Abstract
Multiple myeloma (MM) is a neoplastic plasma cell disorder that results in end-organ damage (hypercalcemia, renal insufficiency, anemia, or skeletal lesions). Patients should not be treated unless they have symptomatic (end-organ damage) MM. They should be classified as having high-risk or standard-risk disease. Patients are classified as high risk in the presence of hypodiploidy or deletion of chromosome 13 (del[13]) with conventional cytogenetics, the presence of t(4:14), t(14;16), t(14;20) translocations or del(17p) with fluorescence in situ hybridization. High-risk disease accounts for about 25% of patients with symptomatic MM. If the patient is deemed eligible for an autologous stem cell transplantation (ASCT), 3 or 4 cycles of lenalidomide and low-dose dexamethasone, or bortezomib and dexamethasone, or thalidomide and dexamethasone are reasonable choices. Stem cells should then be collected and one may proceed with an ASCT. If the patient has a complete response or a very good partial response (VGPR), the patient may be followed without maintenance therapy. If the patient has a less than VGPR, a second ASCT is encouraged. If the patient is in the high-risk group, a bortezomib-containing regimen to maximum response followed by 2 additional cycles of therapy is a reasonable approach. Lenalidomide and low-dose dexamethasone is another option for maintenance until progression. If the patient is considered ineligible for an ASCT, then melphalan, prednisone, and thalidomide is suggested for the standard-risk patient, and melphalan, prednisone, and bortezomib (MPV) for the high-risk patient. Treatment of relapsed or refractory MM is covered. The novel therapies—thalidomide, bortezomib, and lenalidomide—have resulted in improved survival rates. The complications of MM are also described. Multiple myeloma is a plasma cell neoplasm that is characterized by a single clone of plasma cells producing a monoclonal protein (M-protein). The malignant proliferation of plasma cells produces skeletal destruction that leads to bone pain and pathologic fractures. The M-protein might lead to renal failure, hyperviscosity syndrome, or through the suppression of uninvolved immunoglobulins, recurrent infections. Anemia and hypercalcemia are common complications.
Keywords: Autologous stem cell transplantation, Bortezomib, Lenalidomide, Thalidomide
Introduction
Multiple myeloma (MM) accounts for about 1% of all types of malignancy and slightly more than 10% of the hematologic malignancies. The incidence of MM is 4-5 per 100,000 in the United States. In Olmsted County, Minnesota, the incidence has not changed for the past 56 years.1 The reported increased incidence during the past several decades is probably related to the increased availability of medical facilities for elderly patients and to improved diagnostic techniques than to an actual increased incidence. Multiple myeloma occurs in all races, but rates are higher among black populations and lower among Asian populations.2,3
Criteria for Diagnosis and Therapy
The criteria for diagnosis of myeloma require clonal bone marrow plasma cells, presence of serum and/or urinary monoclonal protein except in patients with true nonsecretory MM, and evidence of end-organ damage that can be attributed to the underlying plasma cell proliferative disorder, specifically hypercalcemia (serum calcium > 0.25 mmol/L above the upper limit of normal or > 2.75 mmol/L), renal insufficiency (serum creatinine > 1.73 mmol/L), anemia (hemoglobin < 10 g/dL or > 2 g/dL below the lower limit of normal), or bone lesions (lytic lesions, severe osteopenia with compression fractures or pathologic fractures; CRAB).4-6 Bone marrow involvement may be focal rather than diffuse, requiring repeated bone marrow examinations for diagnosis. Multiparametric flow cytometry or identification of a monoclonal protein in the cytoplasm of plasma cells by immunoperoxidase or immunofluorescence is required in differentiating a monoclonal plasma cell proliferation from reactive plasmacytosis due to connective tissue disorders, chronic liver disease, chronic infections, or metastatic carcinoma. No level of serum M-protein or urine M-protein is included in the diagnostic criteria, but the majority of patients with MM have an M-protein > 3 g/dL. It is important to remember that 97% of patients with MM will have an M-protein in the serum or in the urine by immunofixation.
Smoldering (asymptomatic) MM must be excluded. It is characterized by an M-protein ≥ 3 g/dL and/or bone marrow plasma cells ≥ 10% but no evidence of anemia, hypercalcemia, renal insufficiency, or lytic bone lesions (CRAB).7 They should be recognized and followed up closely because symptomatic myeloma develops at a rate of 10% per year for the first 5 years of follow-up.
mSMART Classification and Approach to Therapy
Patients with symptomatic MM may be classified into high-risk or standard-risk disease.8,9 Patients may be classified as high risk in the presence of hypodiploidy or deletion of chromosome 13 (del[13]) with conventional cytogenetics, the presence of t(4;14), t(14;16), t(14;20), or del(17p) on fluorescence in situ hybridization (FISH; Figure 1). High-risk disease accounts for about 25% of patients with symptomatic MM. Lactate dehydrogenase and β2-microglobulin levels are additional important risk factors.
Figure 1. Classification of Multiple Myeloma at Diagnosis Into High Risk or Standard Risk8.
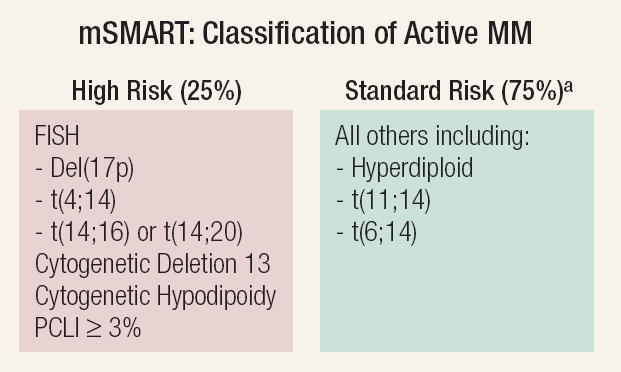
aPatients with t(4;14), β2-microglobulin < 4 mg/L, and Hb ≥ 10 g/dL may have intermediate-risk disease.
Abbreviations: FISH = fluorescence in situ hybridization; Hb = hemoglobin; MM = multiple myeloma; mSMART = Stratification for Myeloma and Risk-Adapted Therapy; PCLI = plasma cell labeling index
From Mayo Clin Proc.,8 with permission.
We strongly recommend clinical trials, but if the patient is not eligible or if clinical trials are not available, one may separate the patients into those who are autologous stem cell transplantation (ASCT) eligible or ineligible (Figure 2).9 The results of selected randomized trials that have had a major effect are shown in Table 1. Eligibility for ASCT in MM varies from country to country. In most European countries, ASCT is offered mainly to patients aged < 65 years, whereas in the United States, decisions are made on a patient-by-patient basis depending upon “physiologic age” rather than chronologic age. In most institutions, patients aged > 70 years, serum creatinine > 2.5 mg/dL, Eastern Cooperative Oncology Group (ECOG) performance status of 3 or 4, or New York Heart Association functional status class III or IV are considered ineligible for ASCT. Patients with renal failure may undergo transplantation, but the morbidity and mortality is higher.10
Figure 2. Algorithm Outlining the Current Approach to the Treatment of Newly Diagnosed Myeloma9.
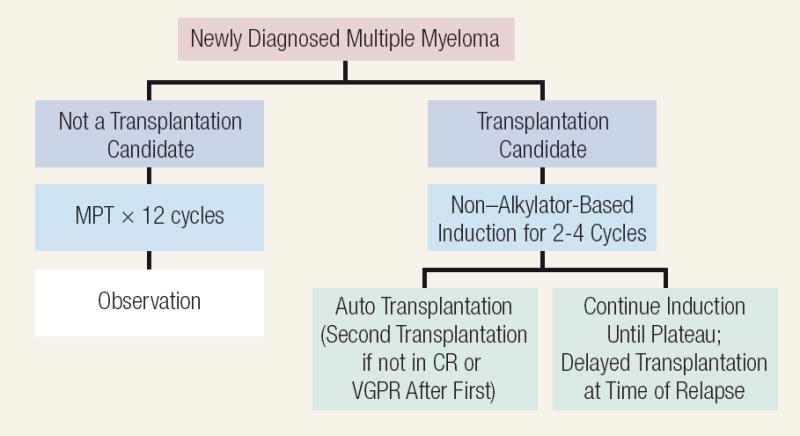
Abbreviations: CR = complete response; MPT = melphalan/prednisone/thalidomide; VGPR = very good partial response
Table 1.
