibly the presence of effusion during the acute phase. The severity is variable, ranging from asymptomatic pericardial thickening found incidentally to cardiac tamponade requiring urgent pericardiocentesis. For those patients with recurrent effusion, some authorities recommend subtotal pericardiectomy to prevent the development of severe constrictive pericarditis, which can be difficult to treat effectively at a later stage (17).
Radiation-related myocardial fibrosis is often asymptomatic and is picked up only incidentally on echocardiography more than 10 years after radiation therapy (18). Clinically significant ventricular dysfunction is uncommon but can occur, particularly in the context of anthracycline chemotherapy and high radiation doses (>30 Gy) to large volumes of the heart. The management of radiation-related cardiomyopathy currently follows guidelines for the management of cardiac failure due to other causes.
Radiotherapy has been associated with valvular heart disease, which is common in some series (18). The incidence has been related to mediastinal radiation doses of >30 Gy 19, 20 and 21 and younger age at irradiation 22, 23 and 24, with an average latency of >10 years for asymptomatic and longer (median, 22 years) for symptomatic disease (20). Aortic disease usually consists of mixed stenosis and regurgitation and is more common than mitral and right-sided disease. Conduction system abnormalities have also been reported, often in association with other types of RRHD. A variety of arrhythmias and conduction blocks have been observed, but these are rarely clinically serious. Autonomic nervous system dysfunction with a persistent nonvariable tachycardia has also been described (25).
Thoracic RT is now firmly established as a risk factor for CAD. Radiation-related CAD is usually not detected until at least 10 years after exposure, and the magnitude of the risk with modern RT techniques is not yet well defined. Relative risks increase with higher radiotherapy doses 19 and 26, younger age at irradiation 23 and 26, and the presence of conventional risk factors for coronary artery disease (20). Radiation-related CAD is managed conventionally, with medical control of risk factors and percutaneous coronary intervention or surgical bypass if indicated. Surgery may be technically challenging due to the presence of mediastinal scarring, friability of the vessels, and a possible increased restenosis rate of irradiated internal mammary vessels when they are chosen for coronary artery bypass grafting (27). Patients most commonly presenting with RRHD are survivors of breast cancer or HL, although RRHD has also been reported after radiotherapy for testicular cancer (28) and peptic ulcer disease (29).
Studies of Patients with Hodgkin Lymphoma
In the general population, early changes indicative of atherosclerosis can be seen in young adults, but clinical manifestations, such as angina and MI, rarely present until patients are at least in their 50s. In contrast, young patients without conventional cardiac risk factors who received mediastinal irradiation for HL can present with CAD in their 20s. In such individuals the likely cause is clear, and they may provide an example of the hypothesis of accelerated atherosclerosis referred to above.
In older people, among whom heart disease in the absence of radiotherapy is common, RRHD is harder to identify, and much of our information about the risk comes from studies in which a large population of HL patients treated with RT have been identified and followed over many years. Information about heart disease is obtained either from patients’ medical records or from disease registers or death certificates, and the risk of developing or dying from heart disease in the HL group can then be compared with that of the general population or with another suitable control group.
A recent study of 1,474 5-year survivors treated for HL before age 40 found relative risk (RR) values of 3 to 5 for cardiac morbidity of all types compared with the general population, suggesting that 66 to 80% of all cardiac disease in the HL population was due to therapy (30), while estimates of the RR of death from MI in HL patients compared with the general population are in the range 2 to 9 31 and 32.
Both the radiotherapy techniques and the chemotherapy regimens used to treat HL are evolving with time. Therefore, the impact of cardiac disease in HL survivors requires periodic reevaluation. The modification of radiotherapy techniques has resulted in a reduction in the risk of death from some cardiac causes. For example, among 2,232 HL patients treated at Stanford from 1960 to 1991, the RR for non-MI cardiac death fell from 5.3 to 1.4 when subcarinal blocking was introduced (26). However, the RR for fatal MI was not changed significantly in this series, possibly due to continued RT exposure of the proximal coronary arteries. Patients treated during the 1990s in Canada still had an increased risk of hospitalization for cardiac disease that was more marked in those who received combination therapy with anthracyclines (33), suggesting that an increased risk of late cardiac effects had not yet been eliminated.
