Abstract
Murein polysaccharides may contribute to a considerable part of the dry matter of bacterial cells. Their utilization by protozoa inhabiting the rumen is, however, poorly recognized. The objective of this study was to examine the ability of three species of ciliates, i.e., Eudiplodinium maggii, Diploplastron affine, and Entodinium caudatum of digest, and ferment these saccharides. The cultivation experiments showed that the enrichment of growth medium with bacterial cell wall β-glycans increased the ciliate number (p < 0.05). A statistically significant increase (p < 0.01) was followed by a continuous decrease (p < 0.01) in the percentage of individuals containing β-glycans particles after 4- and 24-h incubation of ciliates with this substrate, respectively. The enzymatic experiments confirmed the ability of the examined protozoa to digest murein. E. caudatum exhibited the highest activity (8.2 unit (U)/mg protein per min), and E. maggii, the lowest (3.0 U/mg protein per min). The production rates of volatile fatty acids by starved and fed ciliate species were 0.7 and 1.6 (E. caudatum) pmol/ciliate cell per h, 30.5 and 42.5 (E. maggii) pmol/ciliate cell per h, and 8.3 and 19.2 (D. affine) pmol/ciliate cell per h (p < 0.05).
Introduction
Protistan bacterivory in marine and freshwater has been studied in extensive ecosystems (Vaqué et al. 1994; Sherr and Sherr 2002). A similar phenomenon has also been observed in the rumen (Coleman 1988). The biocenosis of this ecosystem is composed of numerous microorganisms, the most abundant of with are bacteria followed by ciliate protozoa (Williams and Coleman 1992). The population density of bacteria ranges between 1010 and 1012 (Stewart et al. 1997), whereas that of ciliate protozoa, up to 106 cells per g of rumen content (Jouany and Ushida 1990). Rumen ciliates have been reported to be highly bacterivorous organisms (Coleman 1988). They digest engulfed bacteria and utilize the resultant products to cover their nutritional requirements (Bonhomme 1990). It is commonly accepted that bacteria are the main source of protein for ciliates (Coleman 1988). Comprehensive studies on utilization of the structural polysaccharides of the bacterial cell wall are, however, lacking. These saccharide components of the cell wall peptidoglycan commonly called murein. Gram-positive species contain 20–60 % of murein, whereas the Gram-negative ones only up to 10 % of cell dry matter (Litzinger and Mayer 2010). Chemically, murein consists of the saccharide strands cross-linked by oligopeptides (Ling 1990). Each strand is composed of acetylglucosamine and N-acetylmuramic acid residues linked by β-1,4-glucosidic bounds (Litzinger and Mayer 2010). Two types of enzymes are involved in the hydrolytic degradation of these saccharides. The first is lysozyme, i.e., endo-N-acetyl-muramidase the second β-N-acetylglucosaminidase (Ling 1990). The available data concerning digestion of murein saccharides by rumen ciliates are limited to either a mixed population or to Entodinium caudatum (Morgavi et al. 1996) and Diploplastron affine (Belzecki et al. 2010). Moreover, information on utilization of the degradation products is restricted to D. affine (Belzecki et al. 2010).
Taking the above into account, we undertook the study reported here. Its aim was (1) to examine the influence of murein saccharides on the population density of three common species of rumen ciliates, i.e., E. caudatum, Eudiplodinium maggii, and D. affine grown in vitro; (2) to assess saccharide ingestion by the examined protozoa species; (3) to characterize of the mureinolytic activity of all three ciliate species; and (4) to determine the ability of examined species of ciliates to ferment murein saccharides.
Material and methods
Isolation of rumen ciliates and maintaining stock cultures
The ciliates, E. maggii, D. affine, and E. caudatum, were identified according to Dogiel (1927). The stock cultures of particular ciliate species were initiated by picking 20–30 individuals of typical features and by inoculating them into separate Erlenmeyer flasks (50 mL) containing 40 mL of 2-week-old cultures of ruminal bacteria growing on “caudatum” salt solution (Coleman et al. 1972). The initiated cultures were supplied with a mixture consisting of powdered meadow hay (60 %), wheat gluten (16 %), and barley flour (12 %) microcrystalline cellulose (12 %). The first two components were obtained by grinding: chopped hay and fine barley grits, respectively. Wheat gluten was prepared according to Pace (1955). Microcrystalline cellulose was supplied by Sigma. The protozoa were maintained according to Michalowski et al. (1986) and used to initiate the experiment performed under in vitro conditions. Some stock cultures of each ciliate species were transferred separately to the rumen of three ciliate-free sheep (Michalowski et al. 1999). They were multiplied there and used to perform the enzymatic and fermentation experiments.