Results of Selected Randomized Trials Having a Major Effect on Myeloma Therapy67
| Trial | Disease Stage | Treatment Comparison | Total Number of Patients Studied | CR/VGPR, % | Median PFS, Months | Median OS, Months | Comments |
|---|---|---|---|---|---|---|---|
| Stem Cell Transplantation | |||||||
| IFM 9082 | Newly diagnosed | Conv. chemo | 100 | 13 | 18 | 44 | Established role of ASCT |
| ASCT | 100 | 38 | 28 | 57 | |||
| MRC VII33 | Newly diagnosed | Conv. chemo | 201 | 8 | 20 | 42 | Confirmed role of ASCT |
| ASCT | 200 | 44 | 32 | 54 | |||
| MAG 9031 | Newly diagnosed | Delayed ASCT | 94 | 21 | 13 | 65 | Demonstrated delayed ASCT as an alternative |
| Early ASCT | 91 | 32 | 39 | 64 | |||
| United States Intergroup S932145 | Newly diagnosed | Delayed ASCT | 255 | 15a | 21 | 64 | Demonstrated delayed ASCT as an alternative; dampened enthusiasm for allogeneic SCT |
| Early ASCT | 261 | 17a | 25 | 58 | |||
| Allogeneic SCT | 36 | 17a | NR | 6 | |||
| IFM 9434 | Newly diagnosed | Single ASCT | 199 | 42 | 25 | 48 | Established role of tandem ASCT if CR/VGPR not achieved with first ASCT |
| Double ASCT | 200 | 50 | 30 | 58 | |||
| PETHEMA83 | Postinduction (responding patients only) | Conv. chemo | 83 | 11a | 33 | 66 | Demonstrated that ASCT may have limited value in patients responding well to induction |
| ASCT | 81 | 30a | 42 | 61 | |||
| Italian48 | Newly diagnosed | Tandem ASCT | 82 | N/A | 29 | 54 | Demonstrated efficacy of single ASCT followed by nonmyeloablative SCT in selected patients |
| Single ASCT followed by nonmyeloablative SCT | 80 | N/A | 35 | 80 | |||
| New Therapies | |||||||
| Italian GIMEMA Group50 | Newly diagnosed | MP | 126 | 12 | 27 | 64% at 3 years | Demonstrated potential value of MPT over the classic MP regimen |
| MPT | 129 | 36 | 54 | 80% at 3 years | |||
| IFM 99-0651 | Newly diagnosed | MP | 196 | 7 | 18 | 33 | Changed standard of care in elderly to MPT (after 3 decades of MP) |
| MPT | 125 | 47 | 28 | 52 | |||
| Tandem intermediate-dose ASCT | 126 | 43 | 19 | 38 | |||
| APEX84 | Relapsed, refractory (1-3 previous therapies) | Bortezomib | 333 | 13b | 6c | NR | Led to full approval of bortezomib in the US |
| Dex | 336 | 2b | 3c | NR | |||
| MM-01064 | Relapsed, refractory (1-3 previous therapies) | Len/Dex | 176 | 24b | 11c | Not reached | Pivotal trial establishing role of lenalidomide |
| Placebo/Dex | 175 | 5b | 5c | 21 | |||
| MM-00963 | Relapsed, refractory (1-3 previous therapies) | Len/Dex | 177 | 24b | 11a | 30 | Led to approval of lenalidomide in the US |
| Placebo/Dex | 176 | 2b | 5a | 20 | |||
Denotes CR only; VGPR not reported.
Denotes CR or near CR.
PFS data not available; numbers reflect median time to progression.
Abbreviations: ASCT = autologous hematopoetic stem cell transplantation; Conv. chemo = conventional chemotherapy; CR = complete response; Dex = dexamethasone; GIMEMA = Gruppo Italiano Malattie Ematologiche dell’Adulto; IFM = InterGroupe Francophone du Myélome; Len = lenalidomide; MAG = Myélome Autogreffe; MM = multiple myeloma; MP = melphalan plus prednisone; MPT = melphalan/prednisone/thalidomide; MRC = Medical Research Council; NR = not reported; OS = overall survival; PETHEMA = Programa para el Estudio y Tratamiento de las Hemopatias Malignas; PFS = progression-free survival; SCT = stem cell transplantation; VGPR = very good partial response
Initial Therapy for Transplantation-Eligible Patients
Alkylating agents have been associated with damage to the hematopoietic stem cells. In a prospective study of peripheral blood progenitor cell collection, Goldschmidt et al reported that adequate hematopoietic stem cells were collected in only 30% of patients who had received melphalan compared with 80% of those who had not.11 Elderly patients who have refractory MM after extensive therapy or those who have received long-term alkylator-based chemotherapy are generally unable to mobilize sufficient stem cells for successful transplantation.12 Plerixafor is helpful in the mobilization of hematopoietic stem cells.13
Lenalidomide Plus Low-Dose Dexamethasone
Patients who are candidates for stem cell transplantation may be treated with lenalidomide. Lenalidomide is an aminosubstituted variant of thalidomide and is an immunomodulatory agent. Patients who are candidates for stem cell transplantation may be treated with lenalidomide plus low-dose dexamethasone. In a randomized trial of 445 previously untreated symptomatic MM, patients were assigned to lenalidomide (25 mg daily days 1-21) plus high-dose dexamethasone (40 mg daily on days 1-4, 9-12, and 17-20) or to lenalidomide in the same dose and schedule plus dexamethasone 40 mg given weekly for each 28-day cycle.14 Preliminary results presented in abstract form show that the 1-year survival was 96% for lenalidomide/low-dose dexamethasone compared with 88% for lenalidomide plus standard-dose dexamethasone. The 2-year survival probability was 87% and 75%, respectively.15 The risk of deep-venous thrombosis was less in the low-dexamethasone regimen (6% vs. 25%), and the incidence of infection was also reduced (7% vs. 14%). In another study, Niesvizky et al reported that lenalidomide, clarithromycin, and weekly dexamethasone produced an objective response in 90%.16 Pregnancy must be avoided in all patients receiving lenalidomide.
Thalidomide Plus Dexamethasone
Thalidomide was introduced as a sedative in the late 1950s and was withdrawn from the market after reports of teratogenecity in 1961. Though trials with thalidomide as an anticancer agent were unsuccessful in the 1960s, the recognition of increased angiogenesis in MM and the awareness of antiangiogenic properties of thalidomide led to the first clinical trial of this agent for the treatment of MM at the University of Arkansas.17 In a randomized study, 470 patients with symptomatic previously untreated MM were randomized to receive thalidomide in a dosage of 50 mg daily with escalation to 200 mg daily if tolerated plus dexamethasone 40 mg (days 1-4, 9-12, and 17-20) versus placebo plus dexamethasone 40 mg (days 1-4, 9-12, and 17-20). A response (CR/PR) was attained in 63% of patients receiving thalidomide/dexamethasone compared with 40% for those receiving only dexamethasone. The time to progression was 22.6 months versus 6.5 months, favoring the thalidomide/dexamethasone regimen. Deep-venous thrombosis occurred in 12% of patients receiving thalidomide/dexamethasone compared with 2% for the dexamethasone-alone regimen.18 In another study, pegylated liposomal doxorubicin (40 mg/m2 intravenously [I.V.] on day 1) plus thalidomide 100 mg daily plus dexamethasone 40 mg on days 1-4 and 9-12 produced an overall response of 98%, while a complete response (CR)/near-complete response (nCR) occurred in 48% after 4 cycles of therapy. The major side effects were infection, thromboembolic events, and neutropenia.19 Prophylactic prevention of thromboembolic phenomena is necessary. The System for Thalidomide Education and Prescribing Safety (STEPS) program must be followed when thalidomide is given.