The proportional increase in the cardiac death rate is greater in survivors of childhood HL than in adults. In a report from the U.S. Childhood Cancer Survivor Study (22), which included 2,717 5-year survivors of childhood HL, the RR of death from all cardiac causes compared with that of the general population was 11.9 (95% confidence interval [CI], 9.1–15.3), while the RR of death from MI following mediastinal irradiation in childhood has been estimated to be as high as 41.5 (95% CI, 18.1–82.1) with RT doses of ≥42 Gy (23).
Studies of Patients with Breast Cancer
In studies of patients with HL, the death rates from heart disease have, in the past, often been so large that comparisons with rates in the general population gave a clear indication of the magnitude of the risk. For breast cancer patients, cardiac doses from RT have been lower and risks smaller. Therefore, the phenomenon of RRHD is less obvious in breast cancer patients than in HL patients. In breast cancer, much of our knowledge of the effect of RT on cardiovascular disease has come from long-term follow-up of women entered into trials in which all women received similar treatment in terms of surgery and drugs, and then half of the women were allocated at random also to receive adjuvant radiotherapy.
One of the first studies to examine the effect of RT on long-term survival in breast cancer was published by Cuzick et al. in 1987 (34). This meta-analysis of randomized trials showed that survival beyond 10 years was significantly worse for those receiving RT. This study was unable to determine the disease responsible for the detrimental effect on survival, but subsequent meta-analyses by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) have shown that mortality from heart disease was increased by 27% (2p = 0.0001) in women randomized to surgery plus RT compared with women randomized to surgery alone (35). Most of the increase was due to CAD. Aspects of the radiotherapy techniques used in the earlier trials that contributed to the increased cardiac mortality included field placement near the heart (particularly anterior fields used to treat the internal mammary nodes), orthovoltage radiation that delivered high doses to the anterior part of the heart, large daily fractions, and high total doses (36).
Recently, a preliminary analysis of updated EBCTCG data has related mortality from heart disease to estimated cardiac doses in over 30,000 women followed for up to 20 years. There is clear evidence that the radiation-related increase is higher in trials with larger mean cardiac RT doses and that the risk of death from heart disease increases by 3% per Gy (95% CI, 2%–5%; 2p < 0.00001) (37). That estimate can only be taken as an approximate indication of the risk, as individual treatment plans were not available for the women in those trials. Nevertheless, the data provide strong evidence that risk of RRHD was related to cardiac dose in irradiated breast cancer patients.
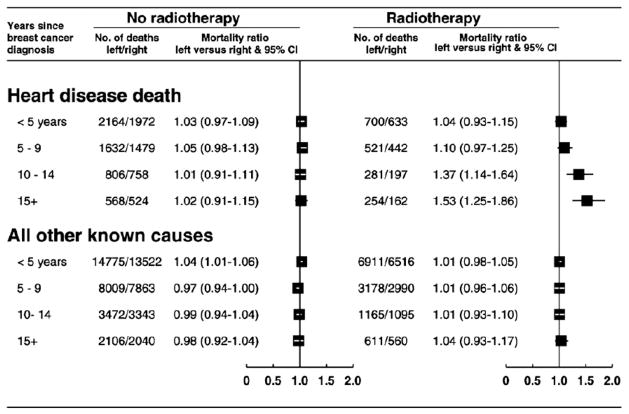
Outside the context of a randomized trial, comparisons of mortality after various different treatment regimens are often misleading because the prognosis of patients given different treatments will vary (38). In breast cancer, however, a reliable indication of the effect of radiotherapy on heart disease can be obtained by comparing the experience of irradiated women with left-sided tumors with that of women with right-sided tumors (39). This can be done because cardiac radiation doses in women given radiotherapy for left-sided tumors are usually larger than the cardiac radiation doses in women with right-sided tumors, and breast cancer laterality has, in the past, played little part in determining who should be given radiotherapy.
As shown in Fig. 5, for breast cancer patients who were not treated with radiotherapy, the subsequent risk of heart disease was independent of tumor laterality, while for irradiated patients, the heart disease mortality ratio, left-sided vs. right-sided tumors, increased with increasing time since diagnosis (i.e., with increasing time since irradiation). The increase was specific to heart disease as, for mortality from all other known causes, the left-sided vs. right-sided mortality ratio was close to unity in both irradiated and unirradiated patients. That suggests that the increasing trend in the left-sided vs. right-sided mortality ratio for heart disease shown in Fig. 5 is caused by radiotherapy, with the bulk of the risk occurring more than a decade after exposure. The proportional increase was higher for women irradiated at ages 20 to 49 than at older ages, but the trend did not reach statistical significance.
