Abstract
Age-related hearing loss (AHL) is a multifactorial disorder characterized by a decline in peripheral and central auditory function. Here, we examined synaptic transmission in DBA/2 mice, which carry the AHL8 gene, at the identifiable glutamatergic synapse in the medial nucleus of the trapezoid body (MNTB), a nucleus in the superior olivary complex critical for acoustic timing. Mice exhibited raised auditory brainstem thresholds by P14, soon after hearing onset. Excitatory postsynaptic currents were prolonged, however, postsynaptic excitability was normal. By P18, high-frequency hearing loss was evident. Coincident with the onset of hearing loss, MNTB principal neurons displayed changes in intrinsic firing properties. These results suggest that changes in transmission in the superior olivary complex are associated with early onset hearing loss.
Keywords: MNTB, auditory system, AHL, DBA/2, auditory brainstem responses
1. Introduction
Genetic animal models of hearing loss arising from congenital or early onset deafness exhibit pathophysiology in the auditory brainstem. In the deaf mouse strain (dn/dn), synaptic currents at end-bulb synapses in the anterior ventral cochlear nucleus are enhanced, and high frequency stimulation evokes strong synaptic depression [15]. In congenitally deaf cats, nerve terminals at end-bulb synapses are more compact with fewer synaptic vesicles [16].
The DBA/2 mouse which expresses the AHL8 (age-related hearing loss 8) mutation [6, 14] is a frequently studied model of hearing loss. Hearing loss, measured with tones up to 32 kHz, begins around P21, and progresses rapidly to an almost complete loss by P45 [23]. In the ventral cochlear nucleus, glutamatergic transmission is compromised following complete deafness at >P60, however, transmission is normal during the second postnatal week, close to hearing onset [21].
We examined synaptic transmission in the medial nucleus of the trapezoid body (MNTB), a component of the superior olivary complex, in the DBA/2 mouse. The glutamatergic MNTB synapse is made between afferents from the cochlear nucleus and MNTB principal neurons [12]. Auditory brainstem thresholds increased soon after hearing onset, by P13, with early high-frequency hearing loss at > 48kHz, by P16. Prolonged synaptic responses and loss of onset spiking in MNTB principal neurons indicated a predisposition to abnormal transmission.
2. Methods
Animal husbandry and experimental procedures were approved by the Institutional Animal Care and Use Committees at Northeast Ohio Medical University and were performed in accordance with guidelines published by the U.S. National Institutes of Health.
2.1 Slice preparation
We compared activity in the MNTB of DBA/2 mice with control CBA or CBA/Ca mice. 9–25 day old pups were anesthetized with isoflurane, the brain removed, and 200 μm thick transverse slices made through the MNTB. Slices were cut, stored and recorded from at 35°C [17] in artificial cerebrospinal fluid (ACSF) containing (in mM): 120 NaCl, 3 KCl, 2 CaCl2, 1.3 MgSO4, 1 NaH2PO4, 10 NaHCO3, 25 glucose, pH 7.35. Whole-cell patch clamp recordings were made under visual control using Nomarski optics and a 40x objective fitted to an upright Zeiss Axioskop. Trapezoid fibers were stimulated extracellularly with a bipolar tungsten electrode and recordings made under current- and voltage-clamp from the soma of contralateral MNTB principal neurons. Principal neurons were identified visually and by the presence of a pre-spike that preceded the EPSC.
Patch pipettes (4–5 MΩ contained (in mM): 120 K Gluconate, 10 KCl, 5 NaCl, 0.2 EGTA, 0.1 CaCl2, 0.3 Na-GTP, 2 Mg-ATP, 10 HEPES, pH 7.35; ~110 nM free Ca++. A junction potential correction of −11 mV was applied to all recordings. Series resistances were ≤ 9 MΩ and compensated by >80% (10 μs lag). EPSC decay time constants in control CBA MNTB neurons were similar in recordings with 6 MΩ and 9 MΩ series resistances (t15 = 0.34; p = 0.74). Average series resistances in the population were similar for CBA and DBA/2 MNTB neuron recordings (6.7 ± 2.2 and 6.5 ± 2.5 MΩ respectively). Data from CBA and CBA/Ca mice were not statistically different and were pooled into one group. Data are reported from 138 neurons; 24 from 18 CBA mice, 36 from 12 CBA/Ca mice, and 62 from 28 DBA/2 mice.