Bortezomib plus Dexamethasone
Bortezomib plus dexamethasone is another option for induction therapy. In a report of 48 patients with untreated symptomatic myeloma, Jagannath et al gave bortezomib 1.3 mg/m2 twice weekly for 2 weeks every 3 weeks plus dexamethasone 40 mg on the day of and the day after bortezomib. The CR/nCR rate was 19%, and the partial response (PR) was 71%, giving a 90% response rate.20 Bortezomib plus dexamethasone is recommended for patients with renal insufficiency. Bortezomib also appears to overcome the adverse effect of unfavorable cytogenetic abnormalities.21 In a phase II trial, 6 cycles of alternating bortezomib and dexamethasone produced a 65% response rate, with the majority occurring during the first 2 cycles of therapy.22
VTD: Bortezomib, Thalidomide, and Dexamethasone
Bortezomib/thalidomide/dexamethasone (VTD) has shown high activity in relapsed refractory myeloma and in newly diagnosed disease. Cavo and colleagues compared thalidomide/dexamethasone to VTD in a randomized controlled trial in 256 patients.23 As pretransplantation induction therapy, the CR plus very good PR (VGPR) rate was significantly higher with VTD compared with thalidomide/dexamethasone (60% vs. 27%; P < .001). The VTD regimen was well tolerated and permitted adequate stem cell collection.
VAD: Vincristine, Doxorubicin, and Dexamethasone
Vincristine/doxorubicin/dexamethasone (VAD) has been used for initial chemotherapy before ASCT. It is typically given intravenously continuously for 96 hours plus dexamethasone 40 mg daily on days 1-4, 9-12, and 17-20 each month.24 Because high-risk dexamethasone accounts for 80% of the effect of VAD, many physicians have used it as a single agent because of the inconvenience and side effects of vincristine and doxorubicin. Significant side effects from VAD include recurrent infections, worsening of diabetes, myopathy, gastrointestinal bleeding, cardiac damage, and neuropathy. The vincristine-produced neuropathy might compromise the ability of the patient to receive treatment with thalidomide or bortezomib in the future. In one study, the overall response was 84% with a CR rate of 27%.25 However, this regimen is infrequently used now because of the risk of sepsis and thromboembolic events from the central venous catheter as well as neurotoxicity from the vincristine. Bolus injection of vincristine and doxorubicin avoids some of these problems.
Autologous Stem Cell Transplantation
Following 3-4 months of induction therapy with lenalidomide, thalidomide, or bortezomib, all with dexamethasone, one must collect the stem cells. In our experience with 472 patients with newly diagnosed MM who underwent an ASCT, we found no significant difference in response rates, posttransplant complications, or treatment-related mortality in patients who received VAD; dexamethasone alone; thalidomide plus dexamethasone; or lenalidomide plus dexamethasone.26 Though we have failed to show that the depth of response before transplantation is associated with improved long-term posttransplantation outcomes, 2 recent studies suggest that induction therapies associated with greater depth of response (VGPR or CR) before transplantation do translate into superior progression-free survival (PFS) following transplantation. Cavo and colleagues reported this in a trial comparing VTD with thalidomide plus dexamethasone.27 A similar finding was also reported by Harousseau and colleagues in a trial comparing bortezomib/dexamethasone to VAD chemotherapy.28
The stem cells must be collected before the patient is exposed to alkylating agents. One may use granulocyte colony-stimulating factor (G-CSF) with or without cyclophosphamide. There are no randomized trials comparing these two approaches. G-CSF plus cyclophosphamide usually produces more stem cells than G-CSF alone, but it is associated with a longer time to begin collection and greater neutropenia. We attempt to collect 6 × 106 CD34+ cells/kg, which is sufficient for 2 ASCTs.
Theoretically, purging of tumor cells would be beneficial, but this has produced no apparent benefit even after removal of 2-3 logs of tumor cells. Furthermore, delayed engraftment may result. In a report of 190 previously treated patients with stable or responsive disease, CD34+ selection resulted in a 3.1-log reduction of tumor cells. There were no differences in median survival or disease-free survival after 37 months follow-up between those patients receiving CD34+ selected or unselected stem cells.29 Bourhis et al,30 in a prospective study of 111 patients with newly diagnosed MM who were randomly assigned to infusion of either CD34+ selected stem cells or unselected autologous stem cells, reported a median reduction of tumor load of 2.2 logs but no significant difference in event-free survival (EFS), relapse rate, or 5-year overall survival (OS). Consequently, purging is not advised at the present time.
The advantages of an ASCT are that the mortality is low (about 1%) and is available for up to one half of patients with MM. In fact, approximately 40% of our patients undergo an ASCT entirely as an outpatient. Most importantly, the procedure is not curative because of the failure to eradicate the tumor and patients must be told that they will likely relapse. The median duration of response is approximately 2 years.26
Timing of Transplantation
The initial ASCT may be given when the patient recovers from hematopoietic stem cell collection or delayed until relapse. In one study, 185 patients were treated with 3-4 cycles of vincristine, doxorubicin, and methylprednisolone and then randomized to high-dose chemotherapy and an autologous transplantation or to receive conventional therapy with the autologous transplantation performed if resistant to chemotherapy or at relapse. The median survival was virtually the same in the two groups (64 months vs. 65 months). The advantages of early autologous transplantation were a superior quality of life, a shorter period of chemotherapy, and a longer EFS (39 months vs. 13 months).31 For those reasons, we prefer an early ASCT.
Conditioning Regimens
In a randomized trial, 282 newly diagnosed symptomatic myeloma patients aged < 65 years were randomized to receive melphalan 140 mg/m2 plus 8 Gy total body irradiation or melphalan 200 mg/m2.32 Patients receiving melphalan 200 mg/m2 had a more rapid hematologic recovery, lower incidence of severe mucositis, fewer transfusions, and shorter hospitalizations. Survival at 45 months was better in patients receiving melphalan 200 mg/m2 (66% vs. 46%). Autologous stem cell transplantation has been shown to be superior to combination chemotherapy. An example is a study from the UK in which 401 patients with newly diagnosed symptomatic MM were randomized to receive a preparative regimen of melphalan 200 mg/m2 followed by stem cell rescue or chemotherapy (doxorubicin, carmustine, cyclophosphamide, and melphalan). The CR rate was 44% for the transplantation group and 8% for the chemotherapy regimen. The PFS (32 months vs. 20 months) and the OS (54 months vs. 42 months) also favored transplantation.33 Most studies have been performed on patients whose serum creatinine was < 2.0 mg/dL. Autologous stem cell transplantation can be performed in patients with renal failure. In one report, 60 patients with creatinine levels > 2 mg/dL received melphalan 200 mg/m2, but this was associated with excessive toxicity. Subsequently, 21 patients were given melphalan 140 mg/m2. Those who received the higher dose of melphalan had higher rates of pulmonary toxicity and mucositis. However, transplantation-related mortality and OS were not different between the two melphalan doses.10 Studies are ongoing now to improve on melphalan-based conditioning by adding bortezomib to melphalan 200 mg/m2.
Single Versus Tandem Transplantation
The question of a single or a tandem (double) ASCT is controversial. Attal et al, in a randomized study comparing single and tandem transplantation, reported a superior EFS (21% vs. 10%) and OS (42% vs. 21%) favoring a tandem transplantation after 7 years’ follow-up.34 They concluded that those patients who obtained a CR or a VGPR with the first autologous transplantation did not benefit from a second transplantation. Most investigators use this approach. In another randomized study of 321 patients, Cavo et al reported an OS of 71 months for a tandem transplantation versus 65 months for the single transplantation.35 On the other hand, Fermand et al, in a smaller, randomized study of 193 patients, reported no difference in OS or EFS.36 Sirohi et al in a retrospective study of 451 patients with a single autologous transplantation concluded that results were comparable to those with a tandem transplantation.37,38
Maintenance Therapy Following Autologous Stem Cell Transplantation
A total 597 patients aged < 65 years in an Intergroupe Français du Myélome (IFM) trial received a tandem autologous transplantation and then were randomized to no maintenance, pamidronate maintenance, or pamidronate plus thalidomide maintenance. A CR or a VGPR was obtained in 55, 57, and 67% of the patients, while 4-year OS rates were 77%, 74%, and 87%, respectively. Although thalidomide improved the response rate and OS, it often resulted in peripheral neuropathy.39 Though the IFM trial showed an OS benefit with thalidomide, with longer follow-up, this difference has lost its statistical significance (JL Harousseau, personal communication).