Fig. 5.
Left-sided vs. right-sided breast cancer. Mortality ratios by radiotherapy status, cause, and years since diagnosis in 300,000 women with breast cancer and registered in the Surveillance Epidemiology and End Results (SEER) cancer registries, 1973 to 2001 (From Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective study of about 300,000 women in US SEER cancel registries. Lancet Oncol 2005;6:557–565: with permission.)
A recent study examining the incidence of CAD following breast irradiation (40) revealed a higher prevalence of stress test abnormalities in left-sided than in right-sided tumor patients (59% vs. 8%; p = 0.001). Among left-sided tumor patients, the disease distribution differed from that expected in women, with a preponderance of left anterior descending artery disease. The anterior portion of the heart and the left anterior descending artery territory are the parts of the heart most often within the tangential radiation fields used to treat breast cancer. Hence, this finding provides direct evidence of a causal effect of radiotherapy on the development of CAD.
Imaging Studies
The long latency between radiation exposure and development of symptomatic RRHD prevents a direct assessment of the cardiovascular risks of current or planned radiotherapy regimens within a practical time scale. Therefore, early surrogate endpoints predictive of the development of later cardiac events would be useful.
Nuclear medicine imaging can assess quantitatively acute and early chronic myocardial perfusion changes and functional deficits due to ischemic heart disease (41). Marks et al.(42) initiated a prospective study to assess changes in myocardial perfusion and function following tangential photon irradiation of left-sided breast cancer. Patients underwent preradiotherapy and serial 6-month postradiotherapy cardiac single-photon emission computed tomography (SPECT) scans. The frequency of perfusion defects was related to the percentage of the left ventricle within the tangential photon fields. Wall motion abnormalities were more common in patients with perfusion abnormalities than in those without. Typically, perfusion defects and the associated wall motion abnormality were in the anterior wall of the left ventricle, corresponding to the region of the heart within the radiotherapy field (Fig. 6).
Fig. 6.
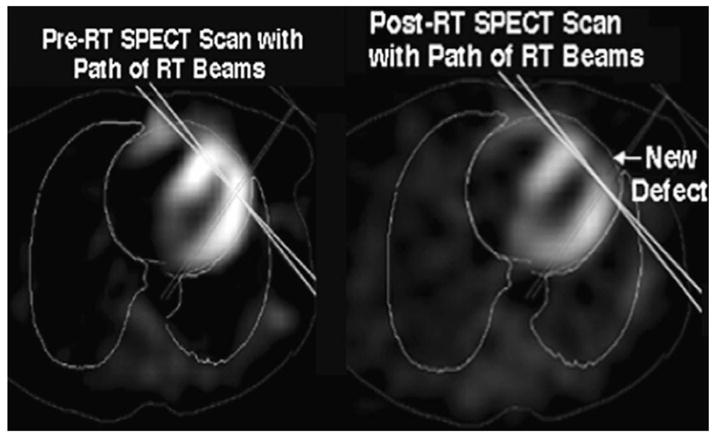
Representative axial images pre-RT (left panel) and post-RT (right panel) cardiac SPECT scans. The deep borders of the tangential RT beams are shown as solid lines. A new perfusion defect in the anterior left ventricle after radiation is seen.
Repeat scanning at 3 to 8 years after radiotherapy of patients with abnormalities at earlier time points demonstrated that perfusion defects persisted (43). Such defects have been associated with modest wall motion abnormalities but at present have not been shown to be predictive of clinical cardiac events such as congestive heart failure or MI. Thus, their clinical significance remains unproven. Nevertheless, as patients irradiated in the past for left-sided breast cancer have been shown to be at increased risk of cardiac events at more than 10 years after breast radiotherapy (39), it is tempting to hypothesize that these perfusion defects are related in some way to the longer-term clinical manifestations.
Since the perfusion abnormalities follow the contour of the radiation treatment field rather than the territorial distribution of coronary arteries, they are likely to represent a radiation-related microvascular injury to the myocardial capillary network. That, if true, would perhaps increase the likelihood or severity of a subsequent ischemic event. One would hypothesize that a later MI in such an irradiated patient may produce a larger perfusion defect and clinical consequence than would have been expected based on the location and severity of their coronary artery lesion due to a reduction in vascular reserve (Fig. 4).