Data collection and analyses were carried out with an EPC-10 amplifier (HEKA Elektroniks/Instrutech Corporation), Patchmaster/Fitmaster (HEKA Electroniks) and Minianalysis (Synaptosoft, Decatur, GA) software. Data were filtered at 5 kHz during acquisition. Chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.2 Analyses of synaptic responses and firing patterns
Synaptic currents were averaged in each slice over 6–10 trials at 1/5s, then averaged across slices. PSC decay time constants (τ) were weighted from double exponential fits: I (t) = Ife−t/τ(f) + Is e−t/τ(s), where If and Is are the peak currents of the fast and slow components of the PSC. The weighted mean of the fast and slow decay time constants, τ(f) and τ(s), were calculated from the equation, τ = τf{If/(If + Is)} + τs {Is/(If + Is)}. Most DBA/2 PSCs were best fit by a single exponential function I(t) = Imax e−t/τ, where Imax is the peak PSC current. In DBA/2 mice at ages <P13, decay times were fit by two exponents; thus weighted means are reported. For each EPSC, goodness of fit, calculated using Origin software, was determined from the r2 value for the first and second time constant. Decay time constants with r2 > 0.98 are reported.
Miniature synaptic currents were identified using threshold detection and analyzed with Minianalysis software (Synaptosoft). Origin software was used to construct amplitude distributions, perform Gaussian fitting and determine means and standard deviations. To determine the threshold for intrinsic MNTB firing, injected currents were altered in 5 pA increments. Threshold voltage was defined as the voltage just prior to the voltage that generated the first full spike and was identified by a small spikelet at the onset. The rate of rise of the membrane potential to threshold was measured at the voltage that evoked the first full spike. Spike widths of intrinsically evoked spikes were measured at half maximum spike height, from baseline to peak.
Results are expressed as mean ± standard error of the mean. Standard deviation, when used, is indicated in the text. Significance was determined using paired t-test or ANOVA; p < 0.05 was used as a criterion for significance and the Bonferroni correction factor applied. Normality was confirmed before statistical testing.
2.3 Measurement of auditory brainstem responses (ABRs)
Mice were anesthetized IM with ketamine (20–40 mg/kg) and xylazine (2.5 mg/kg) and monitored for anesthetic level. ABRs were recorded with two sterile tungsten wires (0.005 inches in diameter, etched to ~0.001 inch in diameter) inserted under the skin, behind an ear, and on top of the head.
Custom software (Batlab; Dr. D. Gans, NEOMED) was used to generate tone bursts and acquire ABRs. Sound was delivered through a loudspeaker placed 10 cm in front of the animal at 15° to the midline. Acoustic stimuli were digitally synthesized and downloaded onto a digital signal processing card, converted to analog signals, filtered, attenuated, summed, amplified, and sent to a loudspeaker (Tucker-Davis Technologies). Acoustic output was calibrated over 10 – 120 kHz with a condenser microphone placed in a position normally occupied by the animal's head. 0 dB attenuation at 4 kHz corresponded to 109 dB SPL, at 40 kHz, to101 dB SPL, at 50 kHz, to 93 dB SPL and at 80 kHz, to 69 dB SPL. We used a maximum tone frequency of 64 kHz. ABRs were recorded in a sound-proof chamber (Gretchken Industries) lined with foam. Sound pressure levels were corrected for speaker drop-off. Harmonic distortion was not detectable 60 dB below the signal intensity using a fast Fourier analysis of the digitized microphone signal.
ABRs were measured using 10–96 dB SPL tones in 5 or 10 dB steps. Tones were 5 ms long with a 0.5 ms cosine rise and fall. Inter-tone intervals were 15 ms. Tone frequencies were sequentially delivered from 4–64 kHz and repeated 300 times at 4/s at each sound level. If the same pup was used more than once, we allowed 4–5 days between recordings. During each recording session, ABR protocols were repeated at least twice. Responses were identified as ABRs if they were ≥ four times the standard deviation of baseline noise.