In a trial of 868 newly diagnosed patients with MM given intensive chemotherapy and a tandem ASCT, patients were randomized to receive or not receive thalidomide during the entire treatment period. The CR rates for thalidomide and the control groups were 62% and 43%, respectively, with an estimated 5-year EFS of 56% and 44%, respectively.40 Severe peripheral neuropathy and deep-venous thrombosis were significantly more common in the thalidomide-treated group. The thalidomide arm of Total Therapy 2 showed a survival benefit only in the subset with high-risk myeloma.40,41 Furthermore, it is hard to attribute this to maintenance therapy because thalidomide was administered in the induction, consolidation, and maintenance phases of the trial in the experimental arm. Two other trials have suggested benefit with thalidomide therapy but have limitations.42,43 In most of these trials, therapy with thalidomide appeared to benefit only those who were not in VGPR after transplantation, and the duration of therapy was short (6-12 months),43 and more appropriately referred to as consolidation rather than maintenance. Results of the recently completed Bone Marrow Transplant Clinical Trials Network (BMT-CTN) trial will shed more light on this issue. Given the adverse effects of thalidomide, lenalidomide maintenance may be more appealing and is being tested in randomized trials.
α-2-Interferon has been used for maintenance therapy. In a large meta-analysis of 24 randomized trials involving 4012 patients, α-2-interferon showed only a modest benefit in PFS and OS.44 A large Intergroup trial showed no benefit with interferon as maintenance therapy.45
At present, we recommend that patients who have obtained a response from an ASCT be followed without maintenance therapy unless they are part of a clinical trial.
Allogenic Transplantation
Allogenic transplantation has the advantage of the absence of contamination of the hematopoietic stem cells by tumor cells and the possibility of producing a cure. On the other hand, more than 90% of patients with MM are ineligible because of their age or lack of an HLA-matched donor. The initial mortality was approximately 40% and currently has been reduced to approximately 25%. In a report of 80 patients who received an allogenic stem cell transplantation, only 5 were alive and without evidence of disease 4-7 years after transplantation.46 Standard allogenic transplantations in MM is not recommended. If an identical twin donor is available, one should proceed with a syngeneic transplantation rather than an ASCT.
Nonmyeloablative Transplantation
Nonmyeloablative (reduced intensity or mini-allogeneic) stem cell transplantation regimens have been used in MM. The goal is to use the improved CR rate and relapse-free mortality seen with an allogenic transplantation while eliminating the high treatment-related mortality. Initially, patients with relapsed or refractory myeloma were treated, but results were disappointing. Consequently, the use of an ASCT followed after recovery with a nonmyeloablative allogeneic stem cell transplantation is preferable. The initial studies reported a mortality of 25% and a 3-year OS and PFS rate of 41% and 21%, respectively.47 Poor OS was associated with chemoresistant disease, > 1 previous transplantation, and absence of chronic graft-versus-host disease (GVHD). It is impossible to separate GVHD from graft-versus-tumor effect. A total of 162 consecutive patients with newly diagnosed MM who were aged ≤ 65 years and had at least one sibling were treated initially with 2 or 3 courses of VAD. This was followed by cyclophosphamide 3-4 g/m2 with or without paclitaxel 250 mg/m2, following which allogeneic stem cells were mobilized after treatment with G-CSF. Those patients with an HLA-identical sibling received an autologous transplantation followed by a nonmyeloablative allogeneic transplantation using the sibling donor. Patients without an HLA-identical sibling received a double ASCT. The mortality rate was 2% for the double autologous transplantation and 10% for the patients receiving an autologous followed by a nonmyeloablative transplantation. With a median follow-up of 45 months, the disease-related mortality was 43% for the tandem autologous transplantation versus 7% for the autologous/allogeneic groups. Grade 2-4 GVHD occurred in 43% of the autologous/allogeneic group. The median OS had not been reached in the autologous/allogeneic group with a median follow-up of 46 months, while median survival was 58 months in the tandem transplanation group.48 A larger trial comparing autologous transplantation with autologous/nonmyeloablative transplantation completed accrual in 2006 by the BMT-CTN and will be analyzed in 2009. A nonmyeloablative transplantation should not be performed unless a patient is in a clinical trial or has high-risk MM.
Initial Therapy for Patients Who Are Ineligible for an Autologous Stem Cell Transplantation
Melphalan/Prednisone
Since the 1960s, melphalan and prednisone have been the standard of therapy. We used melphalan 0.15 mg/kg daily for 7 days plus prednisone 15 mg 4 times daily for the same 7 days. The leukocytes and platelets are measured at 3-week intervals, and the cycle repeated every 6 weeks. The dose of melphalan needs to be altered so that modest cytopenia occurs at midcycle. Even when melphalan and prednisone are used in an ideal fashion, the response rate is only 50%-60%. Consequently, for decades, investigators developed various combinations of chemotherapy. In a large meta-analysis of 4930 symptomatic myeloma patients in 20 prospective trials, the response rate was 60% for various chemotherapy combination regimens compared with 53% for melphalan and prednisone (P < .00001). However, there was no difference in survival and no subsets who benefited from either single or multiple combinations of chemotherapy.49 As discussed below, other options have emerged in recent years for elderly patients, but melphalan plus prednisone might still be the best option for selected patients who are unable to tolerate or have access to new agents such as thalidomide or bortezomib.
Melphalan, Prednisone, and Thalidomide
Palumbo et al reported a randomized trial comparing melphalan, prednisone, and thalidomide (MPT) with melphalan and prednisone (MP) in 255 patients with newly diagnosed myeloma aged 65-85 years who were considered ineligible for an ASCT. The patients were randomized to MPT 100 mg daily for 6 months or MP in the same dose and schedule. The response rate was 76% and CR 16% in the MPT regimen, compared with 48% and 2%, respectively, for MP. The 2-year EFS was 54% versus 27%, while the 3-year survival was 80% versus 64%, favoring the MPT regimen.50 However, grade 3/4 adverse events occurred in 48% with the MPT regimen, compared with 25% for MP. The risk of thromboembolic phenomena (12% vs. 2%), infection (10% vs. 2%), and peripheral neuropathy (8% vs. 0) was greater in MPT compared with MP. The IFM reported on 447 previously untreated patients with myeloma aged 65-70 years who were deemed ineligible for an ASCT. They were randomized to MPT for 1 year with thalidomide given in an increasing dosage to a maximum of 400 mg daily if tolerated; or MP in the same dosage and schedule for 12 months; or VAD followed by melphalan 100 mg/m2 × 2 followed by stem cell rescue. The median PFS times were 28 months, 18 months, and 19 months, respectively, while the OS was 52 months, 33 months, and 38 months.51 MPT was associated with higher rates of grade 3 or 4 neutropenia (48%), thromboembolic phenomena (12%), constipation (10%), and somnolence and fatigue/dizziness (8%). In another IFM study of 229 patients with previously untreated MM who were aged > 75 years, the patients were randomized to melphalan 0.2 mg/kg plus prednisone 2 mg/kg days 1-4 every 6 weeks for 12 months or MP in the same dosage plus thalidomide 100 mg daily.52 The median PFS was 24.2 months and median OS 45.3 months compared with 19 months and 27.7 months, respectively, for the MP alone. Palumbo et al, in a phase II study of MM patients aged ≥ 65 years, determined that the maximum tolerated dose for melphalan was 0.18 mg/kg and lenalidomide 10 mg daily.53 A large, prospective, randomized study comparing melphalan, prednisone, and lenalidomide versus MP is ongoing in Europe.
Bortezomib, Melphalan, and Prednisone
Another option for the nontransplantation candidate is bortezomib, melphalan, and prednisone (VMP): bortezomib 1.3 mg/m2 I.V. days 1, 4, 8, 11, 22, 25, 29, and 32 during cycles 1-4 and bortezomib 1.3 mg/m2 on days 1, 8, 22, and 29 during cycles 5-9 plus melphalan 9 mg/m2 and prednisone 60 mg/m2 days 1-4 of each cycle or to MP in the same dosage and schedule.21 The CR plus VGPR was 45% for VMP compared with 10% for MP. As expected, neutropenia, thrombocytopenia, anemia, and gastrointestinal symptoms were more common with the MPV regimen. Peripheral neuropathy occurred in 44% of the VMP group and in 5% with MP. The peripheral neuropathy resolved or improved in 74% of patients within a median of 2 months.21
Trials directly comparing MPT, VMP, and melphalan/prednisone/lenalidomide (MPR) are needed. Currently, we prefer MPT for the treatment of standard-risk myeloma patients, whereas VMP is recommended for patients with high-risk disease. Though the numbers are small, it appears that VMP overcomes the adverse effect of chromosomal abnormalities.21
We continue the initial chemotherapy regimen until the patient reaches a plateau state. There is no evidence that continued chemotherapy with MP is of benefit after achieving a plateau state. In addition, there is a risk of myelodysplasia from continued treatment with alkylating agents. The role of thalidomide, bortezomib, and lenalidomide for maintenance therapy is not known. Nonalkylating agents should be administered as maintenance therapy only within the context of a clinical trial.