Studies of Atomic Bomb Survivors
Mortality from heart disease has been studied among atomic bomb survivors in the Hiroshima-Nagasaki Life Span Study (LSS) for over 55 years (44). Distinctive features of the LSS are a population of >86,000 survivors who received whole-body uniform doses; individual radiation doses; a dose range of 0 to ~4 Gy; and complete mortality ascertainment and cause-of-death information.
Dose-related increases in heart disease mortality in the LSS occur in both genders, based on >8,400 heart disease deaths. The risk increased by 14% per Gy (95% CI, 6–23%), although it is not certain that there was any increase below about 0.5 Gy (44). It is unclear when the increase started, as there were substantial healthy survivor selection effects for at least a decade. However, even 50 years after irradiation, there is no suggestion that the risk has diminished.
The 14% increase in risk per Gy seen in the LSS is larger than the 3% per Gy seen in women in randomized trials of breast cancer patients. Confounding is unlikely to be responsible for the difference, as controlling for smoking, alcohol consumption, diabetes, obesity, education, and occupation had almost no effect on the estimates in the LSS. Also, the Adult Health Study biennial examinations of a subset of atomic bomb survivors provide additional support for an effect of radiation on heart disease in this population through associations of radiation exposure with alterations in blood pressure (45) and preclinical cardiovascular disease effects, including inflammatory markers (46) and lipid metabolism (47). The nominal difference in risk per Gy may be due to the fact that irradiation of the breast cancer patients is fractionated (2). Other possible reasons include Japanese/Western baseline differences in heart disease mortality rates, differences in age at exposure, and substantially less homogeneous cardiac radiation doses during breast cancer radiotherapy.
Occupational Studies
Radiologists and radiologic technologists undoubtedly received substantial fractionated radiation doses in the early years. Although individual dose estimates are not available, several studies have subdivided them by year of registration to groups likely to have received different doses 48, 49 and 50. In the United Kingdom, cancer mortality was higher in radiologists than in other medical practitioners for those registered during 1897 to 1920, suggesting a radiation-related excess (RR = 1.75; 95% CI, 1.34–2.26), and mortality declined significantly with the increasing year of registration, as would be expected from the declining doses over the 20th Century. In contrast, mortality from circulatory disease for those registered during 1897 to 1921 was lower in radiologists than in other medical practitioners (RR = 0.79; 95% CI, 0.66–0.94), and there was no suggestion of any trend with year of registration. Those data appear incompatible with a substantial radiation-related excess of circulatory disease in the early group. However, a study of 90,000 US radiologic technologists found increased risks of circulatory disease in those starting work prior to 1940 compared with those starting after 1960 (RR = 1.42; 95% CI, 1.04–1.94). This increase was mostly due to cerebrovascular disease (RR = 2.40; 95% CI, 1.09–5.31), rather than ischemic heart disease (RR = 1.22; 95% CI, 0.81–1.82) (50).
FUTURE PROSPECTS
Gaps in knowledge
There are major gaps in current knowledge of the structures at risk and mechanisms of damage in RRHD. It is uncertain whether there is a threshold dose of radiation to the heart below which no risk is incurred. Even at doses sufficient to increase risk, estimates of its magnitude for a given cardiac dose are still subject to considerable uncertainty, and knowledge about the shape of the dose-response relationship and factors that modify it is rudimentary, both for heart disease as a whole and for specific heart diseases. There are substantial dose inhomogeneities within the heart during radiation therapy, but it is not known which part of the heart is the most radiosensitive, nor which structure or structures at risk should be chosen as a reference point for tolerance doses in clinical practice. Additional important gaps in current knowledge include the relationship between short-term effects and long-term risk and the extent to which cardiac risk may be modified by other factors such as irradiation of other organs (e.g., kidneys), preexisting cardiovascular disease or diabetes, lifestyle factors including smoking, and interactions with cardiotoxic drugs used in combination with radiotherapy during cancer treatment.
Experimental models
Experimental models offer the greatest scope for increasing understanding of the cellular and molecular mechanisms of RRHD. A project utilizing high-precision cardiac irradiation of established murine models with prolonged follow-up, in combination with ex vivo and in vitro techniques, is presently underway (51). That project should eventually shed light on whether radiation operates predominantly by accelerating age-related coronary artery atherosclerosis by reducing the heart’s tolerance to acute infarctions through microvascular damage to the myocardium, or whether both mechanisms are important.
