Summary
The active zone protein RIM1α is required both for maintaining normal probability of neurotransmitter release and for long-term presynaptic potentiation at brain synapses. We now demonstrate that RIM1α−/− mice exhibit normal coordination and anxiety-related behaviors but display severely impaired learning and memory. Mice with a synaptotagmin 1 mutation, which selectively lowers release probability, and mice with Rab3A deletion, which selectively abolishes presynaptic long-term potentiation, do not exhibit this abnormality. Our data suggest that a decrease in release probability or a loss of presynaptic LTP alone is not sufficient to cause major behavioral alterations, but the combination of presynaptic abnormalities in RIM1α−/− mice severely alters learning and memory.
Introduction
Presynaptic proteins mediate several forms of short-and long-term synaptic plasticity in the mammalian brain (Brose et al., 2000; Dobrunz and Garner, 2002; Fernandez-Chacon and Sudhof, 1999; Lonart, 2002). However, in contrast to the extensive evidence for a role of post-synaptic proteins and postsynaptically expressed forms of synaptic plasticity in learning and memory (Matynia et al., 2002; Mayford and Kandel, 1999; Tonegawa et al., 2003), relatively little attention has been given to the influence of presynaptic proteins and presynaptic plasticity in cognitive function. We have therefore begun a systematic approach to better understand the role of presynaptic mechanisms in complex behavior.
RIM1α is an active zone protein involved in several aspects of presynaptic function (Castillo et al., 2002; Schoch et al., 2002). RIM1α−/− mice are deficient in a presynaptically expressed form of LTP (long-term potentiation) at the mossy fiber to CA3 pyramidal neuron synapse (mfLTP) in the hippocampus and at the parallel fiber/Purkinje cell synapse in the cerebellum (Castillo et al., 2002; Lonart et al., 2003). Additionally, short-term plasticity is altered in RIM1α−/− mice, which exhibit increased paired pulse facilitation and posttetanic potentiation in area CA1 (Schoch et al., 2002). RIM1α−/− mice also display a ~50% reduction in probability of evoked neurotransmitter release (Pr) (Schoch et al., 2002). In spite of these presynaptic abnormalities, LTP at the Schaffer collateral to CA1 pyramidal neuron synapse remains unchanged in RIM1α−/− mice (Schoch et al., 2002).
Rab3A is a small GTP binding protein associated with synaptic vesicles. Rab3A interacts with RIM1α and is one of several potential RIM1α effector molecules (Fernandez-Chacon and Sudhof, 1999). Like RIM1α−/− mice, Rab3A−/− mice are deficient in mfLTP (Castillo et al., 1997). mfLTP requires protein kinase A (PKA) activity and is thought to be expressed presynaptically as a lasting increase in evoked glutamate release (Huang et al., 1995; Nicoll and Malenka, 1995; Weisskopf and Nicoll, 1995), although the requirements for mfLTP induction are still debated (Mellor and Nicoll, 2001; Yeckel et al., 1999). RIM1α is postulated to be a presynaptic PKA target in mfLTP expression and to require Rab3A to exert its effect. Additionally, Rab3A−/− mice exhibit increased paired pulse facilitation but no change in Pr.
RIM1α also interacts with synaptotagmin 1 (Syt1), a synaptic vesicle protein that is required for fast Ca2+-dependent neurotransmitter release (Coppola et al., 2001; Fernandez-Chacon and Sudhof, 1999). A point mutation in the Ca2+ binding region of Syt1 (Syt1R233Q) results in a ~50% decrease in Ca2+ binding affinity (Fernandez-Chacon et al., 2001). Mice expressing this mutant form of Syt1 (Syt1R233Q+/+) exhibit an approximately 50% decrease in Pr, as well as increased paired pulse facilitation, similar to RIM1α−/− mice.
In this study, we examined the role of presynaptic function in mammalian behavior, including associative learning and memory, locomotor activity, motor coordination, and anxiety-like responses. We found that RIM1α−/− mice, which exhibit the most significant perturbations of presynaptic physiology, display dramatic deficits in associative learning and locomotor responses to novelty but show normal responses in the other behavioral assays. In an effort to better define whether a single electrophysiologic abnormality is responsible for the RIM1α phenotype and to control for the possibility that any alteration of presynaptic function can cause a behavioral deficit, we made use of the overlapping electrophysiologic abnormalities in Rab3A−/− and Syt1R233Q+/+ mice with those of RIM1α−/− mice. These genetic manipulations allow us to reexamine the role of mfLTP in learning and memory and to explore the effects of alterations in Pr on behavior. Rab3A−/− and Syt1R233Q+/+ mice do not recapitulate the RIM1α−/− behavioral phenotype. This finding underscores the central role of RIM1α as a presynaptic regulatory protein that modulates presynaptic mechanisms critical for normal associative learning and novelty responses.
Results
RIM1α−/− Mice Exhibit Impaired Fear Conditioning
To assess the role of RIM1α in emotional learning and memory, RIM1α−/− mice were tested in a one-trial context- and cue-dependent fear conditioning paradigm. In this paradigm, mice learn to associate a novel context (experimental chamber) or cue (auditory tone, 90 dB, 2.8 kHz, 30 s) with a foot shock (0.5 mA, 2 s) after a single pairing. Cue-dependent fear conditioning is dependent on an intact amygdala, while context-dependent fear conditioning requires both hippocampus and amygdala (LeDoux, 2000; Maren, 2001). To reduce genetic and experimental variability, we used a coordinated breeding strategy to generate age- and sex-matched littermate pairs of uniform genetic background for all three mutant mouse lines.
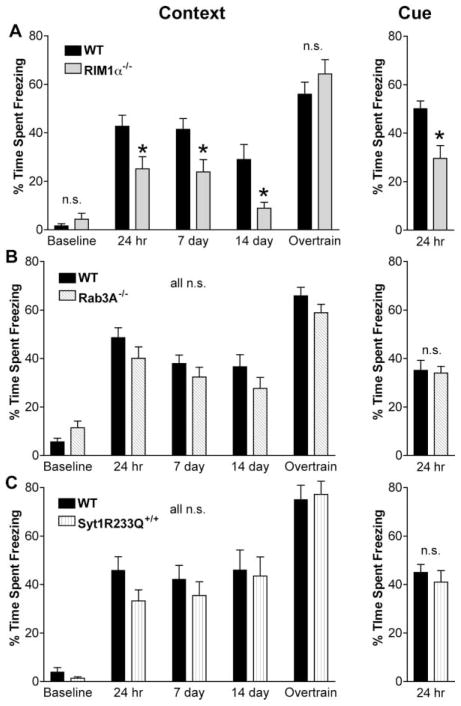
RIM1α−/− mice were significantly impaired in both context- and cue-dependent fear conditioning (Figure 1A). Prior to training, RIM1α−/− mice and their wild-type littermates displayed similar levels of freezing (Figure 1A, baseline). In contrast, 24 hr after training, RIM1α−/− mice spent significantly less time freezing than their littermate controls (context: n = 23, p < 0.01; cue: n =23, p < 0.005). To confirm the contextual fear conditioning deficit, the same group of mice was reexposed to the training context 7 and 14 days after training, at which times the contextual fear conditioning deficit in the RIM1α−/− mice was still apparent (Figure 1A, p < 0.05 in each case). In contrast, immediate short-term memory, assessed by freezing behavior during the 2 min immediately following training on day 1, was not significantly altered between RIM1α−/− mice and littermate controls (not shown, p > 0.05).
Figure 1. RIM1α−/− Mice Exhibit Impaired Fear Conditioning while Rab3A−/− and Syt1R233Q+/+ Do Not.