Murein saccharide preparation
Murein saccharides were isolated from Micrococcus lysodeikticus ATCC Sigma (M3770) according to Glauner (1988). Briefly, 2 g of lyophilized bacteria was suspended in 50 mL of ice-cold distilled water. The solution was diluted to 1 L with 4 % solution of sodium dodecyl sulfate (SDS) and boiled for 30 min. The cell wall polysaccharides forming small vesicles were separated by centrifugation at 100,000×g for 30 min, and this was followed with washing for several times with distilled water until complete removal of SDS. Finally, the saccharides were collected, lyophilized, and stored at −40 °C.
Effect of murein on ciliate growth in vitro
Ciliates were maintained in culture medium consisting of caudatum solution (see above) and food. The food of the control cultures was composed of powdered meadow hay, wheat gluten, and barley flour (E. caudatum) or of only the first components (E. maggii and D. affine). These components were supplied in proportions of 0.3, 0.08, and 0.06 g/L culture per day, respectively. The same food was supplemented with 0.015, 0.03, 0.06, and 0.12 g of murein saccharides/L culture per day and used to feed the experimental cultures. Three control and three experimental cultures were simultaneously initiated and cultivated for 28 days according to Michalowski et al. (1986). Briefly, they were fed every day and were transferred every 4th day to fresh medium. The samples for counting protozoa were taken on transfer days and were preserved with an equal volume of 4 % formaldehyde solution.
Saccharide ingestion and digestion
The experiment was started after 24-h starvation of six cultures of ciliates maintained on the control diet (see above) for 28 days. On the next day, three of them were incubated further for 24 h without feeding (control), whereas the three remaining (experimental) were fed with purified murein saccharides (0.6 g/L) and incubated as usual. All cultures were sampled for estimation of the proportion of individuals with engulfed polysaccharides just before the start of incubation and at 2, 4, 8, 12, 18, and 24 h thereafter.
Measurement of mureinolytic enzyme activity
Ciliates growing in the rumen of selectively faunated sheep were separated, purified, and incubated with antibiotics according to (Belzecki et al. 2007) and were collected, lyophilized, and stored at −80 °C. The frozen protozoa were thawed and homogenized in a glass homogenizer equipped with a Teflon pestle. The homogenate was centrifuged at 22,000 g for 30 min at 4 °C, and the supernatant was collected and used as a crude enzyme preparation.
The mureinolytic activity of ciliates was assessed by measuring the absorbance after 1-h incubation at 40 °C. The reaction mixture consisted of 0.4 mL of crude enzyme preparation; 0.2 mL of 0.1 mol/L citric phosphate buffer, pH 5; and 0.4 mL of substrate. The substrate was obtain by suspension of lyophilized cells of M. lysodeikticus in water and adjusted to give an initial absorbance of 1 at 570 nm.
The enzyme preparation without substrate and substrate without enzyme preparation were always incubated simultaneously as controls. The optimal condition for murein digestion was estimated by measurement of the degradation rate of the substrate at pH and temperature ranges of 3.0–8.0 and 30–60 °C, respectively. The measurements were performed on three different crude enzyme preparations.
Fermentation of murein saccharides by the examined ciliate species
Ciliates isolated from the particular species purified and incubated overnight with antibiotics (see above) were collected, washed two times with caudatum solution, and suspended in 250 mL of warm (40 °C) solution (Hungate 1942). A new portion of antibiotics was added, and 40 mL samples of well-mixed suspension were transferred into six Erlenmeyer flasks. All six cultures were incubated for 12 h at 40 °C under a continuous flow of CO2 to maintain the anaerobic conditions and to mix the flask content. Three control cultures were not fed, whereas 50 mg of murein saccharides was added to each experimental culture at the start of incubation. The samples for counting the protozoa and measuring the concentration of volatile fatty acids (VFA) were collected just before the start of incubation and at 3, 6, 9, and 12 h of thereafter. The samples for counting protozoa were preserved as above and those for determining VFA by acidification with formic acid (Ziolecki and Kwiatkowska 1973). The experiments were repeated three times on three different days in each protozoa species.