Other topics that may be amenable to study experimentally include the sensitivities of specific cardiac substructures and the effect of irradiation of organs such as the kidney on risk of heart disease. Experimental models may also shed light on possible intervention strategies to reduce the risk in patients receiving radiotherapy, although the possibility of appreciable interspecies differences complicates the immediate application of promising findings to humans.
Scientific studies of exposed individuals
Further insight into the way in which the risk of death from RRHD varies with time since exposure and age is expected soon from a multinational study of cause-specific mortality after left-sided vs. right-sided breast cancer irradiation in over 1 million women (52). Information on cardiac morbidity and also on the possible modifying effects of preexisting heart disease and conventional cardiovascular risk factors such as diabetes, kidney disease, and hypertension will be available for a subset of the data 52 and 53.
In some countries it is possible to link information on the subsequent morbidity and mortality of cancer patients with estimates of dose to the whole heart and some cardiac substructures derived from individual radiotherapy charts (36). Although retrospective dose estimates lack the accuracy of prospective studies due to an absence of individual three-dimensional treatment plans, they should provide a broad dose–response relationship for radiation-induced heart disease for doses ranging from about 1 to 20 Gy (53). They should also provide insight into the combined cardiotoxicity of radiation and drugs, such as anthracyclines, taxanes, or trastuzumab.
Studies of irradiated breast cancer patients can avoid selection biases by making use of the left-right comparison. Clearly, however, they can provide no information about the effects of cardiac irradiation in children or in men. It may be possible to derive quantitative risk estimates in patients with childhood cancer or from men with cancers such as HL who have taken part in randomized trials of radiotherapy, but quantitative estimates from nonrandomized studies need to be interpreted with caution unless it can be established that the effects of selection are small.
Clinical studies of the atomic bomb survivors that employ a broad range of physical, imaging, and biomarker measurements are underway. Those studies aim to evaluate prospectively various potential mechanisms of radiation-related subclinical and clinical cardiovascular disease.
Several studies that were initiated to study radiation-related cancer in nuclear industry workers have large population sizes, long follow-up, and generally well-characterized doses, mostly of <0.5 Gy. It is possible that they may eventually provide information on the risks of RRHD. The low doses involved in such studies limit their statistical power. In addition, a major concern is that any dose-response relationship may be subject to confounding by smoking and other lifestyle factors. So far, little has been done to control for such factors in those studies.
Monitoring and prevention of risk in exposed individuals
The cardiac doses delivered by contemporary radiotherapy for cancer are lower than those delivered in previous decades (54). Cardiac risks are therefore likely to be correspondingly lower, but in the absence of reliable dose-response relationships, the extent of any risks from current therapies remains uncertain. The current era of three-dimensional computerized tomography treatment planning for radiotherapy enables the dose delivered to the whole heart and to specific cardiac substructures in any specific treatment plan to be estimated precisely. Hence, those carrying out treatment planning may have more control over cardiac doses than ever before. If reliable dose-response relationships for cardiac risk were available, useful prospective treatment-planning parameters could be derived (55), especially if the dose-response relationships also considered the impact of adjuvant systemic therapies and patient-specific factors.
The recognition and treatment of conventional cardiovascular risk factors, such as hypertension and hypercholesterolemia, can improve outcomes. As studies have suggested that the presence of conventional risk factors is a major modifier in the development of radiation-induced cardiovascular disease (20), there is an argument that those given thoracic radiotherapy may benefit from increased surveillance of these risk factors. Thus, a first step is to increase awareness among practicing physicians of radiation as an additional risk factor. An American Society of Clinical Oncology (ASCO) clinical evidence review published in 2007 (56) emphasized the absence of high-quality evidence regarding the best methods and possible benefits of screening for late cardiac effects of cancer therapies. This deficit in knowledge will become increasingly important as the number of long-term cancer survivors is predicted to increase dramatically over the next decade. Further research is required into surveillance and targeted intervention strategies, so that evidence-based guidelines for long-term cardiac follow-up of those patients at risk can be developed.