(A) RIM1α−/− mice exhibit impaired fear conditioning. Context: Average percent time spent freezing in the context prior to training (baseline) and at various time points after training (24 hr, 7 day, and 14 day) in a one-trial fear conditioning paradigm (n = 23). “Overtrain” represents freezing 24 hr following 5 pairings of context/tone/shock. Results indicate a statistically significant decrease in contextual memory 24 hr after one-trial training that persists for at least 14 days. No difference was observed 24 hr after overtraining. Cue: Cue-dependent freezing tested 24 hr after one-trial training reveals a statistically significant decrease in fear memory (n = 23).
(B) Rab3A−/− mice exhibit normal fear conditioning. Context and Cue: Context- and cue-dependent fear conditioning is not significantly different in Rab3A−/− mice versus wild-type under any condition (n = 23).
(C) Syt1R233Q+/+ mice exhibit normal fear conditioning. Context and Cue: Context- and cue-dependent fear conditioning is not significantly different in Syt1R233Q+/+ mice versus wild-type under any condition (n = 21) (*p < 0.05; n.s., p > 0.05 in this and all subsequent figures).
To test whether the learning deficit in the RIM1α−/− mutants could be overcome with repeated training, the mice were trained again on day 14 with five tone/context/foot shock pairs, and contextual fear conditioning was examined 24 hr later. With this “overtraining” paradigm, RIM1α−/− mice exhibited freezing levels equivalent to controls (Figure 1A). This experiment serves as a within-animal control and demonstrates that the deficit in RIM1α−/− mice is not simply due to an inability to freeze. Furthermore, RIM1α−/− mice can detect the foot shock well enough to accomplish associative learning with multiple pairings. As a further control, RIM1α−/− mice were tested for foot shock sensitivity over a range of stimulus intensity. The foot shock current required to elicit behavioral responses such as flinching and vocalizing were not significantly different in RIM1α−/− mice versus controls (not shown). Finally, the intact immediate freezing following training in the RIM1α−/− mice provides further support for an effect of RIM1α knockout on associative memory rather than a nonspecific effect on the behavioral readout (freezing behavior).
Can the Absence of mfLTP Alone Account for the RIM1α−/− Fear Conditioning Deficit?
Of the physiologic abnormalities shown by the RIM1α−/− mice, the absence of mfLTP would seem an appropriate candidate to explain the associative learning deficit in fear conditioning. mfLTP has been postulated to play a role in hippocampus-based learning and memory, and previous studies have suggested such a role for mfLTP (Otto et al., 2001a; Villacres et al., 1998; Wu et al., 1995). These studies contrast, however, with an exhaustive behavioral study of mice lacking the regulatory or catalytic subunits of PKA and mfLTP, which show no abnormalities in associative learning (Huang et al., 1995).
To better understand the role of mfLTP in associative learning as well as the physiologic basis of the RIM1α−/− phenotype, we tested another knockout line, Rab3A−/− mice, which share a subset of the physiologic deficits of RIM1α−/− mice. These mice also serve to control for the possibility that any alteration of presynaptic function leads to behavioral abnormalities. Rab3A−/− mice are deficient in mfLTP and show increased paired pulse facilitation in hippocampal area CA1, as seen for the RIM1α−/− mutants (Castillo et al., 1997). However, Rab3A−/− mice exhibited no apparent phenotype in context- or cue-dependent fear conditioning (Figure 1B) using the exact same training apparatus and protocol as used for the RIM1α−/− mice. Thus, it is unlikely that the absence of mfLTP alone accounts for the learning deficits seen in the RIM1α−/− mice. Also, these data support previous findings of Huang et al. (1995) and Hensbroek et al. (2003) in suggesting that mfLTP is not required for emotional learning and memory.
Can Decreased Pr Alone Account for the RIM1α−/− Fear Conditioning Phenotype?
One of the more striking physiologic abnormalities of the RIM1α−/− mice is the 50% reduction in Pr. No study to date has examined the role of the Pr set point in cognitive function. One might expect a dramatic decrease in Pr to significantly alter behavior and alone account for the RIM1α−/− behavioral deficits. To assess the contribution of decreased Pr to the RIM1α−/− behavioral phenotype, we examined Syt1R233Q+/+ knockin mice. These mice have an approximately 50% decrease in Pr, similar to that of the RIM1α−/− mice (Fernandez-Chacon et al., 2001). However, Syt1R233Q+/+ mice showed normal context- and cue-dependent fear conditioning compared to wild-type littermate controls (Figure 1C). This indicated that the RIM1α−/− fear conditioning deficits were not simply the result of decreased Pr alone.
Finally, we have made every effort to maintain similar genetic backgrounds across the three lines of mice studied in fear conditioning. Wild-type and mutant mice are littermate progeny of heterozygous matings after back-crossing into an inbred strain and can be compared within an individual line. This level of genetic background control does not hold true for comparisons of the mutant or wild-type mice from one line to another. The possibility of genetic differences across lines of mice may account for some of the apparent differences in wild-type behaviors among the different lines (e.g., compare Syt2R233Q+/+ wild-type mice to RIM1α−/− and Rab3A−/− mice performance on the rotarod, Figure 2). Nevertheless, we have statistically compared our fear conditioning data among the three lines of mice by normalizing the % time spent freezing to the corresponding wild- type control freezing levels. Although such a comparison adds an additional layer of variability, we find that a main effect of genotype was observed for both context-and cue-dependent fear conditioning, and post hoc tests indicate significant differences between RIM1α−/− mice and each of the other two lines [not shown; context: main effect of genotype F(2,59) = 3.58, p < 0.05, post hoc LSD and Duncan’s multiple range tests, RIM1α−/− versus Rab3A−/− p < 0.05 and RIM1α−/− versus Syt1R233Q+/+ p < 0.05; cue: main effect of genotype F(2,64) = 4.5, p < 0.05, post hoc LSD and Duncan’s multiple range tests, RIM1α−/− versus Rab3A−/− p < 0.05 and RIM1α−/− versus Syt1R233Q+/+ p < 0.05]. This analysis further supports our conclusion that RIM1α−/− mice are impaired in associative learning, while the Rab3A−/− and Syt1R233Q+/+ mice are not.
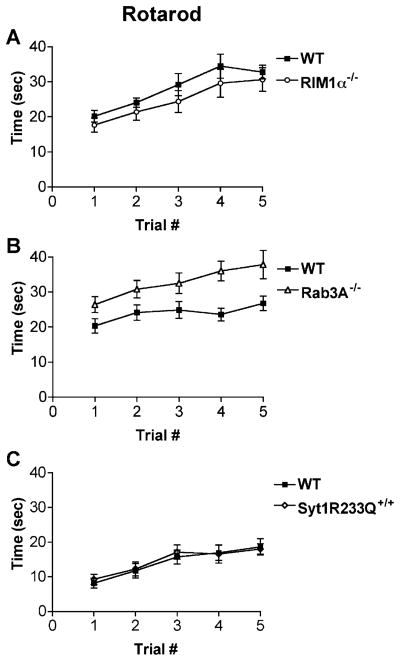
Figure 2. RIM1α−/− and Syt1R233Q+/+ Mice Have Normal Motor Coordination in the Rotarod Apparatus while Rab3A−/− Mice Display Slightly Better Ability.
(A) RIM1α−/− mice exhibit normal motor coordination, staying on the accelerating rotarod as well as littermate controls (n = 20).
(B) Rab3A−/− mice exhibit slightly better motor coordination compared to littermate controls, staying on the accelerating rotarod longer on average than littermate controls (n = 24).
(C) Syt1R233Q+/+ mice exhibit normal motor coordination on the accelerating rotarod (n = 21).
RIM1α−/− Mice Exhibit Normal Coordination and Anxiety-like Behaviors
Because of the profound presynaptic physiologic deficits in RIM1α−/− mice, one might expect fear conditioning deficits to stem from a more global impairment such as decreased motor function or altered anxiety levels. Although RIM1α−/− mice are of normal weight, size, and lifespan (not shown), it was important to determine their gross motor ability and coordination using the accelerating rotarod. RIM1α−/− mice were indistinguishable from their wild-type littermate controls over five trials on the accelerating rotarod (Figure 2A). This is particularly interesting given that RIM1α−/− mice are deficient in a presynaptically expressed form of LTP at the cerebellar parallel fiber/Purkinje cell synapse (Castillo et al., 2002).