3. Results
3.1 Developmental changes in DBA/2 auditory brainstem responses
The DBA/2 mouse exhibits pronounced hearing loss at >P60 [8, 22]. Hearing thresholds increase at ~ P25 with a loss of response at 32 kHz [23]. To determine if hearing loss began earlier, we recorded ABRs over a wider frequency range (4–64 kHz) each day between P9–P30 (3 mice at each postnatal age).
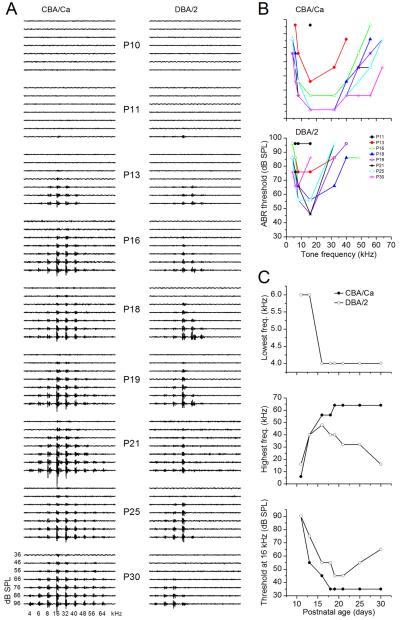
In both CBA/Ca and DBA/2 mice, hearing onset occurred at P11, with an ABR at 16 kHz at 96 dB SPL (Fig. 1). The frequency range of CBA/Ca ABRs increased with age. Responses to 4 kHz first occurred at P16 and were maintained until P30. Responses to 64 kHz were observed by P19, and the 4–64 kHz range was maintained until P30 (Fig. 1B, top; Fig. 1C; top, middle). ABR thresholds decreased with age, reaching the lowest values (e.g. 36 dB SPL to the 16 kHz tone) at P18, and remained low until P30 (Fig. 1C, bottom panel).
Figure 1. DBA/2 mice exhibit early onset, high-frequency hearing loss.
(A) Auditory brainstem responses (ABRs) at postnatal ages indicated. A lack of the response at 56 kHz is apparent at P16, even at 96 dB SPL. (B) ABR thresholds plotted as a function of postnatal age indicate early onset high-frequency hearing loss. Data are from 3 mice at each postnatal age. (C) Lowest (top) and highest (middle) frequencies observed at any sound level, and ABR threshold at 16 kHz (bottom), plotted as a function of postnatal age.
High-frequency hearing loss occurred early in the DBA/2 mouse. Between P11–P13, ABRs were evident between 4–40 or 48 kHz. The frequency range dropped with age, and the 16 kHz tone was the highest frequency that evoked an ABR at P30 (Fig. 1C, top panel). Responses at 4 kHz were normal until P30 (Fig. 1C, middle panel). By P16, the frequency range dropped, and responses to 56 kHz and higher frequencies were absent (Fig. 1C, middle panel).
ABR thresholds increased soon after hearing onset (P11). At P11, the threshold for the 16 kHz response, 96 dB SPL, was similar to control animals. Beginning at P13, ABR thresholds increased. The threshold for the 16 kHz tone, for example, was raised by 20 dB at P13 (Fig. 1B, C; bottom panels; t5 = 6.06; p = 0.0018). Thresholds decreased up to P21, but to a lesser extent than in the CBA/Ca mouse (Fig. 1C, bottom panel). At ages >P21, thresholds increased again (e.g. the threshold for the16 kHz tone at P30 was ~30 dB higher than in the CBA mouse).
3.2 Synaptic transmission is prolonged in the DBA/2 MNTB
To examine changes in glutamatergic synaptic transmission in the MNTB, we measured the kinetics of excitatory postsynaptic currents (EPSCs) evoked by stimulating contralateral trapezoid fibers [1]. Membrane potentials were held at −65 mV, close to the average resting membrane potential in control and DBA/2 mice (−66 ± 6 mV; 62 neurons; t61 = 0.93; p = 0.36; P11–P25). Control and DBA/2 EPSC amplitudes were distributed similarly between P11–25, with most EPSCs between 4–5 nA, as previously reported in the normal MNTB[7, 18] (Mean and SD: CBA, 4.004 nA ± 1.231 nA. DBA/2, 3.52 ± 1.5 nA.; t114 = 1.22; p = 0.22) (Fig. 2A).