Radiological protection
There are approximately 400 million diagnostic medical examinations performed annually in the United States and some 3.6 billion worldwide. In addition, there are almost 4 million workers monitored occupationally for potential radiation exposure in the United States and over 15 million worldwide. Present radiological protection guidelines and regulations are concerned solely with radiation-related cancers and hereditary effects and do not consider RRHD. Virtually all the exposure occurs at doses of below 0.5 Gy, and, at the present time, it is unclear whether such low doses pose any cardiac risk. Therefore, it is as yet unclear whether there will be any future need to take explicit account of RRHD in radiation protection.
CONCLUDING REMARKS
Further knowledge about the nature and magnitude of radiation-related heart disease would have immediate application in radiation oncology. It would also provide a basis for radiation protection policies for use in diagnostic and occupational exposure.
To our knowledge, this review is first time that individuals from such a wide range of disciplines, including pathology, radiobiology, cardiology, radiation oncology, epidemiology, statistics, and diagnostic radiology have collaborated to share knowledge about RRHD. All those different disciplines have roles to play in furthering knowledge and in reducing the future burden of RRHD. Knowledge accumulated in one discipline may often be published in specialized journals that are not regularly read by those working in other disciplines, and technical terminology often impedes ready interpretation by those in other disciplines. We therefore conclude that future multidisciplinary reviews examining the topics raised here in greater depth and summarizing new findings are needed.
Acknowledgments
The authors thank the members and staff of the Nuclear and Radiation Studies Board of the US National Academy of Sciences, which hosted the 2008 Beebe Symposium on this topic, as well as the invited speakers and participants at the Symposium, whose insights contributed greatly to the ideas presented in this paper. The paper has been submitted for publication with permission from the National Academy, but the Academy has not been involved in its preparation, and the views expressed do not necessarily represent the views of the Academy or any of its constituent units. The authors also thank colleagues in the Clinical Trial Service Unit for helpful comments on an earlier draft of the paper.
Footnotes
Conflict of Interest Notification
The authors have no actual or potential conflicts of interest in relation to this work.
References
- 1.Fajardo LF, Berthrong M, Anderson RE. Radiation pathology. New York: Oxford University Press; 2001. [Google Scholar]
- 2.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: Is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 3.Fajardo LF, Stewart JR. Pathogenesis of radiation-induced myocardial fibrosis. Lab Invest. 1973;29:244–257. [PubMed] [Google Scholar]
- 4.Fajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Keynote lecture, 5th Nordic Conference on Radiation Oncology, Bergen, Norway. Acta Oncologica. 2005;44:13–22. doi: 10.1080/02841860510007440. [DOI] [PubMed] [Google Scholar]
- 5.Veinot JP, Edward WD. Pathology of radiation-induced heart disease: A surgical and autopsy study of 27 cases. Hum Pathol. 1996;8:766–773. doi: 10.1016/s0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 6.Lauk S. Strain differences in the radiation response of the rat heart. Radiother Oncol. 1986;5:333–335. doi: 10.1016/s0167-8140(86)80182-7. [DOI] [PubMed] [Google Scholar]
- 7.McChesney SL, Gillette EL, Powers BE. Radiation-induced cardiomyopathy in the dog. Radiat Res. 1988;113:120–132. [PubMed] [Google Scholar]
- 8.Lauk S, Kiszel Z, Buschmann J, et al. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985;11:801–808. doi: 10.1016/0360-3016(85)90314-1. [DOI] [PubMed] [Google Scholar]
- 9.Schultz-Hector S, Sund M, Thames HD. Fractionation sensitivity and repair kinetics of radiation-induced heart failure in rats. Radiother Oncol. 1992;23:33–40. doi: 10.1016/0167-8140(92)90303-c. [DOI] [PubMed] [Google Scholar]