Analytic
The ciliates from the growth experiment were counted under a light microscope according to Michalowski et al. (1986). The proportion of individuals containing cell wall polysaccharides was calculated after counting such individuals among 100 observed ciliates.
Absorbance was measured at 570 nm using a Beckman DU 64 spectrophotometer. Enzyme activity was expressed as a unit of absorbance. One unit (U) was defined as the amount of enzyme responsible for a decrease of absorbance by 0.1 U.
VFA were quantified by gas chromatography, according to Ziolecki and Kwiatkowska (1973) using a Philips PU 4410 gas chromatograph. The protein content in the crude protein preparation was measured with the use of a Microprotein-PR kit (Sigma, 611A).
Statistical analysis
Student’s t test was used to compare mean values, and the correlation coefficient was calculated to determine the relationship between ciliate number and murein saccharide dose in the culture medium. All calculations were made according to Parker (1979).
Results
Effect of murein saccharides on ciliate growth in vitro
The mean results of the growth experiment are presented in Table 1. The E. maggii population was characterized by the lowest density, while that of E. caudatum, by the highest. The concentration of ciliates in control cultures was significantly lower in comparison with cultures fed the diet supplemented with murein saccharides (p < 0.05). The correlation coefficient between the supplement dose and both E. caudatum and D. affine counts was significant at the 1 %, and E. maggii, at the 5 % of probability level.
Table 1.
The concentration of ciliates cultured in vitro on the diets without (A) murein and with murein supplied at a ratio of 0.015 (B) g/L per day, 0.03 (C) g/L per day, 0.06 (D) g/L per day, and 0.12 (E) g/L per day, respectively
| Ciliate species | Diets | SEM | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| Entodinium caudatum | 2,201a | 2,325b | 2,395b | 2,592b | 2,648b | 31.4 |
| Eudiplodinium maggii | 481a | 519b | 580b | 565b | 584b | 11.3 |
| Diploplastron affine | 1,145a | 1,365b | 1,521b | 1,570b | 1,589b | 32.2 |
Values in rows with different superscripts differ significantly (p < 0.05). Mean values (n = 28) are shown
Ingestion of murein by ciliates during 24-h incubation is summarized in Table 2. Only 12–21 % of individuals contained food particles just before feeding (Table 2). The number of such forms increased three- to fivefold during the first 2–4 h after feeding the experimental cultures (p < 0.05) and was followed by continuous decrease up to 52–59 % at the end of the incubation period (p < 0.05). A continuous decrease of such individuals was observed in controls (p < 0.05).
Table 2.
Changes in the proportion of individuals with engulfed food particles (in percent of total number) found during 24-h incubation of protozoa in the absence (control group A) and in the presence of murein saccharides (experimental group B) in the culture medium
| Species | Group | Incubation time | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | 12 | 18 | 24 | SEM | ||
| Entodinium caudatum | A | 13.7ab | 16.3a | 15.7a | 12.3ab | 11.3b | 12.7ab | 8.3c | 2.21 |
| B | 12.0a | 65.3b | 59.7c | 58.3c | 46.0d | 32.0e | 26.0e | 4.23 | |
| Eudiplodinium maggii | A | 19.7a | 17.7a | 15.7a | 17.7a | 15.0a | 13.7ab | 10.3b | 1.95 |
| B | 21.0a | 61.7b | 65.3b | 45.0c | 40.0cd | 36.0cd | 32.0d | 4.23 | |
| Diploplastron affine | A | 16.3a | 15.7ab | 14.7ab | 14.7ab | 14.7b | 12.0ac | 9.7d | 1.74 |
| B | 17.7a | 60.3b | 58.0b | 52.7c | 44.3d | 34.7e | 27.0e | 3.92 | |
Values in rows with different superscripts differ significantly (p < 0.05). Mean values (n = 3) are shown
Enzyme activity
The mean activity of the murein polysaccharide-degrading enzyme(s) of the examined species of protozoa is presented in Table 3. The presented data show that mureinolytic activity depended on the ciliate species. E. caudatum degraded the peptidoglycan over two times and by almost 60 % faster than E. maggii and D. affine, respectively (p < 0.01). E. maggii was also less active than D. affine (p < 0.05). The optimal conditions for enzymatic to degradation of murein were similar.