Surprisingly, Rab3A−/− mice performed slightly better on the accelerating rotarod than wild-type mice [Figure 2B, Rab3A−/− versus wt, F(1,44) = 11.7, p < 0.001; trial #, F(4,176) = 5.2, p < 0.001; no interaction genotype/ trial]. This suggests that Rab3A−/− mice may have increased baseline coordination, although the lack of an interaction between genotype and trial number suggests this is not a difference in motor learning. Follow-up studies will be needed to better define this potentially interesting phenotype. Syt1R233Q+/+ mice were equivalent to littermate controls on the rotarod test (Figure 2C).
Because fear conditioning may be affected by altered baseline emotional states (LeDoux, 2000), we next tested the RIM1α−/− mice in two common measures of anxiety-like behavior. Given the association between GABAergic transmission and anxiety, this was important in the RIM1α−/− mice because they also exhibit increased paired-pulse depression (PPD) at GABAergic inhibitory synapses in hippocampal area CA1 without an apparent change in Pr at inhibitory synapses (Schoch et al., 2002).
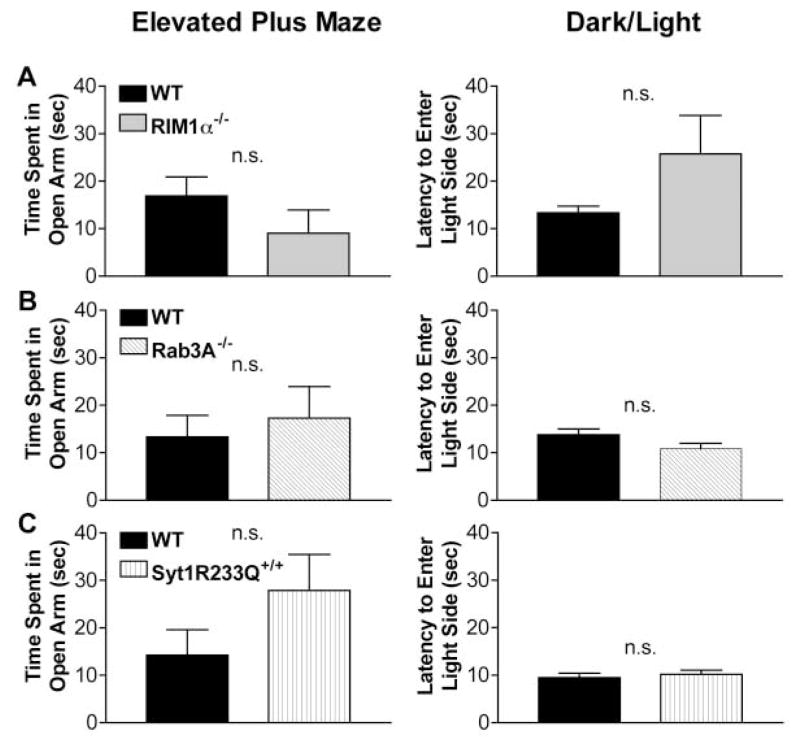
In the elevated plus maze, RIM1α−/− mice showed no difference in time spent in the open arms compared to littermate controls, although a slight trend toward increased anxiety-like behavior was observed (Figure 3A, elevated plus maze, p = 0.22, n = 15). No difference was observed in the number of open arm entries or all arm entries (not shown). In the dark/light test, RIM1α−/− mice were indistinguishable from their littermate controls in their latency to enter the light side of the test chamber (Figure 3A, dark/light, p = 0.14, n = 24), although again a trend toward increased anxiety was observed. These findings indicate that RIM1α−/− mice do not show significant abnormalities in anxiety-like behavior.
Figure 3. RIM1α−/−, Rab3A−/−, and Syt1R233Q+/+ Mice Display Normal Anxiety-like Behaviors in the Elevated Plus Maze and Dark/ Light Apparatus.
(A) RIM1α−/− mice exhibit a nonsignificant trend toward increased anxiety in the elevated plus maze (n = 15) and dark/light tests (n = 24).
(B and C) Rab3A−/− and Syt1R233Q+/+ mice displayed no difference in anxiety-like behavior on the elevated plus maze (Rab3A−/−, n = 12; Syt1R233Q+/+, n = 19) and dark/light tests (Rab3A−/−, n = 24; Syt1R233Q+/+, n = 20).
By comparison, Rab3A−/− mice showed no significant differences from controls in both the elevated plus maze and dark/light tests (Figure 3B). If anything, the Rab3A−/− mice displayed a slight trend toward decreased anxiety-like behavior, opposite that of the RIM1α−/− mice. In the elevated plus maze, Syt1R233Q+/+ mice also showed a nonsignificant trend toward decreased anxiety (Figure 3C, elevated plus maze, p > 0.05, n = 19). In the dark/ light test, Syt1R233Q+/+ mice were indistinguishable from their wild-type littermate controls (Figure 4A, dark/light, p > 0.05, n = 20).
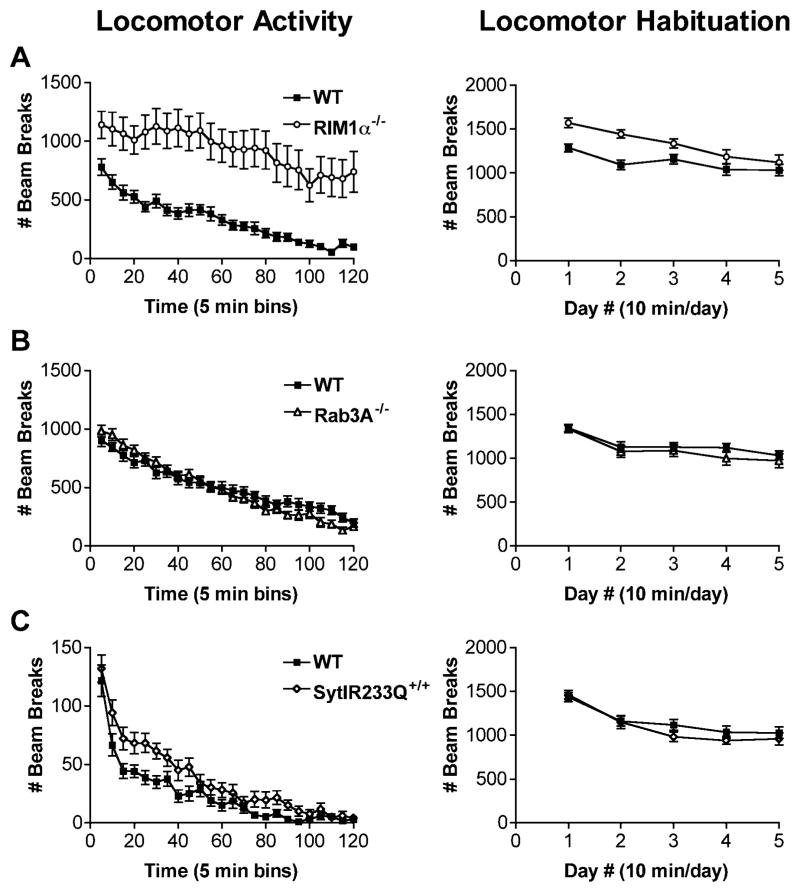
Figure 4. RIM1α−/− Mice Exhibit Increased Locomotor Response to Novelty while Rab3A−/− and Syt1R233Q+/+ Mice Exhibit Normal Locomotor Responses.