Figure 2. Synaptic currents in the DBA/2 MNTB decay slowly.
(A) EPSC amplitudes. Left, middle: Amplitude distributions. P11–25. 57 CBA (24 CBA, 33 CBA/Ca) and 58 DBA/2 synapses. Right: Mean EPSC amplitudes. Errors are SD. (B) EPSC decay time constants. Raw traces from a CBA (left), DBA/2 (middle) and superimposed (right). Asterisk (left trace): prolonged second component. Time constants are indicated by each EPSC. Insets: exponential fits (red dotted lines) of the EPSC decay phase (black solid lines). Right: EPSC decay time constants. τf: fast, τs: slow, and τω: weighted, time constants. τ: time constant from a single exponential fit. 21 CBA (15 CBA, 6 CBA/Ca) and 36 DBA/2 synapses between P12–25. Mean and SD. (C) Left: Developmental changes in EPSC amplitudes at indicated postnatal ages. Right: EPSC amplitudes as a function of postnatal age. 24 CBA and 24 DBA/2 synapses. Sigmoidal fits r2: CBA, 0.87536; DBA/2, 0.91565. (D) EPSC kinetics. Rise times were measured between 10–90% of the peak amplitude. Decay time constants are weighted means of the first and second components. Mean and SD. 24 synapses in each mouse strain. (E) Left: MNTB neuron responses to stimulation of trapezoid fibers. DBA/2: 6 trials from the same synapse are superimposed. Right: postnatal distribution of depolarizing after potentials. Ordinate: fraction of the total synapses recorded in each age group. P9–11: 13 CBA/Ca, 11 DBA/2 synapses; P14–25: 24 CBA/Ca, 19 DBA/2 synapses.
To examine EPSC decay times, we used EPSCs with peak amplitudes within one standard deviation of the average amplitude in data pooled from CBA/Ca and DBA/2 mice (3.86 ± 0.74 nA; 21 CBA/Ca, 36 DBA/2 synapses; P12–25). CBA/Ca EPSCs displayed a double-exponential decay with a characteristic rapid first component and slower second component [7, 18] (Fig. 2B; τ1 = 0.63 ± 0.15 ms; τ2 = 42 ± 12 ms; 21 synapses in 14 mice). DBA/2 EPSCs lacked both the fast and slow decay time constants. EPSC decays were best fit with a single exponent with a time constant of 5.46 ±1.45 ms (36 synapses, 19 mice), which differed from the fast (t56 = 3.07; p = 0.003) or slow (t56 = 3.72; p = 0.0004) decay phases of CBA/Ca EPSCs. A few DBA/2 EPSCs included a second, slower phase to their decay (τ = 9 ± 4 ms; 6 synapses). Weighted averages of DBA/2 EPSC decay time constants (τω = 5.68 ± 1.19 ms) did not differ from single exponential fits (p = 0.51). At holding potentials similar to those used in our recordings (−65 mV), the decay of MNTB EPSCs is slightly shaped by the activation of NMDA receptors [7, 18]. We examined the effect of the NMDAR antagonist APV on CBA and DBA/2 EPSCs. DBA/2 and CBA EPSCs both exhibited a strong NMDAR-mediated component between P9–11, but at later postnatal ages, DBA/2 EPSCs were less susceptible to APV than CBA EPSCs (Supplementary Figure 1), suggesting that the prolonged decay of DBA/2 EPSCs did not arise from an increased NMDAR contribution. Spontaneous miniature synaptic currents recorded in 1 μM tetrodotoxin, at holding potentials similar to those at which EPSCs were recorded, had normal amplitudes and frequencies in the DBA/2 MNTB (Supplementary Figure 2) suggesting that mechanisms underlying spontaneous release were normal.
