Abstract
The AAPM, through its members, meetings, and its flagship journal Medical Physics, has played an important role in the development and growth of x-ray tomography in the last 50 years. From a spate of early articles in the 1970s characterizing the first commercial computed tomography (CT) scanners through the “slice wars” of the 1990s and 2000s, the history of CT and related techniques such as tomosynthesis can readily be traced through the pages of Medical Physics and the annals of the AAPM and RSNA/AAPM Annual Meetings. In this article, the authors intend to give a brief review of the role of Medical Physics and the AAPM in CT and tomosynthesis imaging over the last few decades.
Keywords: computed tomography, tomosynthesis
INTRODUCTION
As the AAPM celebrates its 50th anniversary, it is natural to pause and take stock of the contribution its members, meetings, and publications have made to the field of medical physics. Entire modalities, such as computed tomography (CT) and magnetic resonance imaging, have been conceived and developed into powerful clinical tools in the half century since the founding of the AAPM, often with substantial involvement from AAPM members and journals. In particular, CT and related techniques such as tomosynthesis have been the subject of over 200 publications in the AAPM’s flagship journal Medical Physics.
Certainly, the attempt to trace the role of a single journal and professional organization in the development of an imaging modality such as CT cannot provide a complete and definitive history of the modality itself. Many significant developments in CT were published in other journals or took place at companies who may or may not have divulged proprietary information in the scientific literature. However, the broad outlines of the modality’s history are quite clear from its treatment in the pages of Medical Physics.
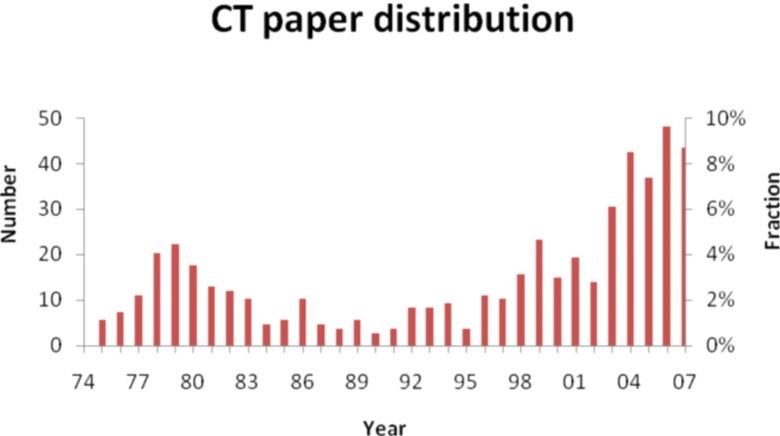
For instance, to gain a sense of the scope of the work that has been published in Medical Physics, we searched the archives of Medical Physics for articles related to x-ray CT and tomosynthesis. Figure 1 shows the number of such papers published per year for the past 35 years.
Figure 1.
Approximate distribution of papers relevant to CT and tomosynthesis published in Medical Physics over the past 35 years. Left axis: Approximate number of araticles. Right axis: Approximate percentage of articles.
The bimodal nature of the plot succinctly tells a story of waxing and waning interest in x-ray tomography, beginning with the initial excitement following the commercial introduction of CT in 1972 by EMI, and then trailing off through the 1980s as the technology matured and emerging technologies, such as magnetic resonance imaging (MRI), won attention from researchers and clinicians alike. The introduction of spiral/helical scanning at the 1989 RSNA/AAPM annual meeting, followed by the introduction of multislice scanners in the mid 1990s, led to a resurgence of interest in CT technology and has also enabled entirely new applications that were impractical with the slower scanners of the past. Similarly, an expanded scope of detector technologies and applications for x-ray tomography is evident in the last decade—e.g., in the use of flat-panel detectors for tomosynthesis and cone-beam CT.
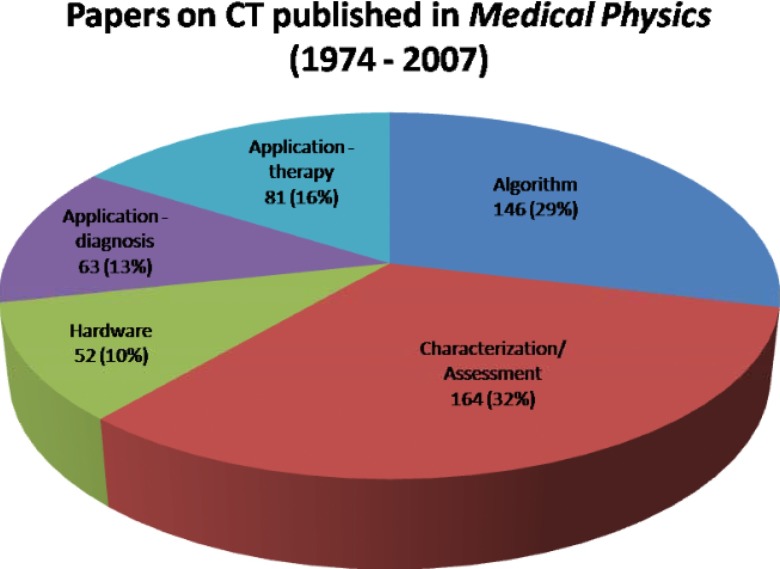
The focus of the x-ray CT papers published in Medical Physics can be divided into five principal categories: hardware, system assessment, algorithm development, diagnostic applications, and therapeutic applications (largely radiation therapy treatment planning and image-guided interventions). Figure 2 illustrates the fraction of the published articles between 1974 and 2007 falling into each category.
Figure 2.
Distribution of topic categories for CT-related papers published in the Medical Physics. Within each sector, the two numbers represent the approximate number and percentage of the articles published for a given category.
Similarly, the AAPM has commissioned a number of Task Groups on the topic of CT, mostly related to the quality assurance and dosimetry of CT or its application in radiation therapy simulation. In fact, the first AAPM Task Group (TG) Report1 was on the topic of quality assurance in CT, which formalized the now familiar concepts of “head” and “body” phantoms as soft-tissue-equivalent cylinders of different diameters. While many Task Group Reports involve CT as integral to the medical imaging arsenal, those dealing specifically with CT in either diagnostic or therapy contexts are summarized in Table 1.
Table 1.
Summary of AAPM Task Group Reports related to CT.
| Year | Report No. | TG No. | Title | Reference |
|---|---|---|---|---|
| 1977 | 1 | N/A1 | Phantoms for performance evaluation and quality assurance of CT scanners | 1 |
| 1978 | 4 | N/A2 | Basic quality control in diagnostic radiology | 2 |
| 1991 | 31 | 8 | Standardized methods for measuring diagnostic x-ray exposures | 3 |
| 1991 | 35 | 6 | Recommendations on performance characteristics of diagnostic exposure meters | 4 |
| 1993 | 39 | 2 | Specification and acceptance testing of computed tomography scanners | 5 |
| 1994 | 46 | 40 | Comprehensive QA for radiation oncology | 6 |
| 1998 | 60 | 4 | Instrumentation requirements of diagnostic radiological physicists | 7 |
| 1998 | 62 | 53 | Quality assurance for clinical radiotherapy treatment planning | 8 |
| 2002 | 74 | 12 | Quality control in diagnostic radiology | 9 |
| 2003 | 83 | 66 | Quality assurance for computed-tomography simulators and the computed-tomography-simulation process: Report of the AAPM Radiation Therapy Committee Task Group No. 66 | 10 |
| 2004 | 86 | N/A3 | Quality assurance for clinical trials: A primer for physicists | 11 |
| 2006 | 91 | 76 | The management of respiratory motion in radiation oncology | 12 |
| 2007 | 95 | 75 | The management of imaging dose during image-guided radiotherapy | 13 |
| 2006 | 108 | 108 | AAPM Task Group 108: PET and PET/CT shielding requirements | 14 |
| 2008 | 96 | 23 | The Measurement, Reporting, and Management of Radiation Dose in CT | 15 |
Work predates AAPM Task Group enumeration—Diagnostic Radiology Committee Task Force on CT Scanner Phantoms.
Work pre-dates AAPM Task Group enumeration—Diagnostic Radiology Committee Task Force on Quality Assurance Protocol.
Reported by the Subcommittee on Quality Assurance Physics for Cooperative Trials, Radiation Therapy Committee.
FOUR DECADES OF X-RAY COMPUTED TOMOGRAPHY
Pre-1970s and 1970s: The Dawn of CT
The 1972 introduction of the first commercial CT scanner by Hounsfield led to a flurry of publications in Medical Physics and other journals by academic and industrial researchers who sought to characterize, improve, and apply the new technology. This accounts for the first peak seen in Fig. 1, and the concerns of the time are well summarized in two review articles on fundamental photon attenuation physics16 and the basic aspects of early CT technology and systems.17 Although CT technology was still in its infancy, researchers of the day were quick to identify and lay the groundwork for new applications, some of which are only just coming to fruition, like dual-energy CT.
Researchers in this era devoted substantial effort to characterizing the physical properties of CT scanners that affect image quality. While much proprietary research took place within the numerous companies developing scanners, a number of academic groups investigated these effects and reported their findings in Medical Physics. They evaluated various physical properties of CT systems that affect image quality, including x-ray beam characteristics,18, 19, 20 focal-spot effects,21 collimators,21, 22 the system modulation transfer function (MTF),23, 24 and image noise properties.25 Additional metrics based on information and detection theory such as noise-power spectrum (NPS), noise equivalent quanta (NEQ), and signal detectability were also developed and applied to evaluating CT image quality.26, 27
Image artifacts also occupied an important body of early research. The deleterious effect of the polychromatic spectrum of conventional x-ray sources on CT image quality—the so-called beam hardening effect—was recognized and described early in this era, along with a number of correction algorithms.28, 29, 30, 31 Meanwhile, other investigators realized that the differences between the polychromatic spectra obtained when the tube is run at two different kVp could be used to develop dual-energy techniques that allow one to extract effective atomic number and electron density information.19, 32 The noise properties of the dual-energy technique were studied first in 1979.33 In fact, these techniques remain the foundation of energy-spectral-based algorithms for modern CT scanners. An article on image reconstruction from truncated data and its implication for metal artifact correction was also published in Medical Physics in 1979.34 Naturally, researchers sought to determine and reduce the radiation dose delivered to the subject during CT imaging. In particular, careful experiments were designed and carried out to measure and calculate exposure/dose in CT imaging.35, 36, 37 Strategies for reducing dose were proposed by Moran38 and Lewitt.34
The original EMI system was a dual-slice first-generation system, acquiring one ray for each of two slices at a time. The source and detector needed to be translated along each section of the patient and then rotated, with the process repeated for 180 projection views. During the 1970s, a more time-efficient fan-beam imaging system involving rotation only was developed. One of the first articles on fan-beam CT published in Medical Physics described a convolution backprojection algorithm, which is now referred to as the fan-beam filtered backprojection (FBP) algorithm.39 This algorithm can fully exploit the data acquired in the divergent-beam scanning configuration and is still used for image reconstruction from data acquired with modern fan-beam CT scanners. Moreover, it forms the basis for the derivation of approximate algorithms for image reconstruction from CT data acquired with the cone-beam configurations introduced later.
Owing to the accurate anatomical information depicted in CT images, CT scans soon became routinely used in many radiation oncology centers for treatment planning, including Co-60 radiotherapy40 and electron beam therapy.41 In fact, Cormack’s original motivation in the “discovery” of CT (contemporary to and independent of Hounsfield) was to image and quantify the electron density within the body for purposes of more accurate dose calculation in radiation therapy. Holden and co-workers studied imaging the continuously-changing distribution of the object, which later evolved into the important technique of four-dimensional CT.42 Overall, CT had established itself as a prime imaging modality in modern radiology by the end of the 1970s.
It deserves mention and has become of topical interest lately that, while so much attention was being devoted to CT, some groups continued to pursue classical tomography, which seeks, through coordinated motion of the source and film, to create x-ray images in which some chosen plane was in focus, with other planes providing a blurred background. Classical tomography is the forebear of the currently thriving tomosynthesis technique. Orphanoudakis and colleagues proposed the mathematical model based on transfer function analysis43 and later validated the assumption of system linearity.44 Harding and colleagues used transfer function analysis to address the problem of optimized blurring.45
The late 1980s: The Doldrums of CT
Following the booming period of CT research in the late 1970s, momentum persisted through the early years of the 1980s before falling off substantially after 1983.
One of the most interesting articles appearing in Medical Physics during this period is Hounsfield’s Nobel address, in which he reviewed the development of CT before the 1980s and predicted the growing importance of MRI.46 In many ways, it is precisely the growth in MRI that accounts for the decline in publications related to CT, as many of the original CT researchers found themselves drawn to this new technology. Moreover, the major focus of the research that was performed on CT in this decade suggested a fairly mature technology, with articles on image artifact reduction, system assessment, and new applications dominating the literature.
Building on work from the 1970s, researchers continued to develop a solid understanding of methods and metrics of system evaluation, with an eye to improving image quality. Glover and Pelc elucidated the nature of CT image artifacts and methods for reducing their impact on image quality, including partial volume effects,47 metal artifacts,48 and x-ray scatter artifacts.49 Understanding the spatial resolution and noise characteristics of CT included evaluation of the MTF,50, 51 noise, NPS, NEQ,52 and signal to noise ratio in CT image for lesion detection.53 Duerinckx analyzed the second order statistics (covariance and correlations) in CT images,54 while Parker tried to relate the amplitude of measurement errors to the maximum image error so that a cost-benefit relationship can be established.55
Naturally, many of these image quality metrics are intimately related to the radiation dose delivered to the patient, and researchers continued to develop new dose figures-of-merit,56, 57 a new ion chamber for dose measurement,58 Monte Carlo simulation for dose calculation,59 and a novel dose calculation approach for heterogeneous media.60 While Table 1 suggests somewhat of a dearth of activity in CT quality assurance and dosimetry during the 1980s, over the course of the decade, ion chambers specifically designed for CT dose measurement had been developed, and dose descriptors such as the CT dose index came to be recognized by federal regulatory bodies and the AAPM.61
Interest in novel system configurations continued, as Drost reported using a xenon detector to obtain dual energy information from a single scan.62 Edwards reported a treatment planning system running on the GE whole body CT scanner.63 A prototype megavolt CT system for clinical use was reported by Simpson and Swindell,64, 65 which appears to be the earliest report of such a system in Medical Physics. On the algorithmic front, researchers began to investigate so-called short scan fan beam trajectories involving rotations less than 360 deg, and one of the earliest articles about short scan was published by Parker.66 The weighting scheme Parker proposed has become a widely used standard. Characterization and algorithmic correction of artifacts expanded into scatter, beginning with the article by Johns and Yaffe67 for fan beam CT and growing to include a number of other approaches.47, 49, 66, 67, 68, 69, 70, 71 Prospective gating was applied to cardiac CT imaging by Moore.72, 73 Finally, this era saw the introduction of the classic Siddon algorithm for calculating the exact radiological path through a three-dimensional (3D) CT array.74
Two important new applications of CT introduced during the 1980s were blood flow measurements and bone mineral measurements. Works pertaining to flow measurement have been reported in Medical Physics.75, 76, 77, 78, 79, 80, 81 Good et al. studied the effect of CT noise and tissue heterogeneity on cerebral blood flow determination for xenon-enhanced CT.80 Articles in Medical Physics addressing bone and cortical mineral measurement typically employed a dual-energy approach.82, 83, 84, 85 Another novel application suggested during this period was to use CT to measure temperature,86 which had potential applications in the area of hyperthermia therapy.
The 1980s also saw the development of early, practical cone-beam CT reconstruction techniques that would capture increased attention in following decades. The now familiar “Feldkamp” algorithm for circular orbit reconstruction made its debut in 1984 and spurred a considerable research effort throughout the field concerning methods for exact reconstruction, cone-beam artifacts, and alternative (noncircular) orbits. By the end of the decade, the ability to reconstruct 3D images from divergent beams—e.g., using 3D filtered backprojection, maximum likelihood estimation, etc.—had become firmly established, and applications in microtomography began to develop.
Finally, in the realm of tomosynthesis, Buonocore reported reconstructing limited angle CT data by using a fast minimum variance estimator.87 Ruttimann designed a new sampling scheme for circular tomosynthesis.88 Two other articles about blood vessel reconstruction from limited-view data also appeared.89, 90
The 1990s: CT Goes Spiral/Helical
As can be seen in Fig. 1, CT research activity continued to be modest in the early nineties, but it began to expand again in the late nineties leading to the second peak in the 2000s. The articles published in the early 1990s, while few in number, were highly significant, as they introduced the spiral, later also referred to as helical, scanning that energized the CT research community and revolutionized radiological practice during the late 1990s.
The new scanning protocols and geometries arising due to spiral/helical and multislice technology demanded new algorithms and new approaches to evaluation. In the early 1990s, the research focus was on validating the use of a spiral/helical scan instead of a step-and-shoot scan for single-slice image acquisition. There was concern that spiral/helical scanning would yield new artifacts since it deliberately introduced motion inconsistencies into the tomographic data set. However, these inconsistencies could be cured through interpolation, and the benefits of the fast volume coverage soon became apparent. Spiral/helical CT was established as the standard CT scan mode by the end of 1990s.
Analysis of the image artifacts resulting from data inconsistency and algorithms to correct for them were described in Medical Physics.91, 92 Later articles deepened the understanding of image-quality issues, including noise, longitudinal resolution, and contrast.93, 94, 95, 96, 97, 98, 99, 100 As the image quality improved due to these investigations, spiral/helical CT scanning began to replace conventional CT scanning in clinics. Patient dose delivered by spiral/helical CT scanning and the methods to reduce dose attracted considerable interest at this point.101, 102, 103, 104
Single-slice spiral/helical CT scanners soon evolved into multislice volume scanners with small voxel size, large scanning range, isotropic spatial resolution, and high speed. Dual-slice spiral/helical CT was first described in Medical Physics by Liang and Kruger, who characterized a two-slice system from Elscint.105 Later, Taguchi and Hu reported advanced interpolation algorithms for multislice spiral/helical CT with careful attention to optimizing the helical pitch.106, 107 McCollough evaluated and systematically compared the performance of a multislice CT system to a single-slice scanner in terms of a variety of image quality and dose parameters and dose.108 Meanwhile, Corrigan et al. described a multirow helical microtomography system for specimen imaging.109 These articles are a prelude to the slice wars that followed in the early 2000s. Interest in dual-energy imaging also continued in this period.110, 111, 112 The faster scanners made possible new applications especially for cardiac,113 lung,114 bone,115 liver,116 and blood flow imaging.117
CT also played an important role in radiation therapy during the 1990s, helping to fuel a revolution in computerized 3D radiotherapy planning. Early interest in the use of CT in radiation therapy was captured in an AAPM symposium edited by Ling et al.,118 and the technology became firmly established in the following decade. With CT scanners developed specifically for radiotherapy planning and integrated with various treatment planning systems, CT provided the important benefits of 3D target and normal tissue delineation, dose calculation (including the dose-volume histogram), and simulation that have become standard to modern radiation therapy. For guidance of radiotherapy as well, CT pointed to more precise delivery through improved soft-tissue localization at the time of treatment. Early interest in megavoltage CT119, 120, 121 suggested the potential for treating the “target-of-the-day” in a manner not possible with two-dimensional portal images, and interest in image-guided radiation therapy continued through the next decade using both megavoltage and kilovoltage CT systems integrated with the treatment machine.
The CT hardware developments reported in Medical Physics during the 1990s mostly relate to detector technology. An image intensifier coupled with a digital TV for tomography122 and a solid-state x-ray detector array consisting of scintillators coupled to Si-photodiodes123 were developed. Holdsworth pursued a related development by coupling an image intensifier to a charge coupled device camera in a benchtop CT scanner.124 Fahrig reported the use of a C-arm CT system with x-ray image intensifiers for vascular imaging and intervention.125 Synchrotron radiation sources began to be investigated and used for CT either in a conventional absorption contrast mode126 or by exploiting different physical phenomena such as refraction.127
Digital tomosynthesis based on a multiple projection method was also investigated,128, 129 along with related studies on sparse object image reconstruction from few-view data sets.130, 131, 132 Finally, Zwicker performed a feasibility study investigating transverse tomosynthesis on a digital radiotherapy simulator with conventional isocentric rotational geometry.133
The 2000s: The Slice Wars and the Renaissance of CT
The CT slice wars began in earnest in the 2000s as manufacturers added more rows to the detectors in an effort to increase the volume that could be scanned in a given time. For example, 16-slice diagnostic CT scanners became available in 2001, and some of the first evaluation studies were reported in Medical Physics.134 At present, state-of-the art clinical scanners have 256 or 320 rows and the first such prototype was developed by Toshiba in 2004. This prototype was compared to a then-standard 16-slice system135 and physical factors such as scatter were investigated.136 As all manufacturers added physical rows to the detector, some also explored further increases in sampling through the use of a z-flying focal spot strategy,137 which makes use of a periodic motion of the focal spot in the longitudinal direction to double the number of simultaneously acquired slices.
An important CT advance in 2000s was the development and widespread applications of CT systems with a flat-panel detector for cone-beam CT imaging. A flat-panel detector eliminates the need for acquiring data slice by slice, thus allowing the acquisition of fully volumetric images. Most CT systems with flat-panel detectors generally do not use a slip-ring technology, thus enjoying a high degree of imaging flexibility, making such systems particularly attractive for applications in image-guided radiation therapy and interventional procedures.138, 139, 140, 141, 142 For image-guided radiation therapy, researchers incorporated flat-panel detector systems on the treatment machine for kilovoltage and megavoltage cone-beam CT and tomosynthesis, now a fairly widespread technology.143, 144, 145, 146 For image-guided surgery and interventional radiology, C-arms featuring flat-panel detectors have been developed for intraoperative cone-beam CT.147, 148, 149, 150
Small-animal imaging has become increasingly important as transgenic and knockout mice are produced to model human diseases. Since the late 1990s, microcomputed tomography (micro-CT) has become a standard imaging technology for in vivo imaging of small animals as well as in vitro imaging of small specimens. For example, micro-CT has been used for imaging vascular specimens with different contrast agents.151 One of the challenges of small animal imaging is that most of their physiological parameters, such as heart rate and respiration rate, are much higher than those of humans while the scanners, for a variety of reasons, typically have worse temporal resolution than human scanners. Johnson and Badea have developed respiratory and cardiac gating techniques that allow for in vivo imaging of cardiopulmonary structures.152
Another important area of application of cone-beam CT over the current decade has been volumetric imaging of the breast. Using benchtop prototype and early clinical cone-beam CT imaging systems, numerous investigators153, 154, 155, 156, 157, 158 have demonstrated exquisite fat-to-fibroglandular soft-tissue discrimination in cone-beam CT images of the breast with total radiation dose no greater than that of a conventional two-view mammogram. Systems developed for early clinical studies utilize a pendant breast geometry, with studies underway to examine the diagnostic performance in breast cancer screening and diagnosis.
In all areas of CT, dose remains an important issue, and because the beam coverage of volumetric imaging devices in the z direction has increased significantly, traditional CT dose metrics and measurement techniques are no longer applicable. New dose metrics have been proposed and they are also appropriate for a direct comparison with the traditional CT dose metrics.159, 160
Another issue of increasing concern in CBCT imaging is the large quantity of scattered radiation generated in the patient and reaching the detector. In some imaging geometries, the scatter fluence may even exceed the primary fluence at the detector. Such a large scatter fraction reduces contrast and creates significant artifacts in the resulting reconstructions.161, 162 The use of postpatient antiscatter grids and the development of robust scatter correction algorithms remain important areas of research.
As discussed above, spiral/helical CT scanners began to adopt multiple detector rows in the late 1990s. At that time the interpolation-based methods approximated the x-ray beams as multiple, parallel fan-beams for up to four-slice scanners. However, when the number of the detector rows increased, the interpolation algorithms became inadequate due to the cone-beam artifacts introduced by the cone-beam angle subtended by the detector rows.163 Since late 1990s, a number of articles have been published in Medical Physics on approximate algorithms based on the FDK algorithm for image reconstruction on spiral/helical CT scanners with multirow detectors.164 These modifications of the FDK algorithm have been quite successful and form the backbone of the current reconstruction algorithms on virtually all commercial diagnostic multislice spiral/helical CT scanners. In parallel to the effort of developing approximate algorithms, active effort has been devoted to exact image reconstruction in spiral/helical cone-beam CT.
In 2001, a breakthrough FBP-based algorithm, now called Katsevich’s algorithm, was introduced for exact reconstruction in spiral/helical cone-beam CT. The original derivation of the Katsevich algorithm was nicely explained in an article published in Medical Physics,165 which also provides an alternative derivation of the Katsevich algorithm. The Katsevich algorithm was subsequently extended to address image reconstruction from spiral/helical cone-beam data acquired with a large detector.166 An alternative approach to reconstructing images on PI-lines or chords was later developed by Zou and Pan for spiral/helical cone-beam CT.167 An important feature of the approach is that algorithms such as the backprojection filtration algorithm can be derived for reconstruction of images within regions of interest from truncated projection data.167 This approach has also sparked considerable effort in the investigation and development of chord-based algorithms for image reconstruction for cone-beam CT.168
The development of algorithms for the correction or suppression of image artifacts remains active in the 2000s. Additional empirical methods have been proposed for scattering correction for flat-panel detector,169, 170 geometric misalignment correction,171, 172, 173 beam-hardening correction,174, 175 and motion-artifact reduction.176 An interesting investigation on local tomography from megavoltage CT may find some applications in radiation therapy.177
After a steady stream of papers on tomosynthesis and classical tomography in the 1970s, 1980s, and 1990s, there was a new surge of interest starting in the 2000s, reflecting the growing availability of digital flat panel x-ray detectors suitable for these techniques.178, 179, 180, 181, 182, 183, 184, 185 The primary application interest in tomosynthesis is in breast imaging, as an extension to mammography, where it may offer better detection rates, with little increase in radiation exposure. Several reconstruction algorithms have been investigated,177, 178, 179, 180, 181, 182, 183 including the backprojection method, FBP algorithm, the simultaneous algebraic reconstruction technique (SART), and a maximum likelihood method. The anisotropy in imaging performance caused by oblique x-ray incidence in detectors for breast tomosynthesis was also investigated.184, 185 The application of tomosynthesis for chest imaging has been proposed.186, 187 Optimization of a tomosynthesis system in terms of system response and lung nodules detection has been investigated in Medical Physics. Recently, there has been increased interest in applying digital tomosynthesis to radiation therapy for localization of radioactive seeds,188 as well as imaging of joints189 and 3D structures of therapy.190 The on-board imager mounted on a treatment system has recently been evaluated as a potential tomosynthesis system for use in image guided radiation therapy.191
DISCUSSION AND A LOOK TO THE FUTURE
While this brief survey of x-ray tomography publications in Medical Physics from the 1970s through the present gives a picture of the history of CT and related modalities, a look at a few recent articles gives a sense of some potential future directions for x-ray tomography.
As summarized above, although there have been tremendous advances in CT technologies and applications in the last two decades, there remains a strong desire and need to develop innovative x-ray detector and source technologies, high performance CT systems dedicated to specific imaging tasks, and advanced applications. As an example, a great deal of effort has been invested to develop highly compact, multiple x-ray sources with sufficient flux. Recently, a new x-ray source has been developed that utilizes carbon nanotubes as “cold cathodes” to replace the thermionic cathode used in the conventional x-ray tube. This development could find important applications in medicine, security scanning, and other fields, as discussed in several presentations at the 2007 AAPM Annual Meeting. It is worth noting that there have also been research efforts on the development of bench-top x-ray sources with (quasi) monochromatic, strong flux for various practical applications. One of the intriguing new directions—inverse geometry volumetric CT—turns traditional CT on its head, by using an extended scannable source or multiple disjoint sources to illuminate a relatively small detector. Pelc’s group has investigated the feasibility of such a system for acquiring volumetric images with negligible cone-beam artifacts.192, 193 While technical challenges remain to develop sources of sufficient flux, the approach has the premise to allow for fast and flexible imaging in a variety of clinical applications.
Another emerging direction seeks to exploit the remarkable physical properties of synchrotron radiation (monochromatic, high-flux, coherent) to optimize traditional absorption CT or to explore whole new forms of contrast based, for example, on detecting phase shifts in the coherent (and even incoherent) beams after passing through a sample. The comparison of conventional micro-CT and synchrotron-radiation CT has been performed by assessing trabecular bone microarchitecture in a large subset of human bone specimens.194
While synchrotron sources are obviously much less widely available than x-ray tubes, there has been emerging interest in simulating some of the properties of synchrotron sources by suitable modification of x-ray tube sources. For instance, quasimonochromatic CT systems using a conventional x-ray source with different filters have been developed with the aim of improving image quality without increasing patient dose. McKinley and co-workers performed a simulation study to compare the image quality obtained with different filter materials and system configurations, thereby showing the feasibility of such a system used for computed mammotomography.195 Saito built such a system using the combination of tungsten target and Er/Yb filters and demonstrated its advantages with phantom images.196
An area that deserves continued research and diligent clinical practice is the radiation dose associated with CT. As indications for diagnostic CT examination have grown, CT has come to contribute an increasingly significant portion of population dose. Thus, as our understanding of biological response to low radiation doses improves, it is incumbent upon the medical physics community not only to develop rigorous quantitative dosimetry techniques suitable to all forms of CT but also to ensure that the radiation dose associated with each exam is as low as reasonably achievable such that the resulting image is sufficient for the imaging task. Improved dosimetry techniques and metrics are under investigation.197, 198, 199, 200, 201 Improved CT scanner technologies, reconstruction techniques, and increasingly task-specific examination will be key to reducing dose in a broad range of applications.202, 203, 204, 205 The tendency toward lower kVp techniques appears a promising direction (e.g., 80 kVp abdominal or chest techniques in place of conventional 100–120 kVp techniques). Patient body habitus—particularly in light of an increasingly overweight population with increased demand on diagnostic medicine—needs to be more rigorously considered in technique selection and better accounted in patient dosimetry.206, 207, 208, 209 Low-dose thoracic CT provides an initial example of the potential for significant dose reduction in task-specific CT examination, motivated by the desire for early cancer detection.210, 211 Similar efforts beckon in all applications of CT—most immediately in head212, 213 and pediatric CT.214, 215, 216, 217
In order to improve the dose efficiency and image quality in CT, the utilization of photon-counting detectors has been proposed. Photon-counting image acquisition has a number of advantages over conventional charge integrating detector. It may allow for image acquisition with effective elimination of electronic noise and Swank noise, and could potentially allow for energy-resolved x-ray imaging. Rejection (either complete or in part) of the scattered radiation would be possible, depending on the spectrum of the input x-ray beam. In the case of a polychromatic x-ray source, signal to noise ratio is further improved due to the fact that the photon-counting detector assigns a more optimal energy-weighting factor to the detected photons.218 At present, photon-counting detectors cannot achieve the count rates necessary for medical CT, but with improvements they could come to replace the energy-weighted, current integrating detectors used at present.
Some of these new directions are certain to flourish and others to fade away. While it is not possible to predict which will suffer which fate without further investigation, it is certain that such research will continue to appear in the pages of Medical Physics and at the Annual Meetings of the AAPM.
ACKNOWLEDGMENTS
The authors would like to thank Xiao Han, Junguo Bian, Erik Pearson, Seungryong Cho, and Dan Xia for gathering some of the materials that have been used for producing the figures and table.
References
- Judy P. F., Balter S., Bassano D., McCullough E. C., Payne J. T., and Rothenberg L., AAPM Report No. 1: Phantoms for Performance Evaluation and Quality Assurance of CT Scanners (American Association of Physicists in Medicine, Chicago, 1977). [Google Scholar]
- Siedband M.et al. , AAPM Report No. 4: Basic Quality Control in Diagnostic Radiology (American Association of Physicists in Medicine, Chicago, 1978). [Google Scholar]
- Chu R. Y. L., Fisher J., Archer B. R., Conway B. J., Goodsitt M. M., Glaze S., Gray J. E., and Strauss K. J., AAPM Report No. 31: Standardized Methods for Measuring Diagnostic X-Ray Wxposures (American Institute of Physics, New York, 1990). [Google Scholar]
- Wagner L. K., Fontenla D. P., Kimme-Smith C., Rothenberg L. N., Shepard J., and Boone J. M., AAPM Report No. 35: Recommendations on Performance Characteristics of Diagnostic Exposure Meters (American Institute of Physics, New York, 1991) [DOI] [PubMed] [Google Scholar]; Printed also as: Wagner L. K., Fontenla D. P., Kimme-Smith C., Rothenberg L. N., Shepard J., and Boone J. M., “Recommendations on performance characteristics of diagnostic exposure meters: Report of AAPM Diagnostic X-Ray Imaging Task Group No. 6,” Med. Phys. 10.1118/1.596904 19, 231–241 (1992). [DOI] [PubMed] [Google Scholar]
- Lin P. -J., Beck T. J., Borras C., Cohen G., Jucius R. A., Kriz R. J., Nickoloff E. L., Rothenberg L. N., Strauss K. J., Villafana T., Cacak R. K., Gray J. E., Hangartner T. N., Hendrick R. E., and Rossi R. P., AAPM Report No. 39: Specification and Acceptance Testing of Computed Tomography Scanners (American Institute of Physics, New York, 1993). [Google Scholar]
- Kutcher G. J., Coia L., Gillin M., Hanson W. F., Leibel S., Morton R. J., Palta J. R., Purdy J., Reinstein L. E., Svensson G. K., Weller M., and Wingfield L., “AAPM Report No. 46: Comprehensive QA for Radiation Oncology (American Institute of Physics, New York, 1994) [DOI] [PubMed] [Google Scholar]; Printed also as: Kutcher G. J., Coia L., Gillin M., Hanson W. F., Leibel S., Morton R. J., Palta J. R., Purdy J. A., Reinstein L. E., Svensson G. K., Weller M., and Wingfield L., “Comprehensive QA for radiation oncology: Report of AAPM Radiation Therapy Committee Task Group 40,” Med. Phys. 10.1118/1.597316 21, 581–618 (1994). [DOI] [PubMed] [Google Scholar]
- Fisher J. R., Lin P. -J. P., Butler P., Conway B. J., Ranallo F., Rossi R., Sheppard J., and Strauss K., AAPM Report No. 60: Instrumentation Requirements of Diagnostic Radiological Physicists (Medical Physics, Madison, WI, 1998). [Google Scholar]
- Fraass B., Doppke K., Hunt M., Kutcher G., Starkschall G., Stern R., and Dyke J. V., “American Association of Physicists in Medicine Radiation Therapy Committee Task Group 53: Quality assurance for clinical radiotherapy treatment planning,” Med. Phys. 10.1118/1.598373 25, 1773–1829 (1998). [DOI] [PubMed] [Google Scholar]
- Shepard S. J.et al. , AAPM Report No. 74: Quality Control in Diagnostic Radiology (Medical Physics, Madison, WI, 2002). [Google Scholar]
- Mutic S., Palta J. R., Butker E. K., Das I. J., Huq M. S., Loo L. -N. D., Salter B. J., McCollough C. H., and Dyk J. V., “Quality assurance for computed-tomography simulators and the computed-tomography-simulation process: Report of the AAPM Radiation Therapy Task Group No. 66,” Med. Phys. 10.1118/1.1609271 30, 2762–2792 (2003). [DOI] [PubMed] [Google Scholar]
- Olch A. J., Kline R. W., Ibbott G. S., Anderson J. R., Deye J., FitzGerald T. J., Followill D., Gillin M. T., Huq M. S., Palta J. R., Purdy J. A., and Urie M. M., Quality Assurance for Clinical Trials: A Primer for Physicists (Medical Physics, Madison, WI, 2004). [Google Scholar]
- Keall P. J., Mageras G. S., Balter J. M., Emery R. S., Forster K. M., Jiang S. B., Kapatoes J. M., Kubo H. D., Low D. A., Murphy M. J., Murray B. R., Ramsey C. R., van Herk M. B., Vedam S. S., Wong J. W., and Yorke E., The Management of Respiratory Motion in Radiation Oncology (American Association of Physicists in Medicine, College Park, MD, 2006). [Google Scholar]
- Murphy M. J., Balter J., Balter S., BenComo J. A., Das I. J., Jiang S. B., Ma C. -M., Olivera G. H., Rodebaugh R. F., Ruchala K. J., Shirato H., and Yin F. -F., “The management of imaging dose during image-guided radiotherapy,” Med. Phys. 10.1118/1.2775667 34, 4041–4063 (2007). [DOI] [PubMed] [Google Scholar]
- Madsen M. T., Anderson J. A., Halama J. R., Kleck J., Simpkin D. J., Votaw J. R., Wendt R. E., Williams L. E., and Yester M. V., “AAPM Task Group 108: PET and PET/CT shielding requirements,” Med. Phys. 10.1118/1.2135911 33, 4–15 (2006). [DOI] [PubMed] [Google Scholar]
- McCollough C.et al. , AAPM Report 96: The Measurement, Reporting, and Management of Radiation Dose in CT (Medical Physics, Madison, WI, 2008). [Google Scholar]
- McCullough E. C., “Photon attenuation in computed tomography,” Med. Phys. 10.1118/1.594199 2, 307–320 (1975). [DOI] [PubMed] [Google Scholar]
- McCullough E. C. and Payne J. T., “X-ray-transmission computed tomography,” Med. Phys. 10.1118/1.594381 4, 85–98 (1977). [DOI] [PubMed] [Google Scholar]
- Szulc M. and Judy P. F., “Effect of x-ray source filtration on dose and image performance of CT scanners,” Med. Phys. 10.1118/1.594609 6, 479–486 (1979). [DOI] [PubMed] [Google Scholar]
- Millner M. R., Payne W. H., Waggener R. G., McDavid W. D., Dennis M. J., and Sank V. J., “Determination of effective energies in CT calibration,” Med. Phys. 10.1118/1.594488 5, 543–545 (1978). [DOI] [PubMed] [Google Scholar]
- Sorenson J. A., “Technique for evaluating radiation beam and image slice parameters of CT scanners,” Med. Phys. 10.1118/1.594554 6, 68–69 (1979). [DOI] [PubMed] [Google Scholar]
- Prasad S. C., “Effects of focal spot intensity distribution and collimator width in reconstructive x-ray tomography,” Med. Phys. 10.1118/1.594570 6, 229–232 (1979). [DOI] [PubMed] [Google Scholar]
- Thomas S. R., Schneider A. J., Kereiakes J. G., Lukin R. R., Chambers A. A., and Tomsick T. A., “An evaluation of the performance characteristics of different types of collimators used with the EMI brain scanner (MKI) and their significance in specific clinical applications,” Med. Phys. 10.1118/1.594419 5, 124–132 (1978). [DOI] [PubMed] [Google Scholar]
- Judy P. F., “The line spread function and modulation transfer function of a computed tomographic scanner,” Med. Phys. 10.1118/1.594283 3, 233–236 (1976). [DOI] [PubMed] [Google Scholar]
- Bischof C. J. and Ehrhardt J. C., “Modulation transfer function of the EMI CT head scanner,” Med. Phys. 10.1118/1.594305 4, 163–167 (1977). [DOI] [PubMed] [Google Scholar]
- Brooks R. A. and Di Chiro G., “Statistical limitations in x-ray reconstructive tomography,” Med. Phys. 10.1118/1.594240 3, 237–240 (1976). [DOI] [PubMed] [Google Scholar]
- Wagner R. F., Brown D. G., and Pastel M. S., “Application of information theory to the assessment of computed tomography,” Med. Phys. 10.1118/1.594559 6, 83–94 (1979). [DOI] [PubMed] [Google Scholar]
- Hanson K. M., “Detectability in computed tomographic images,” Med. Phys. 10.1118/1.594534 6, 441–451 (1979). [DOI] [PubMed] [Google Scholar]
- McDavid W. D., Waggener R. G., Payne W. H., and Dennis M. J., “Spectral effects on three-dimensional reconstruction from x rays,” Med. Phys. 10.1118/1.594200 2, 321–324 (1975). [DOI] [PubMed] [Google Scholar]
- McDavid W. D., Waggener R. G., Payne W. H., and Dennis M. J., “Correction for spectral artifacts in cross-sectional reconstruction from x rays,” Med. Phys. 10.1118/1.594302 4, 54–57 (1977). [DOI] [PubMed] [Google Scholar]
- Kijewski P. K. and Bjärngard B. E., “Correction for beam hardening in computed tomography,” Med. Phys. 10.1118/1.594429 5, 209–214 (1978). [DOI] [PubMed] [Google Scholar]
- Chase R. C. and Stein J. A., “An improved image algorithm for CT scanners,” Med. Phys. 10.1118/1.594486 5, 497–499 (1978). [DOI] [PubMed] [Google Scholar]
- Millner M. R., McDavid W. D., Waggener R. G., Dennis M. J., Payne W. H., and Sank V. J., “Extraction of information from CT scans at different energies,” Med. Phys. 10.1118/1.594555 6, 70–71 (1979). [DOI] [PubMed] [Google Scholar]
- Kelcz F., Joseph P. M., and Hilal S. K., “Noise considerations in dual energy CT scanning,” Med. Phys. 10.1118/1.594520 6, 418–425 (1979). [DOI] [PubMed] [Google Scholar]
- Lewitt R. M., “Processing of incomplete measurement data in computed tomography,” Med. Phys. 10.1118/1.594519 6, 412–417 (1979). [DOI] [PubMed] [Google Scholar]
- Gross G. and McCullough E. C., “Exposure values around an x-ray scanning transaxial tomograph (EMI scanner),” Med. Phys. 10.1118/1.594194 2, 282 (1975). [DOI] [PubMed] [Google Scholar]
- Suzuki A. and Suzuki M. N., “Use of a pencil-shaped ionization chamber for measurement of exposure resulting from a computed tomography scan,” Med. Phys. 10.1118/1.594445 5, 536–539 (1978). [DOI] [PubMed] [Google Scholar]
- Agarwal S. K., Friesen E. J., Bhaduri D., and Courlas G., “Dose distribution from a Delta-25 head scanner,” Med. Phys. 10.1118/1.594632 6, 302–304 (1979). [DOI] [PubMed] [Google Scholar]
- Moran P. R., Perman W. H., and Brown R. L., “Order-of-magnitude dose reduction for the EMI CT-5005,” Med. Phys. 10.1118/1.594397 5, 67–68 (1978). [DOI] [PubMed] [Google Scholar]
- Edelheit L. S., Herman G. T., and Lakshminarayanan A. V., “Reconstruction of objects from diverging x rays,” Med. Phys. 10.1118/1.594370 4, 226–231 (1977). [DOI] [PubMed] [Google Scholar]
- Payne W. H., Waggener R. G., McDavid W. D., and Dennis M. J., “Treatment planning in cobalt-60 radiotherapy using computerized tomography techniques,” Med. Phys. 10.1118/1.594393 5, 48–51 (1978). [DOI] [PubMed] [Google Scholar]
- Datta R., Datta S., McDavid W. D., and Waggener R. G., “Electron beam depth dose scaling by means of effective atomic number reconstructed from CT scans,” Med. Phys. 10.1118/1.594644 6, 526–529 (1979). [DOI] [PubMed] [Google Scholar]
- Holden J. E. and Ip W. R., “Continuous time-dependence in computed tomography,” Med. Phys. 10.1118/1.594458 5, 485–490 (1978). [DOI] [PubMed] [Google Scholar]
- Orphanoudakis S. C. and Strohbehn J. W., “Mathematical model of conventional tomography,” Med. Phys. 10.1118/1.594239 3, 224–232 (1976). [DOI] [PubMed] [Google Scholar]
- Orphanoudakis S. C., Strohbehn J. W., and Metz C. E., “Linearizing mechanisms in conventional tomographic imaging,” Med. Phys. 10.1118/1.594400 5, 1–7 (1978). [DOI] [PubMed] [Google Scholar]
- Harding G., Bertram U., and Weiss H., “Towards optimum blurring in spiral tomography,” Med. Phys. 10.1118/1.594432 5, 280–284 (1978). [DOI] [PubMed] [Google Scholar]
- Hounsfield G. N., “Computed medical imaging,” Med. Phys. 10.1118/1.594709 7, 283–290 (1980). [DOI] [PubMed] [Google Scholar]
- Glover G. H. and Pelc N. J., “Nonlinear partial volume artifacts in x-ray computed tomography,” Med. Phys. 10.1118/1.594678 7, 238–248 (1980). [DOI] [PubMed] [Google Scholar]
- Glover G. H. and Pelc N. J., “An algorithm for the reduction of metal clip artifacts in CT reconstructions,” Med. Phys. 10.1118/1.595032 8, 799–807 (1981). [DOI] [PubMed] [Google Scholar]
- Glover G. H., “Compton scatter effects in CT reconstructions,” Med. Phys. 10.1118/1.595197 9, 860–867 (1982). [DOI] [PubMed] [Google Scholar]
- Verly J. G., “X-ray computed tomography in the presence of arbitrary symmetrical focal spot intensity distributions,” Med. Phys. 10.1118/1.594655 7, 27–34 (1980). [DOI] [PubMed] [Google Scholar]
- Schneiders N. J., “Computer assisted MTF determination in CT,” Med. Phys. 10.1118/1.594769 7, 76–78 (1980). [DOI] [PubMed] [Google Scholar]
- Hanson K. M., “Detectability in computed tomographic images,” Med. Phys. 10.1118/1.594534 6, 441–451 (1979). [DOI] [PubMed] [Google Scholar]
- Judy P. F., Swensson R. G., and Szulc M., “Lesion detection and signal to noise ratio in CT images,” Med. Phys. 10.1118/1.594903 8, 13–23 (1981). [DOI] [PubMed] [Google Scholar]
- Duerinckx A. J. and Macovski A., “Information and artifact in computed tomography image statistics,” Med. Phys. 10.1118/1.594771 7, 127–134 (1980). [DOI] [PubMed] [Google Scholar]
- Parker D. L., Couch J. L., Peschmann K. R., Smith V., Jimbo M., and Wang E. C., “Design constraints in computed tomography: A theoretical review,” Med. Phys. 10.1118/1.595105 9, 531–539 (1982). [DOI] [PubMed] [Google Scholar]
- Shope T. B., Gagne R. M., and Johnson G. C., “A method for describing the doses delivered by transmission x-ray computed tomography,” Med. Phys. 10.1118/1.594995 8, 488–495 (1981). [DOI] [PubMed] [Google Scholar]
- Spokas J. J., “Dose descriptors for computed tomography,” Med. Phys. 10.1118/1.595072 9, 288–292 (1982). [DOI] [PubMed] [Google Scholar]
- Moore M. M., Cacak R. K., and Hendee W. R., “Multisegmented ion chamber for CT scanner dosimetry,” Med. Phys. 10.1118/1.595022 8, 640–645 (1981). [DOI] [PubMed] [Google Scholar]
- Beck J. W., Dunn W. L., and O’Foghludha F., “Monte Carlo model for absorbed dose calculations in computed tomography,” Med. Phys. 10.1118/1.595306 10, 314–320 (1983). [DOI] [PubMed] [Google Scholar]
- Wong J. W. and Henkelman R. M., “A new approach to CT pixel-based photon dose calculations in heterogeneous media,” Med. Phys. 10.1118/1.595294 10, 199–208 (1983). [DOI] [PubMed] [Google Scholar]
- Chu R. Y. L.et al. , AAPM Report No. 31: Standardized Methods for Measuring Siagnostic X-Ray Exposures (American Institute of Physics, New York, 1991). [Google Scholar]
- Drost D. J. and Fenster A., “Experimental dual xenon detectors for quantitative CT and spectral artifact correction,” Med. Phys. 10.1118/1.594672 7, 101–107 (1980). [DOI] [PubMed] [Google Scholar]
- Edwards M., Keller J., Larsen G., Rowberg A., Sandler B., and Whitaker R., “A computed tomography radiation therapy treatment planning system utilizing a whole body CT scanner,” Med. Phys. 10.1118/1.594944 8, 242–248 (1981). [DOI] [PubMed] [Google Scholar]
- Simpson R. G., Chen C. T., Grubbs E. A., and Swindell W., “A 4-MV CT scanner for radiation therapy: The prototype system,” Med. Phys. 10.1118/1.595102 9, 574–579 (1982). [DOI] [PubMed] [Google Scholar]
- Swindell W., “A 4-MV CT scanner for radiation therapy; spectral properties of the therapy beam,” Med. Phys. 10.1118/1.595280 10, 347–351 (1983). [DOI] [PubMed] [Google Scholar]
- Parker D. L., “Optimal short scan convolution reconstruction for fan beam CT,” Med. Phys. 10.1118/1.595078 9, 254–257 (1982). [DOI] [PubMed] [Google Scholar]
- Johns P. C. and Yaffe M., “Scattered radiation in fan beam imaging system,” Med. Phys. 10.1118/1.595076 9, 231–239 (1982). [DOI] [PubMed] [Google Scholar]
- Joseph P. M. and Spital R. D., “The effects of scatter in x-ray computed tomography,” Med. Phys. 10.1118/1.595111 9, 464–472 (1982). [DOI] [PubMed] [Google Scholar]
- Hangartner T. N., “Correction of scatter in computed tomography images of bone,” Med. Phys. 10.1118/1.596089 14, 335–340 (1987). [DOI] [PubMed] [Google Scholar]
- Vetter J. R. and Holden J. E., “Correction for scattered radiation and other background signals in dual-energy computed tomography material thickness measurements,” Med. Phys. 10.1118/1.596187 15, 726–731 (1988). [DOI] [PubMed] [Google Scholar]
- Wagner F. C., Macovski A., and Nishimura D. G., “Dual-energy x-ray projection imaging: Two sampling schemes for the correction of scattered radiation,” Med. Phys. 10.1118/1.596188 15, 732–748 (1988). [DOI] [PubMed] [Google Scholar]
- Moore S. C., Judy P. F., Garnic J. D., Kambic G. X., Bonk F., Cochran G., Margosian P., McCroskey W., and Foote F., “Prospectively gated cardiac computed tomography,” Med. Phys. 10.1118/1.595420 10, 846–855 (1983). [DOI] [PubMed] [Google Scholar]
- Moore S. C. and Judy P. F., “Cardiac computed tomography using redundant-ray prospective gating,” Med. Phys. 10.1118/1.596134 14, 193–196 (1987). [DOI] [PubMed] [Google Scholar]
- Siddon R. L., “Fast calculation of the exact radiological path for a three-dimensional CT array,” Med. Phys. 10.1118/1.595715 12, 252–255 (1985). [DOI] [PubMed] [Google Scholar]
- Nassi M. and Brody W. R., “Regional myocardial flow estimation using computed tomography,” Med. Phys. 10.1118/1.594875 8, 302–307 (1981). [DOI] [PubMed] [Google Scholar]
- Kearfott K. J., Lu H. C., Rottenberg D. A., and Deck M. D. F., “The effects of CT drift on xenon CT measurement of regional cerebral blood flow,” Med. Phys. 10.1118/1.595552 11, 686–689 (1984). [DOI] [PubMed] [Google Scholar]
- Guthaner D. F., Nassi M., Bradley B., Gould E. B., Mai C. H., and Schmidt K. E., “Quantitative evaluation of left ventricular function using computed tomography,” Med. Phys. 10.1118/1.595692 12, 333–338 (1985). [DOI] [PubMed] [Google Scholar]
- Jaschke W., Gould R. G., Assimakopoulos P. A., and Lipton M. J., “Flow measurements with a high-speed computed tomography scanner,” Med. Phys. 10.1118/1.596076 14, 238–243 (1987). [DOI] [PubMed] [Google Scholar]
- Good W. F., Gur D., Yonas H., and Herron J. M., “Errors in cerebral blood flow determinations by xenon-enhanced computed tomography due to estimation of arterial xenon concentrations,” Med. Phys. 10.1118/1.596051 14, 377–381 (1987). [DOI] [PubMed] [Google Scholar]
- Good W. F. and Gur D., “The effect of computed tomography noise and tissue heterogeneity on cerebral blood flow determination by xenon-enhanced computed tomography,” Med. Phys. 10.1118/1.596067 14, 557–561 (1987). [DOI] [PubMed] [Google Scholar]
- Good W. F., Gur D., Herron J. M., and Kennedy W. H., “The development of a xenon/computed tomography cerebral blood flow quality assurance phantom,” Med. Phys. 10.1118/1.596014 14, 867–869 (1987). [DOI] [PubMed] [Google Scholar]
- Kalender W. A., Perman W. H., Vetter J. R., and Klotz E., “Evaluation of a prototype dual-energy computed tomographic apparatus. I. Phantom studies,” Med. Phys. 10.1118/1.595958 13, 334–339 (1986). [DOI] [PubMed] [Google Scholar]
- Vetter J. R., Perman W. H., Kalender W. A., Mazess R. B., and Holden J. E., “Evaluation of a prototype dual-energy computed tomographic apparatus. II. Determination of vertebral bone mineral content,” Med. Phys. 10.1118/1.595951 13, 340–343 (1986). [DOI] [PubMed] [Google Scholar]
- D. D.Robertson, Jr. and Huang H. K., “Quantitative bone measurements using x-ray computed tomography with second-order correction,” Med. Phys. 10.1118/1.595971 13, 474–479 (1986). [DOI] [PubMed] [Google Scholar]
- Sandor T., Weissman B., and Brown E., “Effect of intervertebral changes of the spinal trabecular and cortical mineral content on the precision requirements in longitudinal single and dual energy computed tomography examinations,” Med. Phys. 10.1118/1.596417 16, 218–224 (1989). [DOI] [PubMed] [Google Scholar]
- Fallone B. G., Moran P. R., and Podgorsak E. B., “Noninvasive thermometry with a clinical x-ray CT scanner,” Med. Phys. 10.1118/1.595117 9, 715–721 (1982). [DOI] [PubMed] [Google Scholar]
- Buonocore M. H., Brody W. R., and Macovski A., “Fast minimum variance estimator for limited angle CT image reconstruction,” Med. Phys. 10.1118/1.594838 8, 695–702 (1981). [DOI] [PubMed] [Google Scholar]
- Ruttimann U. E., Qi X. -L., and Webber R. L., “An optimal synthetic aperture for circular tomosynthesis,” Med. Phys. 10.1118/1.596348 16, 398–405 (1989). [DOI] [PubMed] [Google Scholar]
- Liu J., Nishimura D., and Macovski A., “Vessel imaging using dual-energy tomosynthesis,” Med. Phys. 10.1118/1.595998 14, 950–955 (1987). [DOI] [PubMed] [Google Scholar]
- Kruger R. A., Reinecke D. R., Smith S. W., and Ning R., “Reconstruction of blood vessels from x-ray subtraction projections: Limited angle geometry,” Med. Phys. 10.1118/1.595997 14, 940–949 (1987). [DOI] [PubMed] [Google Scholar]
- Crawford C. R. and King K. F., “Computed tomography scanning with simultaneous patient translation,” Med. Phys. 10.1118/1.596464 17, 967–982 (1990). [DOI] [PubMed] [Google Scholar]
- Kalender W. A. and Polacin A., “Physical performance characteristics of spiral CT scanning,” Med. Phys. 10.1118/1.596607 18, 910–915 (1991). [DOI] [PubMed] [Google Scholar]
- Wang G. and Vannier M. W., “Helical CT image noise-analytical results,” Med. Phys. 10.1118/1.596950 20, 1635–1640 (1993). [DOI] [PubMed] [Google Scholar]
- Wang G. and Vannier M. W., “Spatial variation of section sensitivity profile in spiral computed tomography,” Med. Phys. 10.1118/1.597199 21, 1491–1497 (1994). [DOI] [PubMed] [Google Scholar]
- Polacin A., Kalender W. A., Brink J., and Vannier M. A., “Measurement of slice sensitivity profiles in spiral CT,” Med. Phys. 10.1118/1.597251 21, 133–140 (1994). [DOI] [PubMed] [Google Scholar]
- Hsieh J., “A general approach to the reconstruction of x-ray helical computed tomography,” Med. Phys. 10.1118/1.597706 23, 221–229 (1996). [DOI] [PubMed] [Google Scholar]
- Hsieh J., “Nonstationary noise characteristics of the helical scan and its impact on image quality and artifacts,” Med. Phys. 10.1118/1.598026 24, 1375–1384 (1997). [DOI] [PubMed] [Google Scholar]
- Hu H., “Helical CT reconstruction with longitudinal filtration,” Med. Phys. 10.1118/1.598409 25, 2130–2138 (1998). [DOI] [PubMed] [Google Scholar]
- Yen S. Y., Rubin G. D., and Napel S., “Spatially varying longitudinal aliasing and resolution in spiral computed tomography,” Med. Phys. 10.1118/1.598801 26, 2617–2625 (1999). [DOI] [PubMed] [Google Scholar]
- Pan X., “Optimal noise control in and fast reconstruction of fan-beam computed tomography image,” Med. Phys. 10.1118/1.598574 26, 689–697 (1999). [DOI] [PubMed] [Google Scholar]
- Huda W. and Atherton J. V., “Energy imparted in computed tomograpy,” Med. Phys. 10.1118/1.597564 22, 1263–1269 (1995). [DOI] [PubMed] [Google Scholar]
- Atherton J. V. and Huda W., “Energy imparted and effective doses in computed tomography,” Med. Phys. 10.1118/1.597667 23, 735–741 (1996). [DOI] [PubMed] [Google Scholar]
- Kalender W. A., Wolf H., and Suess C., “Dose reduction in CT by anatomically adapted tube current modulation. II. Phantom measurements,” Med. Phys. 10.1118/1.598738 26, 2248–2253 (1999). [DOI] [PubMed] [Google Scholar]
- McNitt-Gray M. F., Cagnon C. H., Solberg T. D., and Chetty I., “Radiation dose in spiral CT: The relative effects of collimation and pitch,” Med. Phys. 10.1118/1.598532 26, 409–414 (1999). [DOI] [Google Scholar]
- Liang Y. and Kruger R. A., “Dual-slice spiral versus single-slice spiral scanning: Comparison of the physical performance of two computed tomography scanners,” Med. Phys. 10.1118/1.597705 23, 205–220 (1996). [DOI] [PubMed] [Google Scholar]
- Taguchi K. and Aradate H., “Algorithm for image reconstruction in multi-slice helical CT,” Med. Phys. 10.1118/1.598230 25, 550–561 (1998). [DOI] [PubMed] [Google Scholar]
- Hu H., “Multi-slice helical CT: Scan and reconstruction,” Med. Phys. 10.1118/1.598470 26, 5–18 (1999). [DOI] [PubMed] [Google Scholar]
- McCollough C. H. and Zink F. E., “Performance evaluation of a multi-slice CT system,” Med. Phys. 10.1118/1.598777 26, 2223–2230 (1999). [DOI] [PubMed] [Google Scholar]
- Corrigan N. M., Chavez A. E., Wisner E. R., and Boone J. M., “A multiple detector array helical x-ray microtomography system for specimen imaging,” Med. Phys. 10.1118/1.598662 26, 1708 (1999). [DOI] [PubMed] [Google Scholar]
- Cardinal H. N. and Fenster A., “An accurate method for direct dual-energy calibration and decomposition,” Med. Phys. 10.1118/1.596512 17, 327–341 (1990). [DOI] [PubMed] [Google Scholar]
- Goodsitt M. M. and Johnson R. H., “Precision in quantitative CT: Impact of x-ray dose and matrix size,” Med. Phys. 10.1118/1.596820 19, 1025–1036 (1992). [DOI] [PubMed] [Google Scholar]
- Steenbeek J. C. M., van Kuijk C., Grashuis J. L., and van Panthaleon van Eck R. B., “Selection of fat-equivalent materials in postprocessing dual-energy quantitative CT,” Med. Phys. 10.1118/1.596823 19, 1051–1056 (1992). [DOI] [PubMed] [Google Scholar]
- Kachelriess M. and Kalender W. A., “Electrocardiogram-correlated image reconstruction from subsecond spiral computed tomography scans of the heart,” Med. Phys. 10.1118/1.598453 25, 2417–2432 (1998). [DOI] [PubMed] [Google Scholar]
- Kemerink G. J., Kruize H. H., Lamers R. J. S., and van Engelshoven J. M. A., “Density resolution in quantitative computed tomography of foam and lung,” Med. Phys. 10.1118/1.597757 23, 1697–1708 (1996). [DOI] [PubMed] [Google Scholar]
- Durand E. P. and Ruegsegger P., “High-contrast resolution of CT images for bone structure analysis,” Med. Phys. 10.1118/1.596847 19, 569–573 (1992). [DOI] [PubMed] [Google Scholar]
- Judy P. F., Swensson R. G., Nawfel R. D., Chan K. H., and Seltzer S. E., “Contrast-detail curves for liver CT,” Med. Phys. 10.1118/1.596791 19, 1167–1174 (1992). [DOI] [PubMed] [Google Scholar]
- Polacin A., Kalender W. A., and Eidloth H., “Simulation study of cerebral blood flow measurements in xenon-CT: Evaluation of washin/washout procedures,” Med. Phys. 10.1118/1.596738 18, 1025–1031 (1991). [DOI] [PubMed] [Google Scholar]
- Ling C., Rogers C. C., and Morton R. J., Computed Tomography in Radiation therapy (Raven, New York, 1983). [Google Scholar]
- Swindell W., Morton E. J., Evans P. M., and Lewis D. G., “The design of megavoltage projection imaging systems: Some theoretical aspects,” Med. Phys. 10.1118/1.596735 18, 855–866 (1991). [DOI] [PubMed] [Google Scholar]
- Mosleh-Shirazi M. A., Swindell W., and Evans P. M., “Optimization of the scintillation detector in a combined 3D megavoltage CT scanner and portal imager,” Med. Phys. 10.1118/1.598377 25, 1880–1890 (1998). [DOI] [PubMed] [Google Scholar]
- Jaffray D. A., Battista J. J., Fenster A., and Munro P., “X-ray source of medical linear accelerators: Focal and extra-focal radiation,” Med. Phys. 10.1118/1.597106 20, 1417–1427 (1993). [DOI] [PubMed] [Google Scholar]
- Takahashi M., Yoshioka S., Bussaka H., Higashida Y., Kamiya M., and Tsuneoka M., “Digital TV tomography: Description and physical assessment,” Med. Phys. 10.1118/1.596466 17, 681–685 (1990). [DOI] [PubMed] [Google Scholar]
- Takahashi T., Nakagawa M., Yoshida M., and Takeuchi H., “Highly stable solid-state x-ray detector array,” Med. Phys. 10.1118/1.596919 19, 1161–1166 (1992). [DOI] [PubMed] [Google Scholar]
- Holdsworth D. W., Drangova M., and Fenster A., “A high-resolution XRII-based quantitative volume CT scanner,” Med. Phys. 10.1118/1.597038 20, 449–462 (1993). [DOI] [PubMed] [Google Scholar]
- Fahrig R. and Holdsworth D. W., “Three-dimensional computed tomographic reconstruction using a C-arm mounted XRII: image-based correction of gantry motion nonidealities,” Med. Phys. 10.1118/1.598854 27, 30–38 (2000). [DOI] [PubMed] [Google Scholar]
- Salome M., Peyrin F., Cloetens P., Odet C., Laval-Jeantet A. -M., Baruchel J., and Spanne P., “A synchrotron radiation microtomography system for the analysis of trabecular bone samples,” Med. Phys. 10.1118/1.598736 26, 2194–2204 (1999). [DOI] [PubMed] [Google Scholar]
- Yagi N., Suzuki Y., Umetani K., Kohmura Y., and Yamasaki K., “Refraction-enhanced x-ray imaging of mouse lung using synchrotron radiation source,” Med. Phys. 10.1118/1.598735 26, 2190–2193 (1999). [DOI] [PubMed] [Google Scholar]
- Kolitsi Z., Panayiotakis G., Anastassopoulos V., Scodras A., and Pallikarakis N., “A multiple projection method for digital tomosynthesis,” Med. Phys. 10.1118/1.596822 19, 1045–1050 (1992). [DOI] [PubMed] [Google Scholar]
- Kolitsi Z., Panayiotakis G., and Pallikarakis N., “A method for selective removal of out-of-plane structures in digital tomosynthesis,” Med. Phys. 10.1118/1.597060 20, 47–50 (1993). [DOI] [PubMed] [Google Scholar]
- Henri C. J., Collins D. L., and Peters T. M., “Analysis of projection geometry for fiew-view reconstruction of sparse objects,” Med. Phys. 10.1118/1.597117 20, 1537–1547 (1993). [DOI] [PubMed] [Google Scholar]
- Henri C. J. and Peters T. M., “Three-dimensional reconstruction of vascular trees: Experimental evaluation,” Med. Phys. 10.1118/1.597815 23, 617–627 (1996). [DOI] [PubMed] [Google Scholar]
- Robert N., Peyrin F., and Yaffe M. J., “Binary vascular reconstruction from a limited number of cone beam projections,” Med. Phys. 10.1118/1.597223 21, 1839–1851 (1994). [DOI] [PubMed] [Google Scholar]
- Zwicker R. D. and Atari N. A., “Transverse tomosynthesis on a digital simulator,” Med. Phys. 10.1118/1.598006 24, 867–871 (1997). [DOI] [PubMed] [Google Scholar]
- Flohr T., Stierstorfer K., Bruder H., Simon J., Polacin A., and Schaller S., “Image reconstruction and image quality evaluation for a 16-slice CT scanner,” Med. Phys. 10.1118/1.1562168 30, 832–845 (2003). [DOI] [PubMed] [Google Scholar]
- Mori S., Endo M., Tsunoo T., Kandatsu S., and Tanada S., “Physical performance evaluation of a 256-slice CT-scanner for four-dimensional imaging,” Med. Phys. 10.1118/1.1747758 31, 1348–1356 (2004). [DOI] [PubMed] [Google Scholar]
- Endo M., Mori S., and Tsunoo T., “Magnitude and effects of x-ray scatter in a 256-slice CT scanner,” Med. Phys. 10.1118/1.2239366 33, 3359–3368 (2006). [DOI] [PubMed] [Google Scholar]
- Flohr T. G., Stierstorfer K., Ulzheimer S., Bruder H., Primak A. N., and McCollough C. H., “Image reconstruction and image quality evaluation for a 64-slice CT scanner with z-flying focal spot,” Med. Phys. 10.1118/1.1949787 32, 2536–2547 (2005). [DOI] [PubMed] [Google Scholar]
- Jaffray D. A. and Siewerdsen J. H., “Cone-beam computed tomography with a flat-panel imager: Initial performance characterization,” Med. Phys. 10.1118/1.599009 27, 1311–1323 (2000). [DOI] [PubMed] [Google Scholar]
- El-Mohri Y., Jee K. -W., Antonuk L. E., Maolinbay M., and Zhao Q., “Determination of the detective quantum efficiency of a prototype, megavoltage indirect detection, active matrix flat-panel imager,” Med. Phys. 10.1118/1.1413516 28, 2538–2550 (2001). [DOI] [PubMed] [Google Scholar]
- Groh B. A., Siewerdsen J. H., Drake D. G., Wong J. W., and Jaffray D. A., “A performance comparison of flat-panel imager-based MV and kV cone-beam CT,” Med. Phys. 10.1118/1.1477234 29, 967–975 (2002). [DOI] [PubMed] [Google Scholar]
- Fahrig R. and Holdsworth D. W., “Three-dimensional computed tomographic reconstruction using a C-arm mounted XRII: Image-based correction of gantry motion nonidealities,” Med. Phys. 10.1118/1.598854 27, 30–38 (2000). [DOI] [PubMed] [Google Scholar]
- Siewerdsen J. H., Moseley D. J., Burch S., Bisland S. K., Bogaards A., Wilson B. C., and Jaffray D. A., “Volume CT with a flat-panel detector on a mobile, isocentric C-arm: Pre-clinical investigation in guidance of minimally invasive surgery,” Med. Phys. 10.1118/1.1836331 32, 241–254 (2005). [DOI] [PubMed] [Google Scholar]
- Sonke J. J., Zijp L., Remeijer P., and Van Herk M., “Respiratory correlated cone beam CT,” Med. Phys. 10.1118/1.1869074 32, 1176–1186 (2005). [DOI] [PubMed] [Google Scholar]
- Sharpe M. B., Moseley D. J., Purdie T. G., Islam M., Siewerdsen J. H., and Jaffray D. A., “The stability of mechanical calibration for a kV cone beam computed tomography system integrated with linear accelerator,” Med. Phys. 10.1118/1.2143141 33, 136–144 (2006). [DOI] [PubMed] [Google Scholar]
- Yoo S., Kim G. Y., Hammoud R., Elder E., Pawlicki T., Guan H., Fox T., Luxton G., Yin F. F., and Munro P., “A quality assurance program for the on-board imagers,” Med. Phys. 10.1118/1.2362872 33, 4431–4447 (2006). [DOI] [PubMed] [Google Scholar]
- Siewerdsen J. H., Moseley D. J., Burch S., Bisland S. K., Bogaards A., Wilson B. C., and Jaffray D. A., “Volume CT with a flat-panel detector on a mobile, isocentric C-arm: Pre-clinical investigation in guidance of minimally invasive surgery,” Med. Phys. 10.1118/1.1836331 32, 241–254 (2005). [DOI] [PubMed] [Google Scholar]
- Daly M. J., Siewerdsen J. H., Moseley D. J., Jaffray D. A., and Irish J. C., “Intraoperative cone-beam CT for guidance of head and neck surgery: Assessment of dose and image quality using a C-arm prototype,” Med. Phys. 10.1118/1.2349687 33, 3767–3780 (2006). [DOI] [PubMed] [Google Scholar]
- Fahrig R., Dixon R., Payne T., Morin R. L., Ganguly A., and Strobel N., “Dose and image quality for a cone-beam C-arm CT system,” Med. Phys. 10.1118/1.2370508 33, 4541–4550 (2006). [DOI] [PubMed] [Google Scholar]
- Schmidgunst C., Ritter D., and Lang E., “Calibration model of a dual gain flat panel detector for 2D and 3D x-ray imaging,” Med. Phys. 10.1118/1.2760024 34, 3649–3664 (2007). [DOI] [PubMed] [Google Scholar]
- Marxen M., Thornton M. M., Chiarot C. B., Klement G., Koprivnikar J., Sled J. G., and Henkelman R. M., “MicroCT scanner performance and considerations for vascular specimen imaging,” Med. Phys. 10.1118/1.1637971 31, 305–313 (2004). [DOI] [PubMed] [Google Scholar]
- Badea C., Hedlund L. W., and Johnson G. A., “Micro-CT with respiratory and cardiac gating,” Med. Phys. 10.1118/1.1812604 31, 3324–3329 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. and Ning R., “Cone-beam volume CT breast imaging: Feasibility study,” Med. Phys. 10.1118/1.1461843 29, 755–770 (2002). [DOI] [PubMed] [Google Scholar]
- McKinley R. L., Tornai M. P., Samei E., and Bradshaw M. L., “Simulation study of a quasi-monochromatic beam for x-ray computed mammotomography,” Med. Phys. 10.1118/1.1668371 31, 800–813 (2004). [DOI] [PubMed] [Google Scholar]
- Kwan A. L. C., Boone J. M., and Shah N., “Evaluation of x-ray scatter properties in a dedicated cone-beam breast CT scanner,” Med. Phys. 10.1118/1.1954908 32, 2967–2975 (2005). [DOI] [PubMed] [Google Scholar]
- Gong X., Glick S. J., Liu B., Vedula A. A., and Thacker S., “A computer simulation study comparing lesion detection accuracy with digital mammography, breast tomosynthesis, and cone-beam CT breast imaging,” Med. Phys. 10.1118/1.2174127 33, 1041–1052 (2006). [DOI] [PubMed] [Google Scholar]
- Kwan A. L. C., Boone J. M., Yang K., and Huang S., “Evaluation of the spatial resolution characteristics of a cone-beam breast CT scanner,” Med. Phys. 10.1118/1.2400830 34, 275–281 (2007). [DOI] [PubMed] [Google Scholar]
- Lai C. J., Shaw C. C., Chen L., Altunbas M. C., Liu X., Han T., Wang T., Yang W., Whitman G. J., and Tu S., “Visibility of microcalcification in cone beam breast CT: Effects of x-ray tube voltage and radiation dose,” Med. Phys. 10.1118/1.2745921 34, 2995–3004 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakonechny K. D., Fallone B. G., and Rathee S., “Novel methods of measuring single scan dose profiles and cumulative dose in CT,” Med. Phys. 10.1118/1.1835571 32, 98–190 (2005). [DOI] [PubMed] [Google Scholar]
- Fahrig R., Dixon R., Payne T., Morin R. L., Ganguly A., and Strobel N., “Dose and image quality for a cone-beam C-arm CT system,” Med. Phys. 10.1118/1.2370508 33, 4541–4550 (2006). [DOI] [PubMed] [Google Scholar]
- Siewerdsen J. H., Moseley D. J., Bakhtiar B., Richard S., and Jaffray D. A., “The influence of antiscatter grids on soft-tissue detectability in cone-beam computed tomography with flat-panel detectors,” Med. Phys. 10.1118/1.1819789 31, 3506–3520 (2004). [DOI] [PubMed] [Google Scholar]
- Graham S. A., Moseley D. J., Siewerdsen J. H., and Jaffray D. A., “Compensators for dose and scatter management in cone-beam computed tomography,” Med. Phys. 10.1118/1.2740466 34, 2691–2703 (2007). [DOI] [PubMed] [Google Scholar]
- La Rivière P. J. and Pan X., “Favorable noise uniformity properties of Fourier-based interpolation and reconstruction approaches in single-slice helical computed tomography,” Med. Phys. 10.1118/1.1477229 29, 943–951 (2002). [DOI] [PubMed] [Google Scholar]
- Kachelrieß M., Schaller S., and Kalender W. A., “Advanced single-slice rebinning in cone-beam spiral CT,” Med. Phys. 10.1118/1.598938 27, 754–772 (2000). [DOI] [PubMed] [Google Scholar]
- Chen G. H., “An alternative derivation of Katsevich’s cone-beam reconstruction formula,” Med. Phys. 10.1118/1.1628413 30, 3217 (2003). [DOI] [PubMed] [Google Scholar]
- Bontus C., Köhler T., and Proksa R., “A quasiexact reconstruction algorithm for helical CT using a 3-Pi acquisition,” Med. Phys. 10.1118/1.1601913 30, 2493–2502 (2003). [DOI] [PubMed] [Google Scholar]
- Pan X., Zou Y., and Xia D., “Image reconstruction in peripheral and central regions-of-interest and data redundancy,” Med. Phys. 10.1118/1.1844171 32, 673–684 (2005). [DOI] [PubMed] [Google Scholar]
- Yu H., Zhao S., Ye Y., and Wang G., “Exact BPF and FBP algorithms for nonstandard saddle curves,” Med. Phys. 10.1118/1.2074207 32, 3305–3312 (2005). [DOI] [PubMed] [Google Scholar]
- Ning R., Tang X., and Conover D., “X-ray scatter correction algorithm for cone beam CT imaging,” Med. Phys. 10.1118/1.1711475 31, 1195–1202 (2004). [DOI] [PubMed] [Google Scholar]
- Siewerdsen J. H., Daly M. J., Bakhtiar B., Moseley D. J., Richard S., Keller H., and Jaffray D. A., “A simple, direct method for x-ray scatter estimation and correction in digital radiography and cone-beam CT,” Med. Phys. 10.1118/1.2148916 33, 187–197 (2006). [DOI] [PubMed] [Google Scholar]
- Karolczak M. and Kalender W., “Implementation of a cone-beam reconstruction algorithm for the single-circle source orbit with embedded misalignment correction using homogeneous coordinates,” Med. Phys. 10.1118/1.1406514 28, 2050–2069 (2001). [DOI] [PubMed] [Google Scholar]
- von Smekal L., Kachelrieß M., Stepina E., and Kalender W. A., “Geometric misalignment and calibration in cone-beam tomography,” Med. Phys. 10.1118/1.1803792 31, 3242–3266 (2004). [DOI] [PubMed] [Google Scholar]
- Sillanpaa J., Chang J., Amols H., and Mageras G., “A method for determining the gantry angle for megavoltage cone beam imaging,” Med. Phys. 10.1118/1.1854776 32, 566–569 (2005). [DOI] [PubMed] [Google Scholar]
- Cho Y., Moseley D. J., Siewerdsen J. H., and Jaffray D. A., “Accurate technique for complete geometric calibration of cone-beam computed tomography systems,” Med. Phys. 10.1118/1.1869652 32, 968–983 (2005). [DOI] [PubMed] [Google Scholar]
- Hsieh J., “An iterative approach to the beam hardening correction in cone beam CT,” Med. Phys. 10.1118/1.598853 27, 23–29 (2000). [DOI] [PubMed] [Google Scholar]
- Alles J. and Mudde R. F., “Beam hardening: Analytical considerations of the effective attenuation coefficient of x-ray tomography,” Med. Phys. 10.1118/1.2742501 34, 2882–2889 (2007). [DOI] [PubMed] [Google Scholar]
- Lu W., Parikh P. J., Hubenschmidt J. P., Politte D. G., Whiting B. R., Bradley J. D., Mutic S., and Low D. A., “Reduction of motion blurring artifacts using respiratory gated CT in sinogram space: A quantitative evaluation,” Med. Phys. 10.1118/1.2074187 32, 3295–3304 (2005). [DOI] [PubMed] [Google Scholar]
- Anastasio M. A., Shi D., and Pan X., “A preliminary investigation of local tomography for megavoltage CT imaging,” Med. Phys. 10.1118/1.1619232 30, 2969–2980 (2003). [DOI] [PubMed] [Google Scholar]
- Stevens G. M., Fahrig R., and Pelc N. J., “Filtered backprojection for modifying the impulse response of circular tomosynthesis,” Med. Phys. 10.1118/1.1350588 28, 372–380 (2001). [DOI] [PubMed] [Google Scholar]
- Li S. and Jiang H., “A practical method for three-dimensional reconstruction of joints using a C-arm system and shift-and-add algorithm,” Med. Phys. 10.1118/1.1915289 32, 1491–1499 (2005). [DOI] [PubMed] [Google Scholar]
- Chen Y., Lo J. Y., and J. T.DobbinsIII, “Importance of point-by-point back projection correction for isocentric motion in digital breast tomosynthesis: Relevance to morphology of structures such as microcalcifications,” Med. Phys. 10.1118/1.2776256 34, 3885–3892 (2007). [DOI] [PubMed] [Google Scholar]
- Wu T., Moore R. H., Rafferty E. A., and Kopans D. B., “A comparison of reconstruction algorithms for breast tomosynthesis,” Med. Phys. 10.1118/1.1786692 31, 2636–2647 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chan H. -P., Sahiner B., Wei J., Goodsitt M. M., Hadjiiski L. M., Ge J., and Zhou C., “A comparative study of limited-angle cone-beam reconstruction methods for breast tomosynthesis,” Med. Phys. 10.1118/1.2237543 33, 3781–3795 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski J. T. and Dennis M. J., “A comparison of reconstruction algorithms for C-arm mammography tomosynthesis,” Med. Phys. 10.1118/1.2219090 33, 3018–3032 (2006). [DOI] [PubMed] [Google Scholar]
- Mainprize J. G., Bloomquist K. A., Kempston M. P., and Yaffe M. J., “Resolution at oblique incidence angles of a flat panel imager for breast tomosynthesis,” Med. Phys. 10.1118/1.2241994 33, 3159–3164 (2006). [DOI] [PubMed] [Google Scholar]
- Badano A., Kyprianou I. S., Jennings R. J., and Sempau J., “Anisotropic imaging performance in breast tomosynthesis,” Med. Phys. 10.1118/1.2779943 34, 4076–4091 (2007). [DOI] [PubMed] [Google Scholar]
- Godfrey D. J., McAdams H. P., and J. T.DobbinsIII, “Optimization of the matrix inversion tomosynthesis (MITS) impulse response and modulation transfer function characteristics for chest imaging,” Med. Phys. 10.1118/1.2170398 33, 655–667 (2006). [DOI] [PubMed] [Google Scholar]
- Pineda A. R., Yoon S., Paik D. S., and Fahrig R., “Optimization of a tomosynthesis system for the detection of lung nodules,” Med. Phys. 10.1118/1.2190329 33, 1372–1379 (2006). [DOI] [PubMed] [Google Scholar]
- Tutar I. B., Managuli R., Shamdasani V., Cho P. S., Pathak S. D., and Kim Y., “Tomosynthesis-based localization of radioactive seeds in prostate brachytherapy,” Med. Phys. 10.1118/1.1624755 30, 3135–3142 (2003). [DOI] [PubMed] [Google Scholar]
- Duryea J., J. T.DobbinsIII, and Lynch J. A., “Digital tomosynthesis of hand joints for arthritis assessment,” Med. Phys. 10.1118/1.1543573 30, 325–333 (2003). [DOI] [PubMed] [Google Scholar]
- Sonke J. J., Zijp L., Remeijer P., and Herk M., “Respiratory correlated cone beam CT,” Med. Phys. 10.1118/1.1869074 32, 1176–1186 (2005). [DOI] [PubMed] [Google Scholar]
- Yan H., Ren L., Godfrey D. J., and Yin F., “Accelerating reconstruction of reference digital tomosynthesis using graphics hardware,” Med. Phys. 10.1118/1.2779945 34, 3768–3776 (2007). [DOI] [PubMed] [Google Scholar]
- Schmidt T. G., Fahrig R., Pelc N. J., and Solomon E. G., “An inverse-geometry volumetric CT system with a large-area scanned source: A feasibility study,” Med. Phys. 10.1118/1.1786171 31, 2623–2627 (2004). [DOI] [PubMed] [Google Scholar]
- Schmidt T. G., Fahrig R., and Pelc N. J., “A three-dimensional reconstruction algorithm for an inverse-geometry volumetric CT system,” Med. Phys. 10.1118/1.2064827 32, 3234–3245 (2005). [DOI] [PubMed] [Google Scholar]
- Chappard C., Basillais A., Benhamou L., Bonassie A., Brunet-Imbault B. A., Bonnet N., and Peyrin F., “Comparison of synchrotron radiation and conventional x-ray microcomputed tomography for assessing trabecular bone microarchitecture of human femoral heads,” Med. Phys. 10.1118/1.2256069 33, 3568–3577 (2006). [DOI] [PubMed] [Google Scholar]
- McKinley R. L., Tornai M. P., Samei E., and Bradshaw M. L., “Simulation study of a quasi-monochromatic beam for x-ray computed mammotomography,” Med. Phys. 10.1118/1.1668371 31, 800–813 (2004). [DOI] [PubMed] [Google Scholar]
- Saito M., “Quasimonochromatic x-ray computed tomography by the balanced filter method using a conventional x-ray source,” Med. Phys. 10.1118/1.1819553 31, 3436–3443 (2004). [DOI] [PubMed] [Google Scholar]
- Atherton J. V. and Huda W., “Energy imparted and effective doses in computed tomography,” Med. Phys. 10.1118/1.597667 23, 735–741 (1996). [DOI] [PubMed] [Google Scholar]
- Caon M., Bibbo G., Pattison J., and Bhat M., “The effect on dose to computed tomography phantoms of varying the theoretical x-ray spectrum: a comparison of four diagnostic x-ray spectrum calculating codes,” Med. Phys. 10.1118/1.598281 25, 1021–1027 (1998). [DOI] [PubMed] [Google Scholar]
- Dixon R. L., “A new look at CT dose measurement: Beyond CTDI,” Med. Phys. 10.1118/1.1576952 30, 1272–1280 (2003). [DOI] [PubMed] [Google Scholar]
- Theocharopoulos N., Damilakis J., Perisinakis K., Tzedakis A., Karantanas A., and Gourtsoyiannis N., “Estimation of effective doses to adult and pediatric patients from multislice computed tomography: A method based on energy imparted,” Med. Phys. 10.1118/1.2349694 33, 3846–3856 (2006). [DOI] [PubMed] [Google Scholar]
- Boone J. M., “The trouble with CTD100,” Med. Phys. 10.1118/1.2713240 34, 1364–1371 (2007). [DOI] [PubMed] [Google Scholar]
- Gies M., Kalender W. A., Wolf H., and Suess C., “Dose reduction in CT by anatomically adapted tube current modulation. I. Simulation studies,” Med. Phys. 10.1118/1.598779 26, 2235–2247 (1999). [DOI] [PubMed] [Google Scholar]
- Papadakis A. E., Perisinakis K., and Damilakis J., “Angular on-line tube current modulation in multidetector CT examinations of children and adults: The influence of different scanning parameters on dose reduction,” Med. Phys. 10.1118/1.2747048 34, 2864–2874 (2007). [DOI] [PubMed] [Google Scholar]
- Brisse H. J., Madec L., Gaboriaud G., Lemoine T., Savignoni A., Neuenschwander S., Aubert B., and Rosenwald J. C., “Automatic exposure control in multichannel CT with tube current modulation to achieve a constant level of image noise: Experimental assessment on pediatric phantoms,” Med. Phys. 10.1118/1.2746492 34, 3018–3033 (2007). [DOI] [PubMed] [Google Scholar]
- Tward D. J., Siewerdsen J. H., Daly M. J., Richard S., Moseley D. J., Jaffray D. A., and Paul N. S., “Soft-tissue detectability in cone-beam CT: evaluation by 2AFC tests in relation to physical performance metrics,” Med. Phys. 10.1118/1.2790586 34, 4459–4471 (2007). [DOI] [PubMed] [Google Scholar]
- Huda W. and Bushong S. C., “In x-ray computed tomography, technique factors should be selected appropriate to patient size,” Med. Phys. 10.1118/1.1388903 28, 1543–1545 (2001). [DOI] [PubMed] [Google Scholar]
- Judy P. F., “Comment on: Med. Phys. (8): 1543–1545 (2001). ‘In x-ray computed tomography, technique factors should be selected appropriate to patient size’,” Med. Phys. 10.1118/1.1415075 28, 2389 (2001). [DOI] [PubMed] [Google Scholar]
- Nickoloff E. L., Dutta A. K., and Lu Z. F., “Influence of phantom diameter, kVp and scan mode upon computed tomography dose index,” Med. Phys. 10.1118/1.1543149 30, 395–402 (2003). [DOI] [PubMed] [Google Scholar]
- Toth T., Ge Z., and Daly M. P., “The influence of patient centering on CT dose and image noise,” Med. Phys. 10.1118/1.2748113 34, 3093–3101 (2007). [DOI] [PubMed] [Google Scholar]
- Huda W., Scalzetti E. M., and Roskopf M., “Effective doses to patients undergoing thoracic computed tomography examinations,” Med. Phys. 10.1118/1.598949 27, 838–844 (2000). [DOI] [PubMed] [Google Scholar]
- Paul N. S., Siewerdsen J. H., Patsios D., and Chung T. B., “Investigating the low-dose limits of multidetector CT in lung nodule surveillance,” Med. Phys. 10.1118/1.2768866 34, 3587–3595 (2007). [DOI] [PubMed] [Google Scholar]
- Huda W., Chamberlain C. C., Rosenbaum A. E., and Garrisi W., “Radiation doses to infants and adults undergoing head CT examinations,” Med. Phys. 10.1118/1.1350435 28, 393–399 (2001). [DOI] [PubMed] [Google Scholar]
- Perisinakis K., Raissaki M., Theocharopoulos N., Damilakis J., and Gourtsoyiannis N., “Reduction of eye lens radiation dose by orbital bismuth shielding in pediatric patients undergoing CT of the head: A Monte Carlo study,” Med. Phys. 10.1118/1.1881852 32, 1024–1030 (2005). [DOI] [PubMed] [Google Scholar]
- Giacco G., Cannata V., Furetta C., Santopietro F., and Fariello G., “On the use of pediatric phantoms in the dose evaluation during computed tomography (CT) thorax examinations,” Med. Phys. 10.1118/1.1344205 28, 199–204 (2001). [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Elliston C. D., Hall E. J., and Berdon W. E., “Comment on: Med. Phys. 2001 28(8):1543–1545. Estimates of the cancer risks from pediatric CT radiation are not merely theoretical: Comment on ‘Point/counterpoint: In x-ray computed tomography, technique factors should be selected appropriate to patient size. Against the proposition’,” Med. Phys. 10.1118/1.1415074 28, 2387–2388 (2001). [DOI] [PubMed] [Google Scholar]
- Tzedakis A., Damilakis J., Perisinakis K., Stratakis J., and Gourtsoyiannis N., “The effect of z overscanning on patient effective dose from multidetector helical computed tomography examinations,” Med. Phys. 10.1118/1.1924309 32, 1621–1629 (2005). [DOI] [PubMed] [Google Scholar]
- Lee C., Lee C., Staton R. J., Hintenlang D. E., Arreola M. M., Williams J. L., and Bolch W. E., “Organ and effective doses in pediatric patients undergoing helical multislice computed tomography examination,” Med. Phys. 10.1118/1.2723885 34, 1858–1873 (2007). [DOI] [PubMed] [Google Scholar]
- Shikhaliev P. M., Xu T., and Molloi S., “Photon counting computed tomography: Concept and initial results,” Med. Phys. 10.1118/1.1854779 32, 427–436 (2005). [DOI] [PubMed] [Google Scholar]