(A) RIM1α−/− mice exhibit increased locomotor response to novelty. Locomotor Activity: RIM1α−/− mice displayed significantly increased locomotor activity over a 2 hr period in a fresh home cage. Both RIM1α−/− and wild-type mice displayed similar rates of habituation over the 2 hr period (n = 21). Locomotor Habituation: RIM1α−/− mice were more active than controls on the first day of a 10 min exposure to a novel chamber (n = 24). Over the next 4 days, RIM1α−/− mice habituated to activity levels equal to wild-type controls. Thus, the RIM1α−/− mice are not continually hyperactive, but exhibit abnormally increased locomotor activity in response to a novel environment.
(B) Locomotor activity and habituation in Rab3A−/− mice are equivalent to wild-type controls. No significant difference was observed between Rab3A−/− mice and littermate controls on either locomotor activity (n = 24) or locomotor habituation (n = 24).
(C) Locomtor activity and habituation in Syt1R233Q+/+ mice are equivalent to wild-type controls. No significant difference was observed in Syt1R233Q+/+ mice and littermate controls on locomotor activity (n = 19) or locomotor habituation (n = 20). The y axis on locomotor activity for the Syt1R233Q+/+ mice differs from that of Rab3A−/− and RIM1α−/− mice because Syt1R233Q+/+ mice were tested using more widely spaced photocells to measure activity. The apparatus, photocells, and absolute activity measurements were the same for locomotor habituation among the three lines of mice.
RIM1α−/− Mice Exhibit Abnormal Locomotor Responses to Novelty
To test the role of RIM1α in regulation of locomotor activity, RIM1α−/− mice were subjected to two different locomotor tests. First, the mice were singly placed in a novel home cage flanked by photobeams to track horizontal activity. Over the 2 hr test period, RIM1α−/− mice exhibited significantly increased locomotor activity, although they habituated at a similar rate compared to wild-type littermates [Figure 4A, locomotor activity, wt versus RIM1α−/−, F(1,40) = 16.4, p < 0.001; habituation, F(19,760) = 9.7, p < 0.001; no interaction habituation/ genotype].
The mild stress of a novel environment is known to increase locomotor activity in normal animals. To distinguish between increased locomotor responses to novelty in the RIM1α−/− mice versus a continual increase in baseline locomotor activity, we tested RIM1α−/− mice in a locomotor habituation paradigm. On the first 2–3 days of exposure to the novel environment, RIM1α−/− mice showed increased activity (Figure 4A, locomotor habituation). By 4 days of exposure to the novel environment, however, activity of the RIM1α−/− mice was indistinguishable from control levels, even though the exposures were limited to only 10 min per day [Figure 4A, RIM1α−/− versus wt, F(1,46) = 10.24, p = 0.002; habituation, F(4,184) = 18.41, p < 0.001; interaction habituation/genotype, F(4,184) = 2.64, p = 0.035]. These findings demonstrate that RIM1α−/− mice are not constitutively hyperactive but have increased locomotor responses to novelty. Furthermore, these data reveal that RIM1α−/− mice were able to acquire habituation, a simple form of nondeclarative memory.
No differences between Rab3A−/− mice and their wild-type littermate controls were observed in locomotor activity or locomotor habituation (Figure 4B). Locomotor activity in Syt1R233Q+/+ mice was equivalent to wild-type controls in the locomotor habituation test (Figure 4C, locomotor habituation), though there was a transiently increased locomotor activity in the 2 hr test of locomotor activity [Figure 4C, locomotor activity, Syt1R233Q−/− versus wt, F(1,36) = 5.56, p < 0.05; time, F(23,828) = 72.46, p < 0.001; interaction, F(23,828) = 1.76, p < 0.05]. Absolute locomotor activity counts were different in the Syt1R233Q+/+ locomotor experiment compared to locomotor activity in the RIM1α−/− and Rab3A−/− experiments, because a different locomotor apparatus with different spacing of photocells was used. RIM1α−/− mice also exhibit a significant increase in locomotor activity using this apparatus (not shown). Locomotor habituation was measured in the same apparatus for all lines and there was again no difference between Syt1R233Q+/+ mice and controls on that apparatus in habituation or in absolute locomotor activity (Figure 4C, locomotor habituation).
RIM1α−/− Mice Are Impaired in Spatial Learning in the Morris Water Maze
While RIM1α−/− mice are impaired in associative learning in the fear conditioning paradigm, this paradigm uses freezing behavior as the primary measure of learning, and differences in locomotor activity could affect this measure. The observation that RIM1α−/− mice are able to freeze at the same levels as controls immediately after training as well as 24 hr after overtraining argues against hyperactivity affecting the freezing responses in the RIM1α fear conditioning experiments. To confirm the RIM1α−/− associative learning deficit, we used the Morris water maze, a task that should not be significantly affected by increased locomotor activity.
Because the increased locomotor activity of the RIM1α−/− mice exhibited habituation, we pre-exposed mutant and wild-type littermate mice to the water maze with visible platform trials (6 trials/day for 5 days) prior to testing them with a hidden (submerged) platform. At the end of this training, RIM1α−/− mice reached the visible platform as rapidly as controls on all six trials (not shown), indicating that their basic neurologic functions (swimming, vision, etc.) are normal.
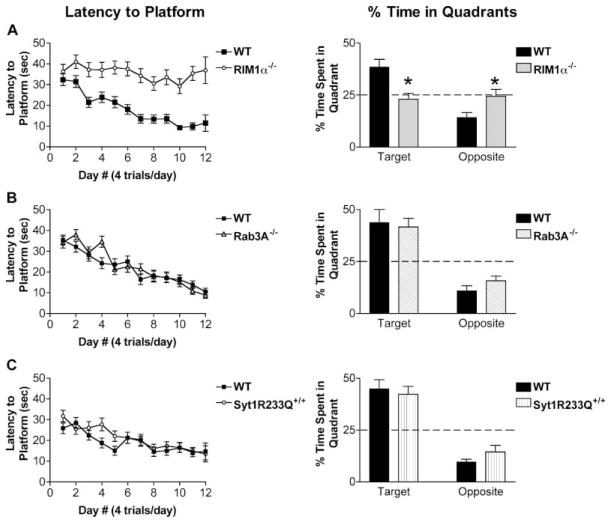
In the hidden platform version of the water maze, RIM1α−/− mice and littermate controls were trained using 4 trials/day for 11 days. Wild-type mice showed maximally decreased latency to reach the hidden platform by day 7 or 8 (Figure 5A, latency to platform). RIM1α−/− mice, however, did not significantly decrease their latency to reach the platform even by day 11 [Figure 5A, latency to platform, RIM1α−/− versus wt, F(1,22) = 20.8, p < 0.001; day #, F(11,242) = 4.0, p < 0.001; interaction genotype/day #, F(1,242) = 2.4, p = 0.007]. This dramatic deficit in the learning curve of RIM1α−/− mice was also observed using the distance traveled prior to reaching the hidden platform, eliminating swimming speed as a concern [not shown; RIM1α−/− versus wt, F(1,22) = 13.7, p < 0.01; day #, F(12,242) = 45.6, p < 0.001; interaction genotype/day #, F(12,242) = 4.3, p < 0.001]. Indeed, the average swim speed of the RIM1α−/− mice and their controls was equivalent [not shown; RIM1α−/− versus wt, F(1,22) = 2.3, p > 0.05], although day-to-day differences in swim speed were apparent in both groups.
Figure 5. RIM1α−/− Mice Are Deficient in a Spatial Learning Task.
(A) RIM1α−/− mice are impaired in spatial learning in the Morris water maze. Latency to Platform: RIM1α−/− mice do not decrease their latency to reach the submerged platform over 12 days of training (n = 12). Littermate wild-type controls do decrease their latency to reach the submerged platform to asymptotic levels by about day 7. % Time in Quadrants: A probe trial was performed on day 12 (n = 12). RIM1α−/− mice spent an equal percentage of time in the target quadrant (in which the submerged platform had been located) as in the quadrant opposite the target quadrant. Littermate controls showed a clear preference for the target quadrant during the probe test and spent significantly more time in the target quadrant than did the RIM1α−/− mice.