Table 3.
The peptidoglycan hydrolases activity (in unit/milligram protein per minute) of particular species of ciliates and the optimum pH and temperature (in degree Celsius) for substrate degradation by the examined enzymes
| Ciliate species | Enzyme activity | pH | Temperature |
|---|---|---|---|
| Entodinium caudatum | 8.2 ± 0.71 | 5.0 | 45 |
| Eudiplodinium maggii | 3.0 ± 0.52 | 5.0 | 40 |
| Diploplastron affine | 4.7 ± 1.09 | 4.5 | 40 |
Mean values ± SD (n = 3) are shown
Fermentation of murein saccharides by the examined ciliate species
The ciliates incubated for 12 h with and without murein saccharides released VFA into the medium (Fig. 1). The incubation of E. caudatum, E. maggii, and D. affine with these saccharides resulted in an increase in the VFA concentration by over 33 % (p < 0.05) and 50 and 75 %, respectively (p < 0.01). The increase in the VFA content in control cultures was 15.5, 51, and 27 %, respectively (p < 0.05), and was significantly lower than that in experimental cultures (Table 4). The calculated net production rate of VFA by the ciliates E. caudatum, E. maggii, and D. affine from control cultures was 0.9 ± 0.23, 12 ± 0.54, and 10.9 ± 0.95 pmol/single ciliate per h.
Fig. 1.
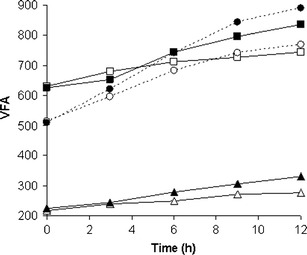
Changes in the concentration of VFA (in millimole per liter) during the incubation of ciliates the E. maggii (filled circle, empty circle), E. caudatum (filled square, empty square) and D. affine (filled triangle, empty triangle) with (black marks) and without (white marks) murein saccharides, respectively. Mean values (n = 3)
Table 4.
The production rate of VFA (in picomole per individual per hour) by three species of rumen ciliates during incubation without murein saccharides (control) and with them (experimental)
| Protozoa | Control | Experimental | SEM |
|---|---|---|---|
| Entodinium caudatum | 0.7a | 1.6b | 0.43 |
| Eudiplodinium maggii | 30.5a | 42.5b | 2.45 |
| Diploplastron affine | 8.3a | 19.2b | 3.64 |
Values in rows marked with different letters differ significantly (p < 0.05). Mean values (n = 3) are shown
Discussion
Murein saccharides stimulated the development of population of E. caudatum, E. maggii, and D. affine population despite their different nutritional preferences. For example, the ciliates E. caudatum use starch to cover their saccharide (Abou Akkada and Howard 1960), and because of this, they were perhaps unable to grow in vitro in the absence of this saccharide. In contrast to E. caudatum, the ciliates E. maggii and D. affine grew well in cultures fed powdered meadow hay and wheat gluten. Similar observations were published earlier by Michalowski et al. (1986, 1991). Thus, the development of their populations in the absence of starch could be the result of the ability of these protozoa to digest and ferment cellulose (Coleman 1985; Michalowski 1997), which is present in hay.