Summary of Treatment Recommendations for Newly Diagnosed Myeloma
Initial chemotherapy for the patient with symptomatic MM depends upon whether the patient is felt to be a candidate for ASCT. The major features that determine eligibility are age, performance status, and the presence of comorbid conditions. For those patients who are not eligible for an ASCT, we recommend MPT. If the patient falls in the high-risk group, VMP is preferable. For the transplantation-eligible patient who is in the standard risk group, we recommend a single ASCT following 3 or 4 cycles of lenalidomide plus low-dose dexamethasone. A second ASCT is recommended if the patient does not achieve a VGPR or CR. If the patient is transplantation eligible and falls in the high-risk group, a bortezomib-containing regimen is indicated. Bortezomib rather than lenalidomide is preferable for patients with renal insufficiency.
Management of Thromboembolic Risks in Patients Receiving Thalidomide or Lenalidomide
Anticoagulation is not needed for patients taking single-agent thalidomide or lenalidomide. If the two agents are used with low-dose dexamethasone (or prednisone), aspirin 81 mg or 325 mg daily is recommended.54
If thalidomide or lenalidomide is given with high-dose dexamethasone, doxorubicin, liposomal doxorubicin, or erythropoietin, the use of prophylactic low-molecular-weight heparin (equivalent of enoxaparin 40 mg subcutaneously daily) or full-dose warfarin to maintain a therapeutic International Normalized Ratio of 2-3 is advised. Low-dose warfarin is not beneficial and should be avoided. It is challenging to maintain a therapeutic level of warfarin while giving corticosteroids or other chemotherapeutic agents intermittently. The risk of bleeding when using anticoagulation agents must be weighed against the risk of bleeding in a given patient. If a patient has a history of a previous thromboembolic event or is on bed rest or is obese or has any other risk factor for thrombosis, one must consider anticoagulation. Bortezomib does not produce a higher level of thromboembolic risk.8
Treatment of Relapsed or Refractory Multiple Myeloma
Multiple myeloma is an incurable disease, and almost all patients will eventually develop resistant or refractory disease. The role of relapse in MM is important. In a report of 578 patients with symptomatic MM who were followed up and monitored throughout their clinical course at Mayo Clinic, the OS rates at 1, 2, and 5 years were 72%, 55%, and 22%, respectively. The median OS was 28.4 months. The median OS of 355 patients who experienced relapse after initial therapy was 17.1 months from institution of the second therapy. The duration of response decreased with each successive relapse. The median duration of time from diagnosis to the first relapse was 9.9 months. Time from diagnosis for the second, third, fourth, fifth, and sixth relapses were 7.3, 6.0, 4.5, 4, and 3.2 months, respectively.55
If relapse occurs more than 6 months after initial therapy is stopped, the initial chemotherapy regimen should be reinstituted in most instances. In the past decade, thalidomide, bortezomib, and lenalidomide have been introduced and have revolutionized the treatment of patients with relapsed MM.
Thalidomide
Singhal et al reported that thalidomide in a dosage of 200 mg daily with escalation to 800 mg daily, if tolerated, produced a response in 32% of 84 patients.17 The responses are durable, with a median duration of approximately 12 months.56 When used with corticosteroids, the response rate with thalidomide increased to approximately 50%. Side effects from thalidomide include sedation, fatigue, constipation, rash, peripheral neuropathy, deep vein thrombosis, edema, bradycardia, and hypothyroidism. The use of thalidomide in pregnancy is contraindicated, and the STEPS program must be followed to prevent teratogenic effects.57
Bortezomib
Bortezomib is a proteasome inhibitor that shows activity against MM. In a phase I study, Orlowski et al reported activity in MM.58 A multicenter phase II trial of bortezomib in 193 patients with refractory MM showed a response rate of 35%. Furthermore, the median duration of response was 12 months.59 A randomized phase II trial of bortezomib versus dexamethasone in 669 myeloma patients who had not responded to treatment or who had relapsed after initial therapy was reported.60 The patients were randomized to bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 every 3 weeks or dexamethasone 40 mg on days 1-4, 9-12, and 17-20. The time to progression was 6.2 months with bortezomib and 3.5 months with dexamethasone. The OS was 29.8 months versus 23.7 months, favoring the bortezomib regimen.60 The response to bortezomib is rapid and usually occurs with 1 or 2 cycles of therapy. In another randomized study of patients with relapsed or refractory myeloma, 646 patients were randomized to bortezomib 1.3 mg/m2 I.V. on days 1, 4, 8, and 11 or to bortezomib in the same dose and schedule plus doxorubicin 30 mg/m2 I.V. on day 4 (pegylated liposomal doxorubicin). The time to progression was 6.5 months for bortezomib and 9.3 months for bortezomib plus doxorubicin. Survival at 15 months was superior in the bortezomib/doxorubicin combination (75% vs. 65%).61 The most common side effects of bortezomib are gastrointestinal symptoms, cytopenias, fatigue, and peripheral neuropathy. The peripheral neuropathy is often very painful and develops in 30%-40% of patients.
Bortezomib appears to overcome the effect of adverse chromosomal features such as hypodiploidy and deletion of chromosome 13 by conventional cytogenetics as well as adverse cytogenetic changes such as t(4;14), t(4;16); or 17p- on FISH. Objective evidence is meager, but the 26 patients with high-risk cytogenetic profiles—t(4;14), t(14;16) translocation or a del(17p)—had the same rate of complete response and similar times to progression as well as OS compared with the 142 patients with normal cytogenetic profiles.21
Lenalidomide
Lenalidomide produces an objective response in approximately 30% of patients with MM.62 In a large randomized study, 704 patients in North America and Europe with relapsed and/or refractory MM were randomized to lenalidomide 25 mg days 1-21 plus dexamethasone 40 mg days 1-4, 9-12, and 17-20, or to placebo plus the same dose and schedule of dexamethasone. The response rate was 60.5% versus 22%, favoring lenalidomide plus dexamethasone over dexamethasone. The time to progression was 11.2 months for lenalidomide/dexamethasone compared with 4.7 months for dexamethasone alone.63,64 The major side effects of lenalidomide are thrombocytopenia, neutropenia, and anemia, whereas neuropathy, sedation, and gastrointestinal effects were not a problem.65-67
Survival of Multiple Myeloma in the Past Decade: Effect of Novel Therapies
The survival of MM was < 1 year before the introduction of alkylating agents.68 The introduction of melphalan was an important advance.69 Introduction of ASCT and the novel agents of thalidomide and bortezomib have improved survival. Kumar et al in 2008 reported that OS improved in patients relapsing after stem cell transplantation when they had received thalidomide, bortezomib, or lenalidomide. In 387 patients from Mayo Clinic, 161 patients who received one of the novel agents had a median survival of 30.9 months compared with 14.8 months for the 226 patients who relapsed and did not receive any of the novel agents (Figure 3).70 A review of 2981 patients from Mayo Clinic who were seen between 1971 and 2006 showed that the median survival of those diagnosed after 1996 was 44.8 months compared with 29.9 months for those diagnosed between 1971 and 1996 (Figure 4).70
Figure 3. Overall Survival From Time of Posttransplantation Relapse Grouped by Whether Patients Received One of the New Drugs (Thalidomide, Bortezomib, or Lenalidomide) or Not70.