(B) Rab3A−/− mice show normal spatial learning in the Morris water maze. Latency to Platform: Rab3A−/− mice displayed a learning curve equal to that of littermate controls (n = 12). % Time in Quadrants: Rab3A−/− mice and littermate controls showed equal preference for the target quadrant in the probe trial on day 12 (n = 12).
(C) Syt1R233Q+/+ mice show normal spatial learning in the Morris water maze. Latency to Platform: The Syt1R233Q+/+ learning curve was not significantly different from that of littermate controls (n = 14). % Time in Quadrants: Equal preference for the target quadrant was observed for Syt1R233Q+/+ mice and littermate controls (n = 14).
A probe trial was performed on day 12 in which the submerged platform was removed and mice were allowed to swim freely for 60 s. RIM1α−/− mice spent an equal percentage of time in the target quadrant (in which the submerged platform had been located) and the quadrant opposite the platform, indicating no spatial preference (Figure 5A, % time in quadrants). The wild-type littermates, by contrast, showed a marked preference for the target quadrant (Figure 5A, % time in quadrants, RIM1α−/− versus wt target, p < 0.01, n = 12; opposite, p < 0.05, n =12). This difference in spatial preference was also observed using number of crossings of theoretical platform areas in each quadrant as a measure of spatial learning (not shown). During training, the RIM1α−/− mice spent more time along the edges of the pool (not shown). This failure to replace such an early thigmotaxic search strategy with other, more advantageous, strategies occurred even after 5 days of successful training in the visible platform version of the water maze. We interpret this as a decrease in learning ability rather than a general inability to tolerate the water maze, since the RIM1α−/− mice performed the same as controls in the final days of the visible platform version of the maze. This is not likely to be an anxiety-related effect since two independent measures of anxiety-like behavior revealed no differences in the RIM1α−/− mice (see Figure 3A). Both mutant and wild-type mice spent an equal amount of time on the submerged platform during training and thus had ample opportunity to learn its spatial location.
It is known that RIM1α expression is relatively specific to the central nervous system (Wang et al., 1997). In situ hybridization experiments reveal, as would be expected, that RIM1α is expressed throughout the brain (not shown). This was confirmed on Western blots of various brain subregions from RIM1α−/− and wild-type mice, including hippocampus and amygdala (not shown). Consistent with RIM1α−/− being a traditional knockout, RIM1α expression is completely lacking throughout the brain in the mutants (Schoch et al., 2002).
Because of conflicting results in the literature regarding a role for mfLTP in spatial learning and memory (Huang et al., 1995; Villacres et al., 1998; Wu et al., 1995; Hensbroek et al., 2003), we also tested the Rab3A−/− mice in the Morris water maze. As with fear conditioning, no phenotype was observed (Figure 5B). Rab3A−/− mice displayed similar latencies to reach the submerged platform, favored the target quadrant over other quadrants, and performed as well as controls on the visible platform test. Possibly consistent with their increased coordination, Rab3A−/− mice had slightly higher swim speeds than controls during the water maze training (not shown, p < 0.05). The Rab3A−/− mice also showed equal spatial preference for the target quadrant in the probe trial; the percentage of time spent in the target quadrant during the probe test would not be expected to be altered by differences in swim speed.
To confirm our lack of effect of decreased Pr on context- and cue-dependent fear conditioning, we examined Syt1R233Q+/+ mice in the Morris water maze. Syt1R233Q+/+ mice displayed similar latencies to reach the submerged platform (Figure 5C, latency to platform), favored the target quadrant over other quadrants (Figure 5C, % time in quadrants), and performed as well as controls on the visible platform test (not shown). Swimming speed was unchanged in the Syt1R233Q+/+ mice as well (not shown). These findings confirm the lack of associative learning and memory deficits in the Syt1R233Q+/+ mice.
Discussion
RIM1α Is Critical for Associative Learning and Memory
Results of the present study establish that the presynaptic active zone protein RIM1α must be present for normal associative learning and memory. RIM1α−/− mice are impaired in two independent tests of associative learning: fear conditioning and Morris water maze. RIM1α, through its multiple presynaptic binding partners, is a key modulator of many presynaptic processes. The aggregate function of RIM1α is the most likely physiologic basis for the observed behavioral deficits because abnormalities in individual presynaptic processes do not account for these deficits.
Of the physiologic abnormalities apparent in RIM1α−/− mice, the absence of mfLTP in area CA3 of the hippo-campus and the decreased Pr in area CA1 of the hippo-campus are obvious candidates for a role in the RIM1α−/− behavioral phenotype. The normal behavioral phenotype in the Rab3A−/− and Syt1R233Q+/+ mice, however, reveals that neither physiologic deficit alone can recapitulate the RIM1α−/− behavioral phenotype. Mice deficient in mfLTP have been shown to exhibit normal cognitive behavior in previous studies (Hensbroek et al., 2003; Huang et al., 1995). To our knowledge, this is the first study demonstrating a lack of effect of a significant, likely global, decrease in Pr. This finding, however, does not entirely rule out the possibility that the decreased Pr seen in RIM1α−/− mice accounts for the RIM1α−/− behavioral abnormalities. First, in area CA1, RIM1α−/− mice have an approximately 50% decrease in Pr at excitatory synapses and decreased PPD at inhibitory synapses, though no change in miniature inhibitory postsynaptic current frequency (Schoch et al., 2002). It is known that Syt1R233Q+/+ mice also have an approximately 50% decrease in Pr at excitatory synapses in hippocampal neuronal cultures. The RIM1α−/− behavioral deficits could be due to subtle differences in the balance between abnormalities at excitatory synapses and inhibitory synapses. The absence of behavioral abnormalities in Syt1R233+/+ mice might be explained by a more balanced decrease in Pr across excitatory and inhibitory synapses. This would still indicate an unprecedented ability of the brain’s plasticity mechanisms to adjust to a new Pr set point to allow relatively normal cognitive function to proceed. It is now of particular interest to determine the role of synaptotagmin 1 and the Syt1R233Q+/+ mutation at inhibitory synapses.
Altered NMDA receptor-dependent LTP in area CA1 of the hippocampus is known to affect both context-dependent fear conditioning and Morris water maze performance (reviewed in Sweatt, 2003). RIM1α−/− mice, however, have been shown to exhibit normal early LTP at the Schaffer collateral to CA1 pyramidal neuron synapse in response to a single 100 Hz tetanic stimulation (Schoch et al., 2002), as have Rab3A−/− mice (Geppert et al., 1994).
RIM1α−/− mice also have increased posttetanic potentiation (PTP) and increased paired pulse facilitation (PPF) in area CA1 of the hippocampus (Schoch et al., 2002), and it is conceivable that these abnormalities in short-term presynaptic plasticity account for the observed behavioral deficits. While mice with increased PTP and PPF alone have not been generated, other studies have examined mice with combined alterations in PTP and PPF. Ca2+/calmodulin-dependent protein kinase II-deficient mice (αCaMKII+/−) exhibit increased PTP and decreased PPF. Ataxin I knockout mice show decreased PPF and no change in PTP. Mice deficient in Synapsin II or Synapsins I and II show decreased PTP and normal PPF, while mice deficient in Synapsin I have increased PPF. When examining the published behavioral phenotypes of these mice in learning and memory paradigms, there is no clear association between increased PTP or increased PPF and learning and memory deficits (Matilla et al., 1998; Silva et al., 1996). Furthermore, the Rab3A−/− and Syt1R233Q+/+ mice in the present study have increased PPF but no behavioral deficits. The increased PPF in RIM1α−/− mice, therefore, is unlikely to be the sole explanation of the RIM1α−/− behavioral phenotype, while we cannot definitively exclude a role for increased PTP in the RIM1α−/− behavioral deficits.