We grouped EPSCs into age ranges, P9–11, 13–16 and 18–25, correlating approximately with pre-hearing, increased ABR thresholds, and onset of high-frequency hearing loss. EPSC rise times changed similarly with postnatal age in control and DBA/2 mice (Fig. 2C; 2D, left, top panel; p = 0.24). DBA/2 EPSC decay times slowed by ~4.4 ms between P13–25 (Fig. 2D, left, bottom panel; p<0.05), in contrast to CBA EPSCs, which decayed faster (Fig. 2D, right panel).
Synaptically-evoked spikes at CBA synapses had after-hyperpolarizations but DBA/2 spikes exhibited prolonged depolarizing after-potentials (30 ± 8 ms; 24 synapses; measured from the stimulus artifact) (Fig. 2E; traces). At early ages (P9–11) control (n = 8/8) and DBA/2 (n = 7/7) synapses had depolarizing after-potentials, however the majority of DBA/2 synapses (88%) continued to express depolarizing after-potentials until P25 (Fig. 2E, right panel), compared with only 8% of CBA synapses.
3.3 Delayed increases in intrinsic excitability of DBA/2 MNTB principal neurons
End-bulb synapses in the auditory system preserve temporal integrity through a postsynaptic cell that fires only at the onset of a stimulus [4, 17]. To determine whether principal MNTB neurons retained their capacity for onset spiking, we examined intrinsic membrane properties. Input resistances, measured at steady state using 300 ms current steps, and time constants, measured with 5–7 mV hyperpolarizations, were similar in CBA and DBA/2 MNTB neurons between P13–P19 (Input resistances: CBA 156 ± 25 MΩ, DBA/2 163 ± 31 MΩ; n = 8 cells each; t15 = 0.5, p = 0.62; membrane time constants: CBA 1.9 ± 0.6 ms, DBA/2 1.67 ± 0.53 ms; n = 8 cells each; t15 = 0.79, p = 0.44). Between P21–P25, DBA/2 neurons had slightly higher input resistances (CBA 139 ± 22 MΩ, DBA/2 205 ± 37 MΩ; n = 6 cells each; t11 = 2.83, p = 0.016) and slower membrane time constants (CBA 1.77 ± 0.5 ms, DBA/2 2.86 ± 0.37 ms; n = 6 cells each; t11 = 3.03, p = 0.011).
Control MNTB neurons responded to depolarizing current injection with a single onset spike at most current strengths (Fig. 3A) up to P25 (31 neurons). Before P19 DBA/2 neurons exhibited well-locked responses to current injection, with one or two onset spikes (Fig. 3B, first column; n=11; second column; n=16). After P20, current steps evoked multiple spikes (Fig. 3B, third column; n = 9). In addition to a short burst of 5–10 spikes following current onset, other spikes occurred randomly during the depolarization (~1–4 spikes/1000 ms). By P25, bursts disappeared; instead an onset spike was followed by broad secondary spikes. Secondary spikes rode on a rising depolarization (e.g. Fig. 3B, fourth column, bars, top and second traces), which contrasted with the steady depolarization during the current step at earlier postnatal ages (Fig. 3B, e.g. first and second columns). Secondary spikes were abolished in reduced external calcium (Fig. 3B, third column; 3rd trace from top), suggesting a calcium-dependence.
Figure 3. Changes in threshold and onset firing in DBA/2 MNTB principal neurons.
(A) CBA MNTB responses to somatic current pulses. Bottom to top: increasing current strengths. (B) DBA/2 MNTB firing patterns. Fourth column: horizontal bars: rising depolarization. Red trace: 0.2 mM external calcium. Dotted trace: expanded view of the onset spike. Arrow, bottom trace: slow rise of membrane potential. (C) Left: expanded traces of onset spikes at just supra-threshold currents. 50 ms scale applies only to the bottom DBA/2 trace. Right: intrinsic membrane parameters as a function of postnatal age. Inset: expanded ordinate to show the decrease in CBA spike width after P22. 5–7 cells at each age for each mouse strain. Mean and s.e.m.