An increase followed by a decrease was observed in the percentage of individuals containing small food particles in the endoplasm when protozoa were incubated for 24 h with murein saccharides. This increase shows that ciliates readily engulfed the particles of murein saccharides added to the growth medium. These findings are in accordance with the earlier observations concerning the engulfment of intact bacteria (Coleman 1988). Moreover, our results suggest that all three species of protozoa digested the engulfed saccharides and subsequently utilized them. This assumption is in line with the results of the enzymatic and fermentation experiments. The first showed that all three ciliate species were able to digest murein with digestion rate varying from 3.2 (E. maggii) to 8.2 (E. caudatum) U/mg protein per min. These findings are comparable with the activity of mixed ciliate fauna (Morgavi et al. 1996). On the other hand, the fermentation experiment showed that incubation of the live protozoa with polysaccharides isolated from murein resulted in a continuous increase in the VFA concentration. Thus, they confirm the ability of the examined species of ciliates to utilize bacterial cell wall saccharides in energy-yielding processes (Czerkawski 1986). To overcome the difference in the number of incubated ciliates, the production rate of VFA per ciliate cell was calculated. The obtained results suggest the existence of a relationship between the cell size of ciliates (Dogiel 1927) and VFA production rate. On the other hand, the examined ciliates differ substantially in cell dry matter (Michalowski 1990), which suggest that production rate of VFA should be expressed per unit dry matter, rather than per cell to compare the fermentation activity of different rumen ciliates.
Finally, the origination of VFA shall be also discussed. As it was given in “Material and methods,” the examined ciliates originated from the rumen of monofaunated sheep. They were separated from food debris and external bacteria by a repeated sedimentation and incubated overnight in culture medium (Hungate 1942) supplemented with streptomycin, ampicillin, and chloramphenicol (50 mg/L). On the next day, the protozoa were separated by the same procedure and suspended in a fresh culture medium containing the same doses of antibiotics. This was done just before the start of experiment. Thus, in our opinion, the measured acids were of ciliates origin. This is because the concentration of bacteria which survived was to low (if any) to affect the fermentation pattern of substrate. It is also noteworthy to emphasize that elimination of E. maggii from the incubated samples resulted in continuous decrease of VFA from 921 to 868 mmol/L. Such an assessment was, however, performed in another experiment, and neither E. caudatum nor D. affine was tested there.
Conclusion
Here, we show that rumen ciliates E. caudatum, E. maggii, and D. affine are able to digest structural saccharides originating from bacterial cell wall and to use the obtained products to cover their requirement for energy. The activity of enzymes involved in the digestion of murein saccharides as well as in the metabolism of the obtained products seems to be species-specific properties.
Acknowledgments
This study was supported by the Ministry of Scientific Research and Information Technology, grant no. 2P06Z05230.
References
- Abou Akkada AR, Howard BH. The biochemistry of rumen protozoa. 3. The carbohydrate metabolism of Entodinium. Biochem J. 1960;76:445–451. doi: 10.1042/bj0760445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzecki G, Newbold CJ, McEwan NR, McIntosh FM, Michalowski T. Characterization of the amylolytic properties of the rumen ciliate protozoan Eudiplodinium maggii. J Anim Feed Sci. 2007;16:590–606. [Google Scholar]
- Belzecki G, Miltko R, Kwiatkowska E, Michalowski T. Mureinolytic ability of the rumen ciliate Diploplastron affine. Folia Microbiol. 2010;55:312–314. doi: 10.1007/s12223-010-0047-0. [DOI] [PubMed] [Google Scholar]
- Bonhomme A. Rumen ciliates: their metabolism and relationships with bacteria and their hosts. Anim Feed Sci Technol. 1990;30:203–266. doi: 10.1016/0377-8401(90)90016-2. [DOI] [Google Scholar]