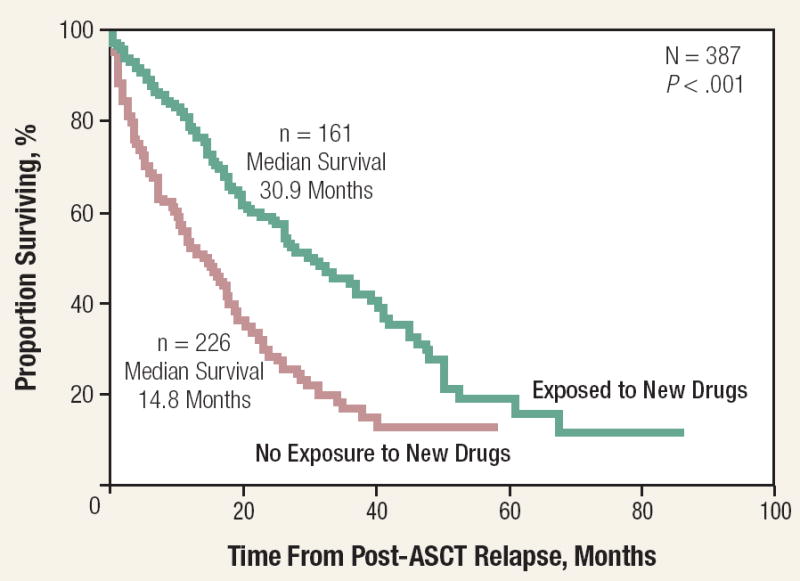
This research was originally published in Blood. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516-20. © the American Society of Hematology.
Figure 4. Overall Survival of Multiple Myeloma From Time of Diagnosis Before and After 199670.
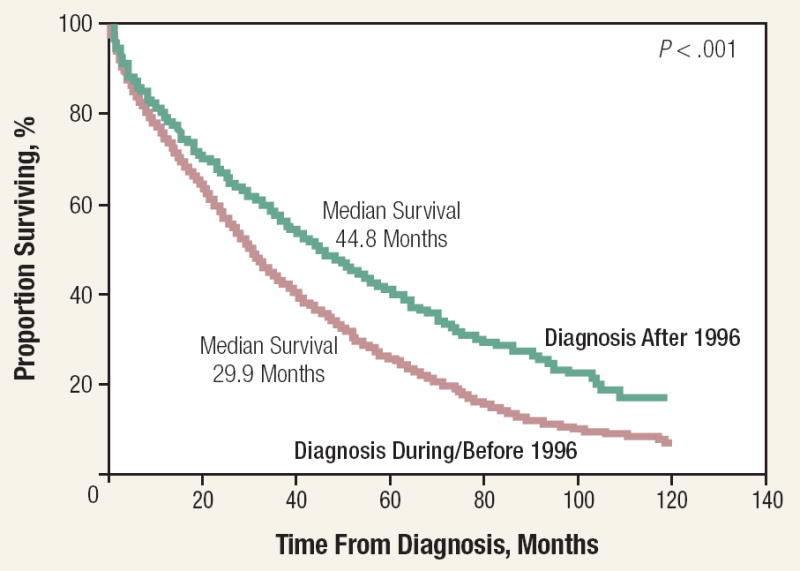
This research was originally published in Blood. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516-20. © the American Society of Hematology.
New phase I or phase II agents include SAHA, a histone deacytlase inhibitor; tanespimycin, a heat-shock protein-90 inhibitor; vatalanib (PTK787), a vascular endothelial growth factor inhibitor; lonafarnib (SCH-66336), farnesyltransferase inhibitor; NPI-005, an oral proteasome inhibitor; dovitinib lactate (CHIR-258), an FGFR3 inhibitor; talmapimod (SCIO 469), a P38 MAPK inhibitor; and carthrozomab. Podar et al published an excellent review concerning new agents for the treatment of MM.71
Treatment of Complications
Management strategies for complications in patients with MM have been published.65
Hypercalcemia
Hypercalcemia is present in 15%-20% of patients with MM at the time of diagnosis. It might be asymptomatic or produce anorexia, nausea, vomiting, polyuria, polydipsia, increased constipation, weakness, confusion, or stupor. Hypercalcemia is a major cause of renal insufficiency. Treatment consists of hydration, preferably with isotonic saline. Prednisone 25 mg 4 times daily is effective in most patients. If the patient has severe hypercalcemia or does not respond to hydration and prednisone, zoledronic acid 4 mg intravenously over 15 minutes is recommended.72
Skeletal Disease
Approximately 80% of patients will have abnormalities on conventional skeletal radiographs at the time of diagnosis of symptomatic MM. Magnetic resonance imaging (MRI) shows lesions in > 90% of patients. Technetium bone scans are inferior to roentgenograms for the detection of lytic lesions.73
Intravenous pamidronate 90 mg delivered over at least 2 hours74 or zoledronic acid 4 mg delivered over at least 15 minutes every 3-4 weeks is recommended for patients with MM who have lytic lesions or osteopenia on the basis of plain radiographs, MRI, or computed tomography (CT).75 Clodronate is an alternative bisphosphonate approved worldwide, except in the United States, and may be given orally.
Pamidronate should be reduced in patients with renal insufficiency. Zoledronic acid should be avoided in patients with severe renal impairment. The bisphosphonates should be continued for 2 years, and at that point, physicians should seriously consider discontinuing bisphosphonates in patients with responsive or stable myeloma.75 Bisphosphonates should be reinstituted in the event of relapsed and progressive skeletal involvement. Intravenous bisphosphonates are not indicated for patients with monoclonal gammopathy of undetermined significance, smoldering MM, or solitary plasmacytoma. Osteonecrosis of the jaw is a potential complication of bisphosphonates.76 It is desirable to have a comprehensive dental examination and appropriate preventive dentistry before bisphosphonate therapy is initiated. When on therapy, patients should maintain good oral hygiene and avoid invasive dental procedures if at all possible in order to reduce the risk of osteonecrosis of the jaw.
Patients with MM should be encouraged to be as active as possible and to avoid trauma. Fixation of fractures or impending fractures of long bones with an intramedullary rod and methyl methacrylate has produced good results.
Vertebroplasty involves the percutaneous injection of methyl methacrylate under fluoroscopic guidance into the collapsed vertebral body. Kyphoplasty is a technique that involves the introduction of an inflatable balloon into the vertebral body. Inflation of the balloon may restore the height of the vertebral body and creates a cavity that can be filled with methyl methacrylate. Both procedures might be beneficial and are discussed in detail by the International Myeloma Working Group.77
Anemia
Anemia is a common feature of MM. Randomized clinical trials have shown that symptomatic anemia is often improved by the administration of erythropoietin. Other causes of anemia such as iron, folate, or vitamin B12 deficiencies must be recognized and treated. Anemia may be secondary to bone marrow involvement or renal insufficiency. Successful treatment of MM often results in reduction of tumor mass and better renal function, leading to higher hemoglobin levels. Erythropoietin should be considered for patients whose hemoglobin is < 10 g/dL. Erythropoietin should not be given if the hemoglobin reaches 11 g/dL. Guidelines for the use of erythropoietin have been published.78 Blood transfusions are indicated for patients with severe anemia that does not respond to other therapy.
Renal Insufficiency
Renal insufficiency is a major problem in MM.79 It is present in approximately one half of patients with MM at diagnosis; 20% have a serum creatinine > 2 mg/dL. The two major causes of renal insufficiency are cast nephropathy, which is due to excessive production of monoclonal light chains or hypercalcemia. Dehydration or hyperuricemia might also produce renal failure. The presence of AL amyloidosis or light chain deposition disease might produce renal failure; they are usually associated with the nephrotic syndrome.
Patients with acute renal failure because of cast nephropathy should be treated with bortezomib and dexamethasone. Plasmapheresis is a consideration for patients with acute renal failure. It is important to encourage patients with Bence Jones proteinuria to drink enough fluids to produce 3 L of urine daily.
Infections
Patients with MM are prone to infections because of suppression of humoral and cell-mediated immunity from the disease and the effects of therapy. Common organisms include Streptococcus pneumoniae and Haemophilus influenzae. Herpes zoster is also common and requires antiviral therapy to prevent dissemination. Intravenous immunoglobulin infusions might be helpful for patients with hypogammaglobulinemia and recurrent serious infections, despite the use of prophylactic antibiotics. Despite the fact that patients with MM develop suboptimal antibody responses, immunization with pneumococcal and influenza vaccine should be given. Guidelines for the management of infections have been published.80
Spinal Cord Compression
Spinal cord compression from an extramedullary plasmacytoma should be suspected in patients with weakness or paresthesias of the lower extremities or bladder or bowel dysfunction or incontinence. MRI or CT myelography of the entire spine must be performed immediately. Dexamethasone and radiation therapy are usually helpful if cord or cauda equina compression is documented. Surgical decompression is usually unnecessary but should be done if the neurologic deficit increases.