RIM1α−/− mice exhibit increased PPD at inhibitory synapses in area CA1 of the hippocampus. However, it is unlikely that such decreased GABA-mediated inhibition is responsible for the RIM1α−/− learning deficits. First, compounds that reduce GABAA receptor activity tend to enhance memory processes, while those that increase GABAA receptor activity tend to decrease learning (Chapouthier and Venault, 2002). Second, RIM1α−/− mice show no difference in measures of anxiety-like behavior such as in the elevated plus maze and dark/ light tests. These tests are sensitive to alterations in GABAergic inhibition. Thus, the change in GABAergic inhibitory function in the RIM1α−/− mice does not affect tests of anxiety-like behavior and would be expected to increase rather than decrease associative learning.
Genetic background does not account for the RIM1α−/− phenotype or for the differences between the RIM1α−/− phenotype and that of Rab3A−/− and Syt1R233Q+/+ mice. The mice used in all of these studies are the littermate progeny of heterozygous crossings. The original SV129/Bl6 background has been back-crossed into a c57/Bl6 background at least four times prior to breeding for the present study. Wild-type and mutant mice are therefore directly comparable since they are littermate progeny of heterozygous matings after backcrossing into an inbred strain.
In any study of traditional knockout mice, one always must consider the possibility of developmental effects of the knockout. While we cannot definitively rule out this possibility, available evidence argues against it. Morphological examination of RIM1α−/− brains revealed no structural abnormalities or changes in brain architecture (Schoch et al., 2002). Electron micrographs from various regions of the hippocampus revealed no abnormalities, and quantitative, unbiased morphometric analysis of area CA1 synapses showed no significant difference in synapse density, size, vesicle density, or number of docked vesicles (Schoch et al., 2002). Furthermore, of 30 synaptic proteins tested in the RIM1α−/− mice, only Munc13-1 showed a change in expression level (Schoch et al., 2002). Munc13-1+/− mice show a similar decrease in Munc13-1 expression levels but show none of the RIM1α−/− physiologic deficits and no obvious synaptic physiology phenotype (Augustin et al., 1999; Schoch et al., 2002). Finally, presynaptic expression of RIM1α can rescue the presynaptic LTP deficits at cerebellar parallel fiber synapses (Lonart et al., 2003). These findings indicate that significant developmental abnormalities are unlikely in the RIM1α−/− mice.
RIM1α−/− mice display decreased context-dependent fear conditioning and spatial learning in the Morris water maze. These behaviors are thought to be dependent on an intact hippocampus, and the electrophysiological abnormalities observed in the hippocampus of RIM1α−/− mice may account for these findings. These electrophysiologic data indicate that RIM1α may be particularly important in the terminals of the mossy fiber pathway of the dentate gyrus neurons and the terminals of the Schaffer collateral pathway of CA3 pyramidal neurons (Castillo et al., 2002; Schoch et al., 2002). The RIM1α−/− deficit in cue-dependent fear conditioning, however, points to the likelihood of additional effects of RIM1α knockout on other brain areas such as the amygdala. In light of our observations, it will be of great interest to explore the role of RIM1α in synaptic function and plasticity in the amygdala. Our in situ hybridization and Western blot data indicate that, consistent with our behavioral findings, RIM1α is expressed in both the hippocampus and amygdala. However, also apparent from this analysis is that RIM1α is expressed throughout the brain. This distribution is not surprising given the role of RIM1α as a critical active zone protein. What is surprising is that global disruption of this protein causes relatively focused abnormalities in associative learning.
The RIM1α−/− deficit in fear conditioning is not as robust as the deficit in the Morris water maze. In fact, the RIM1α−/− mice seemed to exhibit residual fear memory for both the context and the cue as evidenced by their reduced, but present, freezing to the context and tone. Clearly, the absence of RIM1α does not completely abolish fear conditioning but does reduce the strength of the association of the context/cue with the fear response (freezing). As with amygdala lesions, this deficit in fear conditioning in the RIM1α−/− mice could be overcome by overtraining. It is likely that multiple brain pathways contribute to fear conditioning and that RIM1α is critical for some but not all of these pathways.
RIM1α is in a critical position at the presynaptic terminal to regulate multiple forms of presynaptic plasticity. RIM1α interacts with Rab3A, Ca2+ channels, synaptotagmin 1, α-liprins, and Munc13-1 in vitro (Schoch et al., 2002). RIM1α is known to modulate several aspects of presynaptic plasticity. We have now demonstrated that RIM1α is also critical for mammalian learning. The individual RIM1α functions tested (absent mfLTP, increased PPF, and 50% decrease in Pr) do not account for altered learning in the RIM1α−/− mice. Rather, the many physiologic functions of RIM1α in aggregate are likely responsible for the RIM1α−/− behavioral deficits. One attractive explanation of the RIM1α−/− phenotype is that normal cognitive function requires the integration of presynaptic release probability, short-term plasticity, and long-term plasticity. This would explain why changes in presynaptic plasticity (Rab3A−/−) and Pr (Syt1R233Q+/+) alone do not significantly alter behavior. This idea is also consistent with previous studies of altered presynaptic short-term plasticity affecting learning and memory function (Silva et al., 1996). A related view is that presynaptic function is required for cognitive behavior, but one must perturb presynaptic function beyond some threshold, or in specific ways, to observe a behavioral effect.
Altered Locomotor Responses to Novelty in RIM1α−/− Mice
RIM1α−/− mice also demonstrated increased locomotor activity in response to the mild stress of a novel environment. This finding was confirmed in two different contexts: novel home cage for 2 hr and dark/light boxes for 10 min. RIM1α−/− mice demonstrated normal habituation of locomotor activity in both test situations. The increased locomotor response to novelty was observed even in the early seconds of exposure to a novel environment, indicating that this finding was not due to a decrease in very early habituation rates (not shown). Furthermore, there was also no clear evidence of increased anxiety-like behavior in the RIM1α−/− mice. The physiologic and biochemical basis for the abnormal responses to novelty is currently unclear and requires further investigation.
One possible explanation for this effect is that the decreased Pr in the CA1 region of the hippocampus leads to a decreased hippocampal output during development. It is known that early developmental hippocampal lesions lead to increased locomotor activity in response to novelty, amphetamines, and NMDA receptor antagonists like MK-801 (Lipska and Weinberger, 2000). However, a decrease in Pr would not appear to explain the entire locomotor phenotype of the RIM1α mutant mice, since Syt1R233Q+/+ mice also have a decrease in hippocampal Pr but do not exhibit increased locomotor activity in the habituation test and have only transiently increased locomotor activity in the 2 hr test of locomotor activity. This is surprising, since one might expect a global 50% decrease in Pr to affect many behavioral modalities. Yet, Syt1R233Q+/+ mice, like RIM1α−/− mice, live a relatively normal life span, have normal weight, and display essentially normal behavior in the rotarod, elevated plus maze, dark/light, and habituation tests.