- 10.Schulz-Hector S, Balz K. Radiation-induced loss of endothelial alkaline phosphatase activity and development of myocardial degeneration. An ultrastructural study. Lab Invest. 1994;71:252–260. [PubMed] [Google Scholar]
- 11.Stewart FA, Heeneman S, te Poele J, et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE−/− mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol. 2006;168:649–658. doi: 10.2353/ajpath.2006.050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves WC, Cunningham D, Schwiter EJ, et al. Myocardial hydroxyproline reduced by early administration of methylprednisolone or ibuprofen to rabbits with radiation-induced heart disease. Circulation. 1982;65:924–927. doi: 10.1161/01.cir.65.5.924. [DOI] [PubMed] [Google Scholar]
- 13.Yarom R, Harper IS, Wynchank S, et al. Effect of captopril on changes in rats’ hearts induced by long-term irradiation. Radiat Res. 1993;133:187–197. [PubMed] [Google Scholar]
- 14.Kruse JJ, Strootman EG, Wondergem J. Effects of amifostine on radiation-induced cardiac damage. Acta Oncol. 2003;42:4–9. doi: 10.1080/0891060310002168. [DOI] [PubMed] [Google Scholar]
- 15.Boerma M, Roberto KA, Hauer-Jensen M. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Radiat Oncol Biol Phys. 2008;72:170–177. doi: 10.1016/j.ijrobp.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmel RJ, Kaplan HS. Mantle irradiation in Hodgkin’s disease. An analysis of technique, tumor eradication, and complications. Cancer. 1976;37:2813–2825. doi: 10.1002/1097-0142(197606)37:6<2813::aid-cncr2820370637>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Clare GC, Troughton RW. Management of constrictive pericarditis in the 21st century. Curr Treat Options Cardiovasc Med. 2007;9:436–442. doi: 10.1007/s11936-007-0038-x. [DOI] [PubMed] [Google Scholar]
- 18.Heidenreich PA, Hancock SL, Lee BK, et al. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003;42:743–749. doi: 10.1016/s0735-1097(03)00759-9. [DOI] [PubMed] [Google Scholar]
- 19.Glanzmann C, Kaufman P, Jenni R, et al. Cardiac risk after mediastinal irradiation for Hodgkin’s disease. Radiother Oncol. 1998;46:51–62. doi: 10.1016/s0167-8140(97)00125-4. [DOI] [PubMed] [Google Scholar]
- 20.Hull MC, Morris CG, Pepine CJ, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–2837. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- 21.Carlson RG, Mayfield WR, Normann S, et al. Radiation-associated valvular disease. Chest. 1991;99:538–545. doi: 10.1378/chest.99.3.538. [DOI] [PubMed] [Google Scholar]
- 22.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol. 1993;7:1208–1215. [Google Scholar]
- 24.Lipshultz SE, Sallan SE. Cardiovascular abnormalities in longterm survivors of childhood malignancy. J Clin Oncol. 1993;11:1199–1203. [Google Scholar]
- 25.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 26.Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA. 1993;270:1949–1955. [PubMed] [Google Scholar]
- 27.Brown ML, Schaff HV, Sundt TM. Conduit choice for coronary artery bypass grafting after mediastinal radiation. J Thorac Cardiovasc Surg. 2008;136:1167–1171. doi: 10.1016/j.jtcvs.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Fossa° SD, Gilbert E, Dores GM, et al. Noncancer causes of death in survivors of testicular cancer. J Natl Cancer Inst. 2007;99:533–544. doi: 10.1093/jnci/djk111. [DOI] [PubMed] [Google Scholar]
- 29.Carr ZA, Land CE, Kleinerman RA, et al. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol Phys. 2005;61:842–850. doi: 10.1016/j.ijrobp.2004.07.708. [DOI] [PubMed] [Google Scholar]
- 30.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin’s lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 31.Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: A collaborative British cohort study. J Natl Cancer Inst. 2007;99:206–214. doi: 10.1093/jnci/djk029. [DOI] [PubMed] [Google Scholar]
- 32.Henry-Amar M, Hayat M, Meerwaldt JH, et al. EORTC Lymphoma Cooperative Group. Causes of death after therapy for early stage Hodgkin’s disease entered on EORTC protocols. Int J Radiat Oncol Biol Phys. 1990;19:1155–1157. doi: 10.1016/0360-3016(90)90221-5. [DOI] [PubMed] [Google Scholar]
- 33.Myrehaug S, Pintilie M, Tsang R, et al. Cardiac morbidity following modern treatment for Hodgkin lymphoma: Supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk Lymphoma. 2008;49:1486–1493. doi: 10.1080/10428190802140873. [DOI] [PubMed] [Google Scholar]
