Abstract
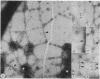
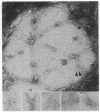
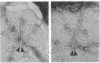
We have obtained clear images of the erythrocyte membrane skeleton from negatively stained preparations that originate directly from the intact cell but in which the spectrin meshwork is artificially spread to allow close inspection. Our procedure requires less than 2 min at 5 degrees C in phosphate buffers. We find 200-nm-long spectrin tetramers crosslinked by junctional complexes. Each junction contains a regular 37-nm rod, probably an actin oligomer of approximately 13 monomers. Densities appear at variable places in the meshwork but distinct globules occur with great frequency 78 nm from the spectrin tetramer's junctional insertion end, very close to the known binding site for ankyrin. Most frequently, five or six spectrin tetramers insert into each junction, producing a meshwork that displays remarkably regular long range order.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V. The molecular basis for membrane - cytoskeleton association in human erythrocytes. J Cell Biochem. 1982;18(1):49–65. doi: 10.1002/jcb.1982.240180106. [DOI] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Cohen C. M. The molecular organization of the red cell membrane skeleton. Semin Hematol. 1983 Jul;20(3):141–158. [PubMed] [Google Scholar]
- Evans E. A., La Celle P. L. Intrinsic material properties of the erythrocyte membrane indicated by mechanical analysis of deformation. Blood. 1975 Jan;45(1):29–43. [PubMed] [Google Scholar]
- Fowler V. M., Bennett V. Erythrocyte membrane tropomyosin. Purification and properties. J Biol Chem. 1984 May 10;259(9):5978–5989. [PubMed] [Google Scholar]
- Fowler V. M., Davis J. Q., Bennett V. Human erythrocyte myosin: identification and purification. J Cell Biol. 1985 Jan;100(1):47–55. doi: 10.1083/jcb.100.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. C., Lin S. High affinity binding of [3H]dihydrocytochalasin B to peripheral membrane proteins related to the control of cell shape in the human red cell. J Biol Chem. 1978 Mar 10;253(5):1415–1419. [PubMed] [Google Scholar]
- Liu S. C., Windisch P., Kim S., Palek J. Oligomeric states of spectrin in normal erythrocyte membranes: biochemical and electron microscopic studies. Cell. 1984 Jun;37(2):587–594. doi: 10.1016/0092-8674(84)90389-1. [DOI] [PubMed] [Google Scholar]
- Lux S. E. Dissecting the red cell membrane skeleton. Nature. 1979 Oct 11;281(5731):426–429. doi: 10.1038/281426a0. [DOI] [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Identification of a factor in conventional muscle actin preparations which inhibits actin filament self-association. Biochem Biophys Res Commun. 1980 Sep 16;96(1):18–27. doi: 10.1016/0006-291x(80)91175-4. [DOI] [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder J. C., Clark S. E., Baines A. J., Morris E., Gratzer W. B. The construction of the red cell cytoskeleton. Prog Clin Biol Res. 1981;55:343–361. [PubMed] [Google Scholar]
- Pinder J. C., Gratzer W. B. Structural and dynamic states of actin in the erythrocyte. J Cell Biol. 1983 Mar;96(3):768–775. doi: 10.1083/jcb.96.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Sawyer D. Triton shells of intact erythrocytes. J Supramol Struct. 1978;8(4):399–412. doi: 10.1002/jss.400080403. [DOI] [PubMed] [Google Scholar]
- Shen B. W., Josephs R., Steck T. L. Ultrastructure of unit fragments of the skeleton of the human erythrocyte membrane. J Cell Biol. 1984 Sep;99(3):810–821. doi: 10.1083/jcb.99.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Siegel D. L., Branton D. Partial purification and characterization of an actin-bundling protein, band 4.9, from human erythrocytes. J Cell Biol. 1985 Mar;100(3):775–785. doi: 10.1083/jcb.100.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Tsukita S., Tsukita S., Ishikawa H. Bidirectional polymerization of G-actin on the human erythrocyte membrane. J Cell Biol. 1984 Mar;98(3):1102–1110. doi: 10.1083/jcb.98.3.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Tsukita S., Ishikawa H. Cytoskeletal network underlying the human erythrocyte membrane. Thin-section electron microscopy. J Cell Biol. 1980 Jun;85(3):567–576. doi: 10.1083/jcb.85.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Hargreaves W. R., Branton D. Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5192–5196. doi: 10.1073/pnas.76.10.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E., Gratzer W. Self-association of human spectrin. A thermodynamic and kinetic study. Eur J Biochem. 1978 Aug 1;88(2):379–385. doi: 10.1111/j.1432-1033.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]
- Wong A. J., Kiehart D. P., Pollard T. D. Myosin from human erythrocytes. J Biol Chem. 1985 Jan 10;260(1):46–49. [PubMed] [Google Scholar]
- Yu J., Fischman D. A., Steck T. L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1(3):233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]