Role of mfLTP in Learning and Memory
Our negative behavioral data in Rab3A−/− mice represent an important observation. mfLTP in area CA3 of the hippocampus does not appear to be required for learning and memory in the specific memory tasks examined. This finding corroborates two previous studies showing that mfLTP is not required for normal fear conditioning or water maze learning and memory (Hensbroek et al., 2003; Huang et al., 1995). Huang and colleagues demonstrated that absence of the regulatory (R1β) or catalytic (Cβ1) subunit of PKA prevents mfLTP while sparing several learning and memory tasks, including the water maze and fear conditioning (Huang et al., 1995). Hensbroek and collaborators showed recently that absence of Rab3A in two different genetic backgrounds does not affect fear conditioning or the Morris water maze (Hensbroek et al., 2003). Mice lacking the metabotropic glutamate receptor (mGluR1) or type I adenylyl cyclase (AC1), however, do show some deficits in the Morris water maze (Villacres et al., 1998; Wu et al., 1995). The reason for this discrepancy is not entirely clear; AC1−/− mice also have deficits in area CA1 LTP, which may explain their water maze abnormality (Wu et al., 1995). A fourth line of mice, PACAP (pituitary adenylyl cyclase activating peptide) type I receptor knockout mice, have absent mfLTP and normal water maze learning but impaired fear conditioning (Otto et al., 2001a, 2001b). However, these mice also exhibit other impairments in emotional behavior (Otto et al., 2001b). While absence of a behavioral phenotype in the Rab3A−/− mice lacking mfLTP cannot stand alone, with the corroborating evidence of Hensbroek et al. (2003) in Rab3A−/− mice using two different genetic backgrounds and with the extensive behavioral analysis of two different PKA subunit knockout mice by Huang et al. (1995), evidence is mounting that mfLTP, as studied in hippocampal slices, is not required for normal emotional learning using the fear conditioning paradigm and may not be required for normal spatial learning.
Role of Pr Set Point in Cognitive Function
A dramatic 50% reduction in Pr at mammalian brain synapses alone does not lead to profound behavioral abnormalities as demonstrated by the relatively normal behavior of Syt1R233Q+/+ mice. This novel finding raises important questions regarding the importance of fidelity and dynamic range of synaptic transmission in cognitive function. Clearly, lack of synaptic transmission is not compatible with life; however, the brain must somehow accommodate to dramatic alterations in Pr. If long-term synaptic plasticity is important for learning and memory, for example, one can imagine that an altered Pr might not interfere with the ability of synapses to undergo plasticity either post- or presynaptically. In Syt1R233Q+/+ mice in particular, it will be important to understand if the decreased Pr occurs in both excitatory and inhibitory synapses. In any case, it is likely that the brain’s ability to accommodate to such a change in Pr is a chronic one, since acute alterations of Pr with rapid changes in serum electrolyte concentrations (e.g., Mg2+) can produce acute cognitive effects in humans.
Conclusions
RIM1α, via its interactions with multiple presynaptic binding partners and the multiple presynaptic plasticity mechanisms it modulates, is required for normal learning and memory. This represents the first genetic demonstration that alteration of a presynaptic protein can lead to relatively selective deficits in cognitive function while sparing motor coordination and other behaviors. Furthermore, the relatively normal behavior across multiple paradigms in the synaptotagmin 1 mutant mice demonstrates an extraordinary level of accommodation to widespread alterations in the Pr set point in the brain. Normal behavior in the Rab3A knockouts confirms that altered presynaptic LTP alone does not lead to significant behavioral deficits. Thus, the brain is able to accommodate significant perturbations in presynaptic function, but when multiple presynaptic functions are altered beyond some threshold, this accommodative capacity breaks down, leading to selective deficits in cognitive function. Perturbation of a single physiologic process is unlikely to be responsible for the RIM1α−/− behavioral phenotype. Rather, RIM1α itself underlies multiple presynaptic processes that are necessary in aggregate for normal learning and memory.
Experimental Procedures
Genetic Manipulations
RIM1α−/− and Rab3A−/− mice were generated as previously described (Geppert et al., 1994; Schoch et al., 2002). Syt1R233Q+/+ mice were created via homologous recombination in embryonic stem cells as described (Fernandez-Chacon et al., 2001). In an effort to reduce genetic and experimental variability, age/sex-matched littermate pairs resulting from heterozygous crossings were used for all experiments. Mice were derived in a hybrid SV129/Bl6 background and subjected to at least four backcrosses into c57/Bl6 prior to behavioral characterization.
Behavioral Overview
Mice were age/sex-matched littermate pairs run through a battery of behaviors in 2 or 3 groups for each genotype. All mice ranged from 3 to 6 months of age during the behavioral testing, and within each group mice were born within 2–4 weeks of each other. Less stressful behaviors were tested first, with more stressful procedures at the end. The order of tests was as follows: dark/light, elevated plus maze, accelerating rotarod, locomotor activity, fear conditioning, water maze, shock threshold. Mice were moved within the animal facility to the testing room and allowed to habituate to the new location for at least 1 hr prior to behavioral testing. The same behavioral apparati were used for each line of mice except that one line of mice was tested on a different apparatus in one of the two locomotor activity measurements. The order of behavioral testing was constant among the different lines of mice.
Fear Conditioning
Age/sex-matched littermate mice were placed in a plexiglass shock box with clear front and rear walls (MedAssociates) for 2 min, and then a 30 s, 90 dB acoustic conditioned stimulus (CS; white noise) coterminating in a 2 s, 0.5 mA foot shock (US) was delivered. Mice remained in the chamber 2 min after pairing before returning to their home cage. Freezing behavior (motionless except respirations) was monitored at 5 s intervals by an observer blind to the genotype. The boxes were cleaned with 70% ethanol, and bedding below the shock grid was changed after each mouse. To test for contextual learning 24 hr, 7 days, and 14 days after training, mice were placed into the same training context and scored for freezing behavior every 5 s. In “overtraining,” the CS/US pairing was performed as above but was repeated five times with 1 min between pairings. To assess cue-dependent fear conditioning, mice were placed in a novel environment with novel vanilla odor in the afternoon following the context test for a 3 min baseline followed by 3 min of the CS (tone). Cue-dependent fear conditioning was determined by subtracting the 3 min baseline freezing from freezing during the tone. In this and all other behavioral experiments, mice ranged in age from 3 to 6 months. Student’s t test was used to analyze the data, although results of an ANOVA using each context fear conditioning test day (day 1, 7, and 14) were similar. Significance was taken as p < 0.05 in this and all experiments.
Locomotor Activity
Mice were placed in a fresh home cage with minimal bedding for 2 hr. Horizontal activity was monitored using photobeams linked to computer data acquisition software (San Diego Instruments). Two-way ANOVA was used to analyze the data.
Accelerating Rotarod
An accelerating rotarod designed for mice (IITC Life Science) was used. The rotarod was activated after placing mice on the motionless rod. The rod accelerated from 0 to 45 revolutions per min in 60 s. The time to fall off the rod or to turn one full revolution was measured. Data were analyzed with two-way ANOVA.
Elevated Plus Maze
Mice were placed in the center of a black, plexiglass elevated plus maze (each arm 33 cm long and 5 cm wide with 25 cm high walls on closed arms) in a dimly lit room for 5 min. Each session was videotaped for later analysis by an observer blind to the genotype of the mice. Time spent in the open and closed arms, number of open and closed arm entries, time spent in the middle, and number of explorations of the open arm (defined as placing head and two limbs into open arm without full entry) was calculated. The apparatus was wiped with 70% ethanol and air-dried between mice. Data were analyzed with Student’s t test.
Dark/Light Apparatus and Locomotor Habituation
The dark/light apparatus consisted of MedAssociates mouse place preference boxes. One side was kept dark (room light entry limited), while the other side was lit by a light built into the top. Mice were placed in the dark side for 2 min, and then the automatic door between the compartments opened and they were allowed to freely explore either the light or dark side for 10 min. Anxiety-like behavior is measured on the initial 10 min exposure based on the latency to enter the light side. Locomotor activity was also measured during the dark/light testing with photobeams and MedAssociates software (MedPC). To measure locomotor habituation, mice were placed in the same apparatus 10 min/day for a total of 5 consecutive days with locomotor activity measured each day. Student’s t test was used to analyze anxiety-like behavior while two-way ANOVA was used to analyze locomotor habituation data.