Hyperviscosity Syndrome
Patients with MM infrequently develop hyperviscosity syndrome.81 It is characterized by blurred vision, oronasal bleeding, neurologic symptoms, confusion, or congestive heart failure. Serum viscosity measurements do not correlate well with symptoms or the clinical findings. Consequently, physicians must examine the fundus for evidence of venous dilatation, presence of hemorrhages or exudates or papilledema. Plasmapheresis promptly relieves the symptoms and should be performed regardless of the viscosity level if the patient is symptomatic. It should be kept in mind that some patients might have a marked increase of relative viscosity but no symptoms or findings of hyperviscosity. These patients should not be treated with plasmapheresis.
Acknowledgments
This work was supported in part by research grants CA107476 and CA62242 from the National Cancer Institute.
Footnotes
Disclosures
Dr. Robert A. Kyle serves on disease monitoring committees for Celgene Corporation, Johnson & Johnson; disease monitoring board for Novartis Pharmaceuticals Corporation; data monitoring committee for Merck & Co.; independent review committee for Kosan Biosciences, Inc.; independent monitoring committee for Bristol-Myers Squibb Company; and has served as a consultant for Millennium Pharmaceuticals, Inc.
Dr. S. Vincent Rajkumar has no relevant disclosures to report.
References
- 1.Kyle RA, Therneau TM, Rajkumar SV, et al. Incidence of multiple myeloma in Olmsted County, Minnesota - Trend over 6 decades. Cancer. 2004;101:2667–74. doi: 10.1002/cncr.20652. [DOI] [PubMed] [Google Scholar]
- 2.Wingo PA, Ries LA, Rosenberg HM, et al. Cancer incidence and mortality, 1973-1995: a report card for the U.S. Cancer. 1998;82:1197–207. doi: 10.1002/(sici)1097-0142(19980315)82:6<1197::aid-cncr26>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Ries LA, et al., editors. SEER cancer statistics review, 1975-2003. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 4.Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–57. [PubMed] [Google Scholar]
- 5.Rajkumar SV, Dispenzieri A, Kyle RA. Monoclonal gammopathy of undetermined significance, Waldenström macroglobulinemia, AL amyloidosis, and related plasma cell disorders: diagnosis and treatment. Mayo Clin Proc. 2006;81:693–703. doi: 10.4065/81.5.693. [DOI] [PubMed] [Google Scholar]
- 6.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582–90. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 8.Dispenzieri A, Rajkumar SV, Gertz MA, et al. Treatment of newly diagnosed multiple myeloma based on Mayo stratification of myeloma and risk-adapted therapy (mSMART): consensus statement. Mayo Clin Proc. 2007;82:323–41. doi: 10.4065/82.3.323. [DOI] [PubMed] [Google Scholar]
- 9.Stewart AK, Bergsagel PL, Greipp PR, et al. A practical guide to defining high-risk myeloma for clinical trials, patient counseling and choice of therapy. Leukemia. 2007;21:529–34. doi: 10.1038/sj.leu.2404516. [DOI] [PubMed] [Google Scholar]
- 10.Badros A, Barlogie B, Siegel E, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol. 2001;114:822–9. doi: 10.1046/j.1365-2141.2001.03033.x. [DOI] [PubMed] [Google Scholar]
- 11.Goldschmidt H, Hegenbart U, Wallmeier M, et al. Factors influencing collection of peripheral blood progenitor cells following high-dose cyclophosphamide and granulocyte colony-stimulating factor in patients with multiple myeloma. Br J Haematol. 1997;98:736–44. doi: 10.1046/j.1365-2141.1997.2783095.x. [DOI] [PubMed] [Google Scholar]
- 12.Morris CL, Siegel E, Barlogie B, et al. Mobilization of CD34+ cells in elderly patients (>/= 70 years) with multiple myeloma: influence of age, prior therapy, platelet count and mobilization regimen. Br J Haematol. 2003;120:413–23. doi: 10.1046/j.1365-2141.2003.04107.x. [DOI] [PubMed] [Google Scholar]
- 13.Giralt S, Stadtmauer E, Harousseau J, et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high dose therapy for multiple myeloma including the role of plerixafor [AMD 3100] Leukemia. 2009 doi: 10.1038/leu.2009.127. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Rajkumar SV, Jacobus S, Callander N, et al. Phase III trial of lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone in newly diagnosed multiple myeloma (E4A08): A trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2007;25(suppl):447s. Abstract LBA8025. [Google Scholar]
- 15.Rajkumar SV, Jacobus S, Callander N, et al. Randomized trial of lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone in newly diagnosed myeloma (ERA03), a trial coordinated by the Eastern Cooperative Oncology Group: Analysis of response, survival, and outcome. J Clin Oncol. 2008;26(suppl):455s. Abstract 8504. [Google Scholar]
- 16.Niesvizky R, Jayabalan DS, Christos PJ, et al. BiRD (Biaxin [clarithromycin]/Revlimid [lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood. 2008;111:1101–9. doi: 10.1182/blood-2007-05-090258. [DOI] [PubMed] [Google Scholar]
- 17.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–71. doi: 10.1056/NEJM199911183412102. erratum appears in N Engl J Med 2000; 342:364. [DOI] [PubMed] [Google Scholar]
- 18.Rajkumar SV, Rosinol L, Hussein M, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26:2171–7. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Offidani M, Corvatta L, Piersantelli MN, et al. Thalidomide, dexamethasone, and pegylated liposomal doxorubicin (ThaDD) for patients older than 65 years with newly diagnosed multiple myeloma. Blood. 2006;108:2159–64. doi: 10.1182/blood-2006-03-013086. [DOI] [PubMed] [Google Scholar]
- 20.Jagannath S, Durie BD, Wolf JL, Jr, et al. Long-term follow-up of patients treated with bortezomib alone and in combination with dexamethasone as frontline therapy for multiple myeloma. Blood. 2006;108:238a–9a. Abstract 796. [Google Scholar]
- 21.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 22.Rosinol L, Oriol A, Mateos MV, et al. Phase II PETHEMA trial of alternating bortezomib and dexamethasone as induction regimen before autologous stem-cell transplantation in younger patients with multiple myeloma: efficacy and clinical implications of tumor response kinetics. J Clin Oncol. 2007;25:4452–8. doi: 10.1200/JCO.2007.12.3323. [DOI] [PubMed] [Google Scholar]
- 23.Cavo M, Patriarca F, Tacchetti P, et al. Bortezomib (Velcade(R))-Thalidomide-Dexamethasone (VTD) vs. Thalidomide-Dexamethasone (TD) in preparation for autologous stem-cell (SC) transplantation (ASCT) in newly diagnosed multiple myeloma (MM) Blood. 2007;110:30A. Abstract 73. [Google Scholar]
- 24.Barlogie B, Smith L, Alexanian R. Effective treatment of advanced multiple myeloma refractory to alkylating agents. N Engl J Med. 1984;310:1353–6. doi: 10.1056/NEJM198405243102104. [DOI] [PubMed] [Google Scholar]
- 25.Anderson H, Scarffe JH, Ranson M, et al. VAD chemotherapy as remission induction for multiple myeloma. Br J Cancer. 1995;71:326–30. doi: 10.1038/bjc.1995.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar SK, Dingli D, Dispenzieri A, et al. Impact of pretransplant therapy in patients with newly diagnosed myeloma undergoing autologous SCT. Bone Marrow Transplant. 2008;41:1013–9. doi: 10.1038/bmt.2008.24. [DOI] [PubMed] [Google Scholar]
- 27.Cavo M, Tacchetti P, Patriarca F, et al. Superior complete response rate and progression-free survival after autologous transplantation with up-front velcade-thalidomide-dexamethasone compared with thalidomide-dexamethasone in newly-diagnosed multiple myeloma. Blood. 2008;112 Abstract 158. [Google Scholar]
- 28.Harousseau J, Mathiot C, Attal M, et al. Velcade/dexamethasone (Vel/D) versus VAD as induction treatment prior to autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma (MM): Updated results of the IFM 2005/01 trial. Blood (Abstract) 2007;110:450. [Google Scholar]
- 29.Stewart AK, Vescio R, Schiller G, et al. Purging of autologous peripheral-blood stem cells using CD34 selection does not improve overall or progression-free survival after high-dose chemotherapy for multiple myeloma: results of a multicenter randomized controlled trial. J Clin Oncol. 2001;19:3771–9. doi: 10.1200/JCO.2001.19.17.3771. [DOI] [PubMed] [Google Scholar]
- 30.Bourhis JH, Bouko Y, Koscielny S, et al. Relapse risk after autologous transplantation in patients with newly diagnosed myeloma is not related with infused tumor cell load and the outcome is not improved by CD34+ cell selection: long term follow-up of an EBMT phase III randomized study. Haematologica. 2007;92:1083–90. doi: 10.3324/haematol.10535. [DOI] [PubMed] [Google Scholar]
- 31.Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–6. [PubMed] [Google Scholar]