- Coleman GS. The cellulase contents of 15 species of entodiniomorphid protozoa, mixed bacteria and plant derbies isolated from the ovine rumen. J Agric Sci. 1985;104:349–360. doi: 10.1017/S0021859600044038. [DOI] [Google Scholar]
- Coleman GS. Protozoal–bacterial interaction in the rumen. In: Nolan JV, Leng RA, Demeyer DI, editors. The roles of protozoa and fungi in ruminant digestion. 1. Armidale: Penambul Books; 1988. pp. 13–26. [Google Scholar]
- Coleman GS, Davies JI, Cash MA. The cultivation of rumen ciliates Epidinium ecaudatum caudatum and Polyplastron multivesiculatum in vitro. J Gen Microbiol. 1972;73:509–521. doi: 10.1099/00221287-73-3-509. [DOI] [PubMed] [Google Scholar]
- Czerkawski JW. An introduction to rumen studies. New York: Pergamon; 1986. [Google Scholar]
- Dogiel VA. Monographie der Familie Ophryoscolecidae. Arch Protistenk. 1927;59:1–288. [Google Scholar]
- Glauner B. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal Biochem. 1988;172:451–64. doi: 10.1016/0003-2697(88)90468-X. [DOI] [PubMed] [Google Scholar]
- Hungate RE. The culture of Eudiplodinium neglectum, with experiments on the digestion of cellulose. Biol Bull. 1942;83:303–319. doi: 10.2307/1538229. [DOI] [Google Scholar]
- Jouany JP, Ushida K. Protozoa and fiber digestion in the rumen. In: Hoshimo S, Onodera R, Minato H, Itabashi H, editors. The rumen ecosystem: the microbial metabolism and its regulation. Tokyo: Japan Scientific Societies Press; 1990. pp. 139–151. [Google Scholar]
- Ling JR. Digestion of bacterial cell walls in the rumen. In: Hoshino S, Onodera R, Minato H, Itabashi H, editors. The rumen ecosystem: the microbial metabolism and its regulation. Tokyo: Japan Scientific Societies Press; 1990. pp. 83–90. [Google Scholar]
- Litzinger S, Mayer C. The murein sacculus. In: König H, Claus H, Varma A, editors. Prokaryotic cell wall compounds: structure and biochemistry. Berlin: Springer; 2010. pp. 3–52. [Google Scholar]
- Michalowski T. The synthesis and turnover of the cellular mater of ciliates in the rumen. Acta Protozool. 1990;29:47–72. [Google Scholar]
- Michalowski T. Digestion and fermentation of the microcrystalline cellulose by the rumen ciliate protozoon Eudiplodinium maggii. Acta Protozool. 1997;36:181–185. [Google Scholar]
- Michalowski T, Szczepkowski P, Muszynski P. The nutritive factors affecting the growth of the rumen ciliate Diploplastron affine in vitro. Acta Protozool. 1986;25:419–426. [Google Scholar]
- Michalowski T, Muszynski P, Landa L. Factor influencing the growth on rumen ciliates Eudiplodinium maggii in vitro. Acta Protozool. 1991;30:115–120. [Google Scholar]
- Michalowski T, Harmeyer J, Belzecki G. The importance of washing the omasum for successful defaunation of sheep. J Anim Feed Sci. 1999;8:611–619. [Google Scholar]
- Morgavi DP, Sakurada M, Tomita Y, Onodera R. Electrophoretic forms of chitinolytic and lysozyme activities in ruminal protozoa. Curr Microbiol. 1996;32:115–118. doi: 10.1007/s002849900020. [DOI] [PubMed] [Google Scholar]
- Pace J. Seed proteins. In: Peach K, Tracey MV, editors. Modern methods of plant analysis. Berlin: Springer; 1955. pp. 69–105. [Google Scholar]
- Parker RE. Introductory statistics for biology. Southampton: The Camelot Press Ltd; 1979. [Google Scholar]
- Sherr EB, Sherr BF. Significance of predation by protists in aquatic microbial food webs. Anton Leeuw Int J G. 2002;81:293–308. doi: 10.1023/A:1020591307260. [DOI] [PubMed] [Google Scholar]
- Stewart CS, Flint HJ, Bryant MP. The rumen bacteria. In: Hobson PJ, Stewart CS, editors. The rumen microbial ecosystem. 2. Madras: Blackie Academic & Professional; 1997. pp. 10–72. [Google Scholar]
- Vaqué D, Gasol JM, Marrasé C. Grazing rates on bacteria: the significance of methodology and ecological factors. Mar Ecol Prog Ser. 1994;109:263–274. doi: 10.3354/meps109263. [DOI] [Google Scholar]
- Williams AG, Coleman GS. The rumen protozoa. Berlin: Springer; 1992. [Google Scholar]
- Ziolecki A, Kwiatkowska E. Gas chromatography of C1 to C5 fatty acids in rumen fluid and fermentation media. J Chromatogr. 1973;80:250–254. doi: 10.1016/S0021-9673(01)85338-3. [DOI] [PubMed] [Google Scholar]