Morris Water Maze
A 1.2 m diameter, white, plastic, circular pool was filled to a depth of 33 cm with 22°C ± 1°C water made opaque with gothic white, nontoxic, liquid tempra paint in a room with prominent extramaze cues. Mice were placed in one of four starting locations facing the pool wall and allowed to swim until finding a 15 cm diameter white platform submerged by 0.5 to 0.75 cm or a maximum of 60 s. On finding the platform, mice remained on the platform for 20 s before being removed to the home cage. If mice did not find the platform within 60 s, they were guided to the platform by the experimenter and remained on the platform for 20 s before being removed to the home cage. Latency to reach the platform, distance traveled to reach the platform, swim speed, time spent in each of four quadrants, and time spent along the walls were obtained using automated video tracking software from Noldus (Ethovision 2.3.19). Mice were trained with 4 trials/day with an intertrial interval of 1–1.5 min for 11 consecutive days between 8 am and 1 pm. A probe trial (free swim with the submerged platform removed) was performed as the first trial of the day on days 5, 9, and 12. Percent time spent in the target quadrant and number of platform location crossings were calculated. Time spent in the target quadrant and number of platform crossings were analyzed with Student’s t test, while latency to platform, distance to platform, and swim speed were analyzed with two-way ANOVA. In visible platform experiments, the platform was marked with a gray foam block (10 × 6.3 × 6.3 cm) atop a 20 cc syringe attached to the middle of the platform, and spatial cues were covered by four white plastic curtains surrounding the maze.
RIM1α In Situ and Western Blotting
In situ hybridization for RIM1α was performed using digoxigenin (DIG)-labeled in vitro transcription products (cRNA). Wistar rats (3 weeks old) were anesthetized and decapitated. The brains were removed and quickly frozen. Sections were cut at 12 μm on a cryostat, thaw-mounted on slides, and fixed with 4% (w/v) paraformaldehyde in PBS. Hybridization and washes were performed according to standard procedures (Chen et al., 2001). Hybridization probes for RIM1α were generated by PCR using primers (5′-CTG TCT CTT CCC AAG ACA CTG CT-3′; 5′-GAC ACG TTT GCG CTC GC-3′) with rat hippocampal cDNA as template and subsequent in vitro transcription. Control sections were hybridized to sense probes without detecting specific signals.
Brain regions were dissected from the brains of adult littermate mice (wild-type and knockout, >2 months of age) and homogenized in PBS/10 mM EDTA/1 mM PMSF. Brain protein (50 μg per lane) was analyzed by SDS-PAGE and immunoblotting following standard procedures (Laemmli, 1970; Towbin et al., 1979) using specific anti-RIM1α antibodies (Wang et al., 1997).
Acknowledgments
We are indebted to several excellent technical assistants including Phillip Williams, Nick Sanchez, Deanna Wallace-Black, and Qiona Young. We also thank Nicole Hamlin and Cathy Steffen for assistance with animal breeding, care, and transfer. This work was supported by K08MH65975 and NARSAD (C.M.P.), HHMI (T.C.S.), and P50MH66172 (E.J.N.).
References
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Brose N, Rosenmund C, Rettig J. Regulation of transmitter release by Unc-13 and its homologues. Curr Opin Neurobiol. 2000;10:303–311. doi: 10.1016/s0959-4388(00)00105-7. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Janz R, Sudhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Chapouthier G, Venault P. GABA-A receptor complex and memory processes. Curr Top Med Chem. 2002;2:841–851. doi: 10.2174/1568026023393552. [DOI] [PubMed] [Google Scholar]
- Chen J, Sochivko D, Beck H, Marechal D, Wiestler OD, Becker AJ. Activity-induced expression of common reference genes in individual CNS neurons. Lab Invest. 2001;81:913–916. doi: 10.1038/labinvest.3780300. [DOI] [PubMed] [Google Scholar]
- Coppola T, Magnin-Luthi S, Perret-Menoud V, Gattesco S, Schi-avo G, Regazzi R. Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP-25, and synaptotagmin. J Biol Chem. 2001;276:32756–32762. doi: 10.1074/jbc.M100929200. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Garner CC. Priming plasticity. Nature. 2002;415:277–278. doi: 10.1038/415277a. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Sudhof TC. Genetics of synaptic vesicle function: toward the complete functional anatomy of an organelle. Annu Rev Physiol. 1999;61:753–776. doi: 10.1146/annurev.physiol.61.1.753. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, Sudhof TC. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- Hensbroek RA, Kamal A, Baars AM, Verhage M, Spruijt BM. Spatial, contextual and working memory are not affected by the absence of mossy fiber long-term potentiation and depression. Behav Brain Res. 2003;138:215–223. doi: 10.1016/s0166-4328(02)00243-7. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER, Varshavsky L, Brandon EP, Qi M, Idzerda RL, McKnight GS, Bourtchouladze R. A genetic test of the effects of mutations in PKA on mossy fiber LTP and its relation to spatial and contextual learning. Cell. 1995;83:1211–1222. doi: 10.1016/0092-8674(95)90146-9. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Lonart G. RIM1: an edge for presynaptic plasticity. Trends Neurosci. 2002;25:329–332. doi: 10.1016/s0166-2236(02)02193-8. [DOI] [PubMed] [Google Scholar]
- Lonart G, Schoch S, Kaeser PS, Larkin CJ, Sudhof TC, Linden DJ. Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell. 2003;115:49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Matilla A, Roberson ED, Banfi S, Morales J, Armstrong DL, Burright EN, Orr HT, Sweatt JD, Zoghbi HY, Matzuk MM. Mice lacking ataxin-1 display learning deficits and decreased hippocampal paired-pulse facilitation. J Neurosci. 1998;18:5508–5516. doi: 10.1523/JNEUROSCI.18-14-05508.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matynia A, Kushner SA, Silva AJ. Genetic approaches to molecular and cellular cognition: a focus on LTP and learning and memory. Annu Rev Genet. 2002;36:687–720. doi: 10.1146/annurev.genet.36.062802.091007. [DOI] [PubMed] [Google Scholar]
- Mayford M, Kandel ER. Genetic approaches to memory storage. Trends Genet. 1999;15:463–470. doi: 10.1016/s0168-9525(99)01846-6. [DOI] [PubMed] [Google Scholar]
- Mellor J, Nicoll RA. Hippocampal mossy fiber LTP is independent of postsynaptic calcium. Nat Neurosci. 2001;4:125–126. doi: 10.1038/83941. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, Grone HJ, Kellendonk C, Tronche F, Maldonado R, et al. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating poly-peptide type I receptor-deficient mice. J Neurosci. 2001a;21:5520–5527. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Martin M, Paul Wolfer D, Lipp H, Maldonado R, Schutz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res. 2001b;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Sudhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Rosahl TW, Chapman PF, Marowitz Z, Friedman E, Frankland PW, Cestari V, Cioffi D, Sudhof TC, Bourt-chuladze R. Impaired learning in mice with abnormal short-lived plasticity. Curr Biol. 1996;6:1509–1518. doi: 10.1016/s0960-9822(96)00756-7. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mechanisms of Memory. San Diego, CA: Academic Press; 2003. [Google Scholar]
- Tonegawa S, Nakazawa K, Wilson MA. Genetic neuroscience of mammalian learning and memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:787–795. doi: 10.1098/rstb.2002.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villacres EC, Wong ST, Chavkin C, Storm DR. Type I adenylyl cyclase mutant mice have impaired mossy fiber long-term potentiation. J Neurosci. 1998;18:3186–3194. doi: 10.1523/JNEUROSCI.18-09-03186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Nicoll RA. Presynaptic changes during mossy fibre LTP revealed by NMDA receptor-mediated synaptic responses. Nature. 1995;376:256–259. doi: 10.1038/376256a0. [DOI] [PubMed] [Google Scholar]
- Wu ZL, Thomas SA, Villacres EC, Xia Z, Simmons ML, Chavkin C, Palmiter RD, Storm DR. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc Natl Acad Sci USA. 1995;92:220–224. doi: 10.1073/pnas.92.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]