- 34.Cuzick J, Stewart H, Peto R, et al. Overview of randomized trials of postoperative adjuvant radiotherapy in breast cancer. Cancer Treat Rep. 1987;71:15–29. [PubMed] [Google Scholar]
- 35.Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 36.Taylor CW, Nisbet A, McGale P, et al. Cardiac exposures in breast cancer radiotherapy: 1950’s–1990’s. Int J Radiat Oncol Biol Phys. 2007;69:1484–1495. doi: 10.1016/j.ijrobp.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 37.Early Breast Cancer Trialists’ Collaborative Group. Long term toxicity of radiation therapy. 2006 Update of the Early Breast Cancer Trialists’ Collaborative group overview of radiation therapy for early breast cancer; 2007 American Society of Clinical Oncology Annual Meeting; June 1–5 2007; Chicago. [Google Scholar]
- 38.McGale P, Darby SC. Commentary: A dose-response relationship for radiation-induced heart disease -C-urrent issues and future prospects. Int J Epidemiol. 2008;37:518–523. doi: 10.1093/ije/dyn067. [DOI] [PubMed] [Google Scholar]
- 39.Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 40.Correa CR, Litt HI, Hwang WT, et al. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol. 2007;25:3031–3037. doi: 10.1200/JCO.2006.08.6595. [DOI] [PubMed] [Google Scholar]
- 41.Goethals I, Dierckx R, De Meerler G, et al. The role of nuclear medicine in the prediction and detection of radiation-associated normal pulmonary and cardiac damage. J Nucl Med. 2003;44:1531–1539. [PubMed] [Google Scholar]
- 42.Marks LB, Xiaoli Y, Prosnitz RG, et al. The incidence and functional consequences of RT associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 43.Prosnitz RG, Hubbs JL, Evans ES, et al. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: Analysis of data 3 to 6 years after treatment. Cancer. 2007;110:1840–1850. doi: 10.1002/cncr.22965. [DOI] [PubMed] [Google Scholar]
- 44.Preston DL, Shimizu Y, Pierce DA, et al. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 45.Yamada M, Wong FL, Fujiwara S, et al. Noncancer disease incidence in atomic bomb survivors, 1958–1998. Radiat Res. 2004;161:622–632. doi: 10.1667/rr3183. [DOI] [PubMed] [Google Scholar]
- 46.Hakoda M, Kasagi F, Kusunoki Y, et al. Levels of antibodies to microorganisms implicated in atherosclerosis and of C-reactive protein among atomic bomb survivors. Radiat Res. 2006;166:360–366. doi: 10.1667/RR3589.1. [DOI] [PubMed] [Google Scholar]
- 47.Wong FL, Yamada M, Sasaki H, et al. Effects of radiation on the longitudinal trends of total serum cholesterol levels in the atomic bomb survivors. Radiat Res. 1999;151:736–746. [PubMed] [Google Scholar]
- 48.Matanoski GM, Sartwell PE, Elliott EA, et al. Cancer risks in radiologists and radiation workers. In: Boice JD, Fraumeni JF, editors. Radiation carcinogenesis: Epidemiology and biological significance. New York: Raven Press; 1984. [Google Scholar]
- 49.Berrington A, Darby SC, Weiss HA, et al. 100 years of observation on British radiologists: Mortality from cancer and other causes 1897–1997. Br J Radiol. 2001;74:507–519. doi: 10.1259/bjr.74.882.740507. [DOI] [PubMed] [Google Scholar]
- 50.Hauptmann M, Mohan AK, Doody MM, et al. Mortality from diseases of the circulatory system in radiologic technologists in the United States. Am J Epidemiol. 2003;157:239–248. doi: 10.1093/aje/kwf189. [DOI] [PubMed] [Google Scholar]
- 51.CARDIORISK -The mechanism of cardiovascular risks after low radiation doses. http://www.cardiorisk.eu/
- 52.Collaborative Group on Observational Studies of Breast Cancer Survivors (COBS) http://www.ctsu.ox.ac.uk/projects/cobs/
- 53.RACE Radiation associated cardiac events. http://www.race.ki.se/
- 54.Taylor CW, Povall JM, McGale P, et al. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys. 2008;72:501–507. doi: 10.1016/j.ijrobp.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 55.Pierce LJ, Butler JB, Martel MK, et al. Post-mastectomy radiotherapy of the chest wall: dosimetric comparison of common techniques. Int J Radiat Oncol Biol Phys. 2002;52:1220–1230. doi: 10.1016/s0360-3016(01)02760-2. [DOI] [PubMed] [Google Scholar]
- 56.Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: Cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]