Abstract
Medial agranular cortex (AGm) has a prominent bilateral projection to the dorsocentral striatum (DCS). We wished to develop a normal baseline by which to assess neuronal plasticity in this corticostriatal system in rats with neglect resulting from a unilateral lesion in AGm, followed by treatment with agents that promote sprouting and functional recovery in other systems. Injections of biotinylated dextran amine were made into AGm in normal rats, and unbiased sampling was used to quantify the density of axons and axonal varicosities present in DCS (the latter represent presynaptic profiles). Labeling density in contralateral DCS is approximately half that seen in ipsilateral DCS (this ratio is 0.50 for axons, 0.55 for varicosities). The ratio of varicosities is stable over a greater than seven-fold range of absolute densities. There is no consistent relationship between the absolute density of axons and axon varicosities; however, the ratio measures are strongly correlated. We conclude that changes in the contralateral/ipsilateral ratio of axon density after experimental treatments do reflect changes in synaptic density, but axon varicosities are likely to be the most sensitive anatomical parameter by which to assess plasticity at the light microscopic level.
Keywords: axon density, synaptic density, striatum, cerebral cortex
Introduction
Corticostriatal projections have been known to exist since Cajal (1891 – see Webster 1961). However, the existence of bilateral corticostriatal projections from single cortical areas was discovered more recently, in the 1960s (see Carman et al., 1965). Due to the presence of bilateral corticostriatal projections in a number of species which have been studied (Kemp and Powell, 1970; Fallon and Ziegler, 1979; Cospito and Kultas-Ilinsky, 1981; Royce, 1982; Fisher et al., 1984; Wilson, 1986), these projections are considered to be a feature typical of mammalian brains.
In rats, medial agranular cortex (area AGm, or Fr2) is the source of a particularly prominent projection to the dorsocentral striatum (DCS) (Reep et al., 1987; Wilson, 1987; Reep and Corwin, 1999; Reep et al., 2003). Two classes of cortical neurons contribute to this projection system. The majority of the projection originates from pyramidal neurons in layer Va and layer III, whereas a much smaller portion originates as collaterals from deeper layer Vb/VI pyramidal cells that project to the thalamus and brainstem (Wilson, 1987; Cowan and Wilson, 1994).
Recent behavioral studies have pointed to the importance of AGm and DCS as components of a cortical-subcortical network subserving directed spