Abstract
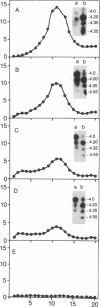
PANC-1 human pancreatic carcinoma cells readily bound and internalized 125I-labeled epidermal growth factor (EGF). Bound 125I-labeled EGF was then partially processed to a number of high molecular weight acidic species. Percoll gradient centrifugation of cell homogenates indicated that the majority of 125I activity localized to several intracellular vesicular compartments. Both intact EGF and its processed species were subsequently released into the incubation medium. A major portion of the released radioactivity was capable of rebinding to the cell. Only a small amount of bound 125I-labeled EGF was degraded to low molecular weight products, and this degradation was completely blocked by methylamine. This lysosomotropic compound did not arrest either the generation or the extrusion of the major high molecular weight species of processed EGF (pI 4.2). These findings suggest that in PANC-1 cells, bound EGF undergoes only limited processing. Both intact EGF and its major processed species bypass the cellular degradative pathways, are slowly released from the cell, and then rebind to the cell.
Full text
PDF
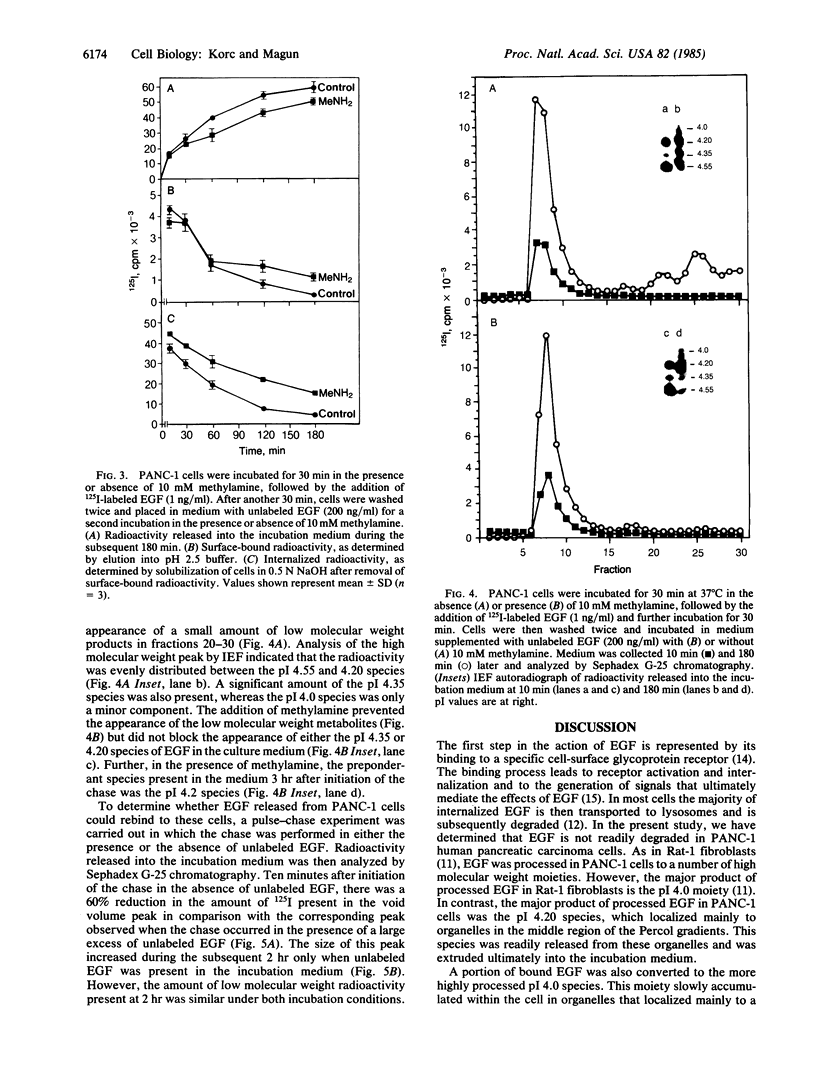
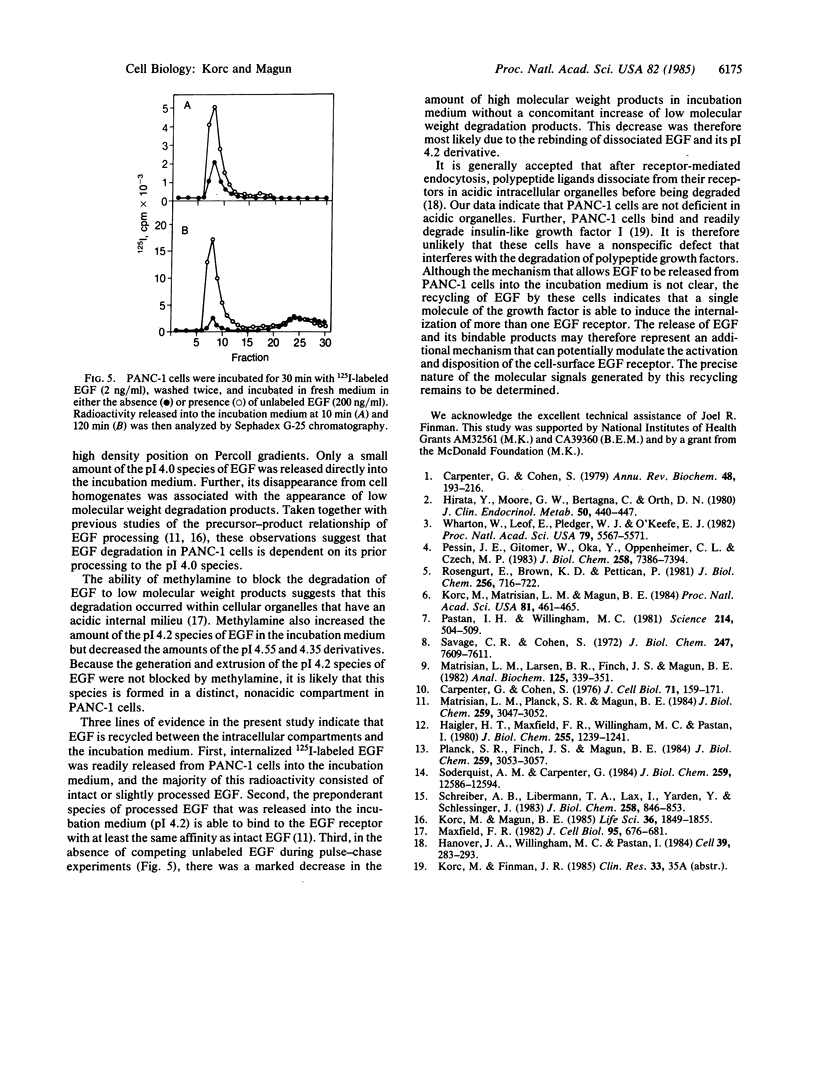
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Hanover J. A., Willingham M. C., Pastan I. Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell. 1984 Dec;39(2 Pt 1):283–293. doi: 10.1016/0092-8674(84)90006-0. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Moore G. W., Bertagna C., Orth D. N. Plasma concentrations of immunoreactive human epidermal growth factor (urogastrone) in man. J Clin Endocrinol Metab. 1980 Mar;50(3):440–444. doi: 10.1210/jcem-50-3-440. [DOI] [PubMed] [Google Scholar]
- Korc M., Magun B. E. Binding and processing of epidermal growth factor in Panc-I human pancreatic carcinoma cells. Life Sci. 1985 May 13;36(19):1849–1855. doi: 10.1016/0024-3205(85)90158-4. [DOI] [PubMed] [Google Scholar]
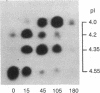
- Korc M., Matrisian L. M., Magun B. E. Cytosolic calcium regulates epidermal growth factor endocytosis in rat pancreas and cultured fibroblasts. Proc Natl Acad Sci U S A. 1984 Jan;81(2):461–465. doi: 10.1073/pnas.81.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Larsen B. R., Finch J. S., Magun B. E. Further purification of epidermal growth factor by high-performance liquid chromatography. Anal Biochem. 1982 Sep 15;125(2):339–351. doi: 10.1016/0003-2697(82)90015-x. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Planck S. R., Magun B. E. Intracellular processing of epidermal growth factor. I. Acidification of 125I-epidermal growth factor in intracellular organelles. J Biol Chem. 1984 Mar 10;259(5):3047–3052. [PubMed] [Google Scholar]
- Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- Pessin J. E., Gitomer W., Oka Y., Oppenheimer C. L., Czech M. P. beta-Adrenergic regulation of insulin and epidermal growth factor receptors in rat adipocytes. J Biol Chem. 1983 Jun 25;258(12):7386–7394. [PubMed] [Google Scholar]
- Planck S. R., Finch J. S., Magun B. E. Intracellular processing of epidermal growth factor. II. Intracellular cleavage of the COOH-terminal region of 125I-epidermal growth factor. J Biol Chem. 1984 Mar 10;259(5):3053–3057. [PubMed] [Google Scholar]
- Rozengurt E., Brown K. D., Pettican P. Vasopressin inhibition of epidermal growth factor binding to cultured mouse cells. J Biol Chem. 1981 Jan 25;256(2):716–722. [PubMed] [Google Scholar]
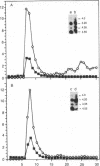
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schreiber A. B., Libermann T. A., Lax I., Yarden Y., Schlessinger J. Biological role of epidermal growth factor-receptor clustering. Investigation with monoclonal anti-receptor antibodies. J Biol Chem. 1983 Jan 25;258(2):846–853. [PubMed] [Google Scholar]
- Soderquist A. M., Carpenter G. Glycosylation of the epidermal growth factor receptor in A-431 cells. The contribution of carbohydrate to receptor function. J Biol Chem. 1984 Oct 25;259(20):12586–12594. [PubMed] [Google Scholar]
- Wharton W., Leof E., Pledger W. J., O'Keefe E. J. Modulation of the epidermal growth factor receptor by platelet-derived growth factor and choleragen: effects on mitogenesis. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5567–5571. doi: 10.1073/pnas.79.18.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]