- 32.Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–5. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 33.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 34.Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–502. doi: 10.1056/NEJMoa032290. erratum appears in N Engl J Med 2004; 350:2628. [DOI] [PubMed] [Google Scholar]
- 35.Cavo M, Tosi P, Zamagni E, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–41. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 36.Fermand JP, Alberti C, Morolleau JP. Single versus tandem high dose therapy (HOT) supported with autologous blood stem cell (ABSC) transplantation using unselected or CD34-enriched ASBC: Results of a two by two designed randomized trial in 230 young patients with multiple myeloma (MM) Hematology J. 2003;4:23–7. [Google Scholar]
- 37.Sirohi B, Powles PR, Mehta J, et al. Prognostic factors at the time of single autotransplantation in 451 myeloma patients treated with 200 mg/m2 Melphalan: Results equivalent to tandem autotransplantation. Blood. 2002;100 Abstract 673. [Google Scholar]
- 38.Sirohi B, Powles PR, Singhal S, et al. Long-term outcome of myeloma patients treated with an elective single autograft with follow-up (≥ 5 years): Results comparable to tandem autotransplantation. Blood. 2002;100 Abstract 671. [Google Scholar]
- 39.Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–94. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 40.Barlogie B, Tricot G, Anaissie E, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354:1021–30. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 41.Barlogie B, Pineda-Roman M, van Rhee F, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008;112:3115–21. doi: 10.1182/blood-2008-03-145235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdelkefi A, Ladeb S, Torjman L, et al. Single autologous stem-cell transplantation followed by maintenance therapy with thalidomide is superior to double autologous transplantation in multiple myeloma: results of a multicenter randomized clinical trial. Blood. 2008;111:1805–10. doi: 10.1182/blood-2007-07-101212. [DOI] [PubMed] [Google Scholar]
- 43.Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27:1788–93. doi: 10.1200/JCO.2008.18.8573. [DOI] [PubMed] [Google Scholar]
- 44.Interferon as therapy for multiple myeloma: an individual patient data overview of 24 randomized trials and 4012 patients. Br J Haematol. 2001;113:1020–34. doi: 10.1046/j.1365-2141.2001.02857.x. [DOI] [PubMed] [Google Scholar]
- 45.Barlogie B, Kyle RA, Anderson KC, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24:929–36. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 46.Bensinger WI, Buckner CD, Anasetti C, et al. Allogeneic marrow transplantation for multiple myeloma: an analysis of risk factors on outcome. Blood. 1996;88:2787–93. [PubMed] [Google Scholar]
- 47.Crawley C, Lalancette M, Szydlo R, et al. Outcomes for reduced-intensity allogeneic transplantation for multiple myeloma: an analysis of prognostic factors from the Chronic Leukaemia Working Party of the EBMT. Blood. 2005;105:4532–9. doi: 10.1182/blood-2004-06-2387. [DOI] [PubMed] [Google Scholar]
- 48.Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–20. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 49.Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. Myeloma Trialists’ Collaborative Group. J Clin Oncol. 1998;16:3832–42. doi: 10.1200/JCO.1998.16.12.3832. [DOI] [PubMed] [Google Scholar]
- 50.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367:825–31. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 51.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–18. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 52.Hulin C, Facon T, Rodon P, et al. Melphalan-Prednisone-Thalidomide (MP-T) demonstrates a significant survival advantage in elderly patients ≥ 75 years with multiple myeloma compared with melphalan-prednisone (MP) in a randomized, double-blind, placebo-controlled trial, IFM 01/01. Blood. 2007;110:31a. Abstract 75. [Google Scholar]
- 53.Palumbo A, Falco P, Corradini P, et al. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA–Italian Multiple Myeloma Network. J Clin Oncol. 2007;25:4459–65. doi: 10.1200/JCO.2007.12.3463. [DOI] [PubMed] [Google Scholar]
- 54.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–23. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- 55.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79:867–74. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 56.Barlogie B, Desikan R, Eddlemon P, et al. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase 2 study of 169 patients. Blood. 2001;98:492–4. doi: 10.1182/blood.v98.2.492. [DOI] [PubMed] [Google Scholar]
- 57.Zeldis JB, Williams BA, Thomas SD, et al. S.T.E.P.S.: a comprehensive program for controlling and monitoring access to thalidomide. Clin Ther. 1999;21:319–30. doi: 10.1016/s0149-2918(00)88289-2. [DOI] [PubMed] [Google Scholar]
- 58.Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–7. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 59.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–17. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 60.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557–60. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 61.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 62.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–7. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 63.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–42. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 64.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–32. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 65.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–73. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 66.Anderson KC, Kyle RA, Rajkumar SV, et al. Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008;22:231–9. doi: 10.1038/sj.leu.2405016. [DOI] [PubMed] [Google Scholar]
- 67.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–72. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Osgood EE. The survival time of patients with plasmocytic myeloma. Cancer Chemother Rep. 1960;9:1–10. [PubMed] [Google Scholar]
- 69.Blokhin N, Larionov L, Perevodchikova N, et al. Clinical experiences with sarcolysin in neoplastic diseases. Ann N Y Acad Sci. 1958;68:1128–32. doi: 10.1111/j.1749-6632.1958.tb42675.x. [DOI] [PubMed] [Google Scholar]
- 70.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23:10–24. doi: 10.1038/leu.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19:558–67. doi: 10.1200/JCO.2001.19.2.558. [DOI] [PubMed] [Google Scholar]
- 73.Wahner HW, Kyle RA, Beabout JW. Scintigraphic evaluation of the skeleton in multiple myeloma. Mayo Clin Proc. 1980;55:739–46. [PubMed] [Google Scholar]
- 74.Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334:488–93. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 75.Kyle RA, Yee GC, Somerfield MR, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25:2464–72. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 76.Raje N, Woo SB, Hande K, et al. Clinical, radiographic, and biochemical characterization of multiple myeloma patients with osteonecrosis of the jaw. Clin Cancer Res. 2008;14:2387–95. doi: 10.1158/1078-0432.CCR-07-1430. [DOI] [PubMed] [Google Scholar]
- 77.Hussein MA, Vrionis FD, Allison R, et al. The role of vertebral augmentation in multiple myeloma: International Myeloma Working Group Consensus Statement. Leukemia. 2008;22:1479–84. doi: 10.1038/leu.2008.127. [DOI] [PubMed] [Google Scholar]
- 78.Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26:132–49. doi: 10.1200/JCO.2007.14.3396. [DOI] [PubMed] [Google Scholar]
- 79.Dimopoulos MA, Kastritis E, Rosinol L, et al. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008;22:1485–93. doi: 10.1038/leu.2008.131. [DOI] [PubMed] [Google Scholar]
- 80.Smith A, Wisloff F, Samson D. Guidelines on the diagnosis and management of multiple myeloma 2005. Br J Haematol. 2006;132:410–51. doi: 10.1111/j.1365-2141.2005.05867.x. [DOI] [PubMed] [Google Scholar]
- 81.Gertz MA, Kyle RA. Hyperviscosity syndrome. J Intensive Care Med. 1995;10:128–41. doi: 10.1177/088506669501000304. [DOI] [PubMed] [Google Scholar]
- 82.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–7. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 83.Blade J, Rosinol L, Sureda A, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. 2005;106:3755–9. doi: 10.1182/blood-2005-03-1301. [DOI] [PubMed] [Google Scholar]
- 84.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]