Figure 3C illustrates onset spikes at just supra-threshold currents at four postnatal ages. The threshold voltage in DBA/2 mice increased greatly after P19 (Fig. 3C, right column, top panel) and the rate of rise of the membrane potential towards threshold slowed (Fig. 3C, right column, second panel). Spike width and onset latency (~108 ± 25.4 ms between P22–25) increased (Fig. 3C, right column). In contrast, thresholds for CBA onset spikes were high at P12, remained high until P19, then decreased (p<0.05). After P19, the membrane potential reached threshold faster and spikes were narrower (p< 0.05). Onset latencies remained unchanged (Anova; p = 0.62).
4. Discussion
We demonstrate early loss of normal synaptic transmission in an auditory nucleus critical for preserving acoustic timing in a mouse model of hearing loss. Hearing thresholds increased by P13 and, by P16, responses > 48kHz were absent. Synaptic currents became prolonged soon after hearing onset, while postsynaptic excitability became abnormal when hearing loss was pronounced.
mEPSCs have similar decay times in control and DBA/2 mice, whereas evoked EPSCs decay slower in DBA/2 mice, suggesting sincreased asynchronous release. We cannot, however, rule out a down-regulation of GluR3/4 subunits correlating with the onset of AHL. Larger than normal EPSCs have been reported in the auditory cortex [9, 19], inferior colliculus [20] and cochlear nucleus [15] of other hearing loss models and may serve to alter the excitatory-inhibitory balance in upstream processing of auditory information.
Higher intrinsic firing thresholds such as we report in the third postnatal week occur in older DBA/2 mice that are completely deaf [21]. The slowly building depolarization in MNTB neurons during a current step suggests stunted development of potassium currents as reported in inner hair cells in AHL-prone rodents [3] or in the MNTB of congenitally deaf mice [10]. Like other murine models with hearing loss, DBA/2 hearing loss genes have the same or neighbouring loci as genes for audiogenic seizure susceptibility [6, 14]. The increases in threshold and latency we observe in the DBA/2 MNTB are not observed in the MNTB of mice that become seizure susceptible following knockout of the Kv1.2 subunit [2], however, and an increased probability of transmitter release commonly associated with seizure susceptibility [5] also does not occur in DBA/2 MNTB. This suggests that down-regulation of potassium currents might be associated with high-frequency hearing loss rather than the seizure phenotype. Calcium channel blockers have been reported to delay AHL [11]and may act by preventing abnormal calcium-mediated spikes such as we report. Decreased calcium buffering capacity reported in mouse models of hearing loss [13] may provide one avenue for generating calcium spikes.
Supplementary Material
Highlights
-
◯
Synaptic transmission was examined in the auditory brainstem of DBA/2 mice with early onset hearing loss.
-
◯
Mice exhibited very early loss of high-frequency hearing and raised hearing thresholds.
-
◯
With the onset of hearing loss, postsynaptic currents and spikes were abnormally prolonged.
-
◯
Potassium currents gradually declined over the course of high-frequency hearing loss.
Acknowledgements
Supported by NIH R01 DC008120 (SS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Barnes-Davies M, Forsythe ID. Pre- and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brainstem slices. The Journal of physiology. 1995;488(Pt 2):387–406. doi: 10.1113/jphysiol.1995.sp020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brew HM, Gittelman JX, Silverstein RS, Hanks TD, Demas VP, Robinson LC, Robbins CA, McKee-Johnson J, Chiu SY, Messing A, Tempel BL. Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. Journal of neurophysiology. 2007;98:1501–1525. doi: 10.1152/jn.00640.2006. [DOI] [PubMed] [Google Scholar]
- [3].Charizopoulou N, Lelli A, Schraders M, Ray K, Hildebrand MS, Ramesh A, Srisailapathy CR, Oostrik J, Admiraal RJ, Neely HR, Latoche JR, Smith RJ, Northup JK, Kremer H, Holt JR, Noben-Trauth K. Gipc3 mutations associated with audiogenic seizures and sensorineural hearing loss in mouse and human. Nature communications. 2011;2:201. doi: 10.1038/ncomms1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Forsythe ID, Barnes-Davies M. The binaural auditory pathway: membrane currents limiting multiple action potential generation in the rat medial nucleus of the trapezoid body. Proceedings. 1993;251:143–150. doi: 10.1098/rspb.1993.0021. [DOI] [PubMed] [Google Scholar]
- [5].Janz R, Goda Y, Geppert M, Missler M, Sudhof TC. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron. 1999;24:1003–1016. doi: 10.1016/s0896-6273(00)81046-6. [DOI] [PubMed] [Google Scholar]
- [6].Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- [7].Joshi I, Wang LY. Developmental profiles of glutamate receptors and synaptic transmission at a single synapse in the mouse auditory brainstem. The Journal of physiology. 2002;540:861–873. doi: 10.1113/jphysiol.2001.013506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Klein BD, Fu YH, Ptacek LJ, White HS. c-Fos immunohistochemical mapping of the audiogenic seizure network and tonotopic neuronal hyperexcitability in the inferior colliculus of the Frings mouse. Epilepsy research. 2004;62:13–25. doi: 10.1016/j.eplepsyres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- [9].Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leao RN, Berntson A, Forsythe ID, Walmsley B. Reduced low-voltage activated K+ conductances and enhanced central excitability in a congenitally deaf (dn/dn) mouse. The Journal of physiology. 2004;559:25–33. doi: 10.1113/jphysiol.2004.067421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lei D, Gao X, Perez P, Ohlemiller KK, Chen CC, Campbell KP, Hood AY, Bao J. Anti-epileptic drugs delay age-related loss of spiral ganglion neurons via T-type calcium channel. Hearing research. 2011;278:106–112. doi: 10.1016/j.heares.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lenn NJ, Reese TS. The fine structure of nerve endings in the nucleus of the trapezoid body and the ventral cochlear nucleus. The American journal of anatomy. 1966;118:375–389. doi: 10.1002/aja.1001180205. [DOI] [PubMed] [Google Scholar]
- [13].Martin del Campo HN, Measor KR, Razak KA. Parvalbumin immunoreactivity in the auditory cortex of a mouse model of presbycusis. Hearing research. 2012;294:31–39. doi: 10.1016/j.heares.2012.08.017. [DOI] [PubMed] [Google Scholar]
- [14].Noben-Trauth K, Zheng QY, Johnson KR, Nishina PM. mdfw: a deafness susceptibility locus that interacts with deaf waddler (dfw) Genomics. 1997;44:266–272. doi: 10.1006/geno.1997.4869. [DOI] [PubMed] [Google Scholar]
- [15].Oleskevich S, Walmsley B. Synaptic transmission in the auditory brainstem of normal and congenitally deaf mice. The Journal of physiology. 2002;540:447–455. doi: 10.1113/jphysiol.2001.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ryugo DK, Pongstaporn T, Huchton DM, Niparko JK. Ultrastructural analysis of primary endings in deaf white cats: morphologic alterations in endbulbs of Held. The Journal of comparative neurology. 1997;385:230–244. doi: 10.1002/(sici)1096-9861(19970825)385:2<230::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- [17].Sivaramakrishnan S, Laurent G. Pharmacological characterization of presynaptic calcium currents underlying glutamatergic transmission in the avian auditory brainstem. J Neurosci. 1995;15:6576–6585. doi: 10.1523/JNEUROSCI.15-10-06576.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Steinert JR, Postlethwaite M, Jordan MD, Chernova T, Robinson SW, Forsythe ID. NMDAR-mediated EPSCs are maintained and accelerate in time course during maturation of mouse and rat auditory brainstem in vitro. The Journal of physiology. 2010;588:447–463. doi: 10.1113/jphysiol.2009.184317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Takesian AE, Kotak VC, Sharma N, Sanes DH. Hearing loss differentially affects thalamic drive to two cortical interneuron subtypes. Journal of neurophysiology. 2013;110:999–1008. doi: 10.1152/jn.00182.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. The European journal of neuroscience. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- [21].Wang Y, Manis PB. Temporal coding by cochlear nucleus bushy cells in DBA/2J mice with early onset hearing loss. J Assoc Res Otolaryngol. 2006;7:412–424. doi: 10.1007/s10162-006-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zheng QY, Johnson KR. Hearing loss associated with the modifier of deaf waddler (mdfw) locus corresponds with age-related hearing loss in 12 inbred strains of mice. Hearing research. 2001;154:45–53. doi: 10.1016/s0378-5955(01)00215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hearing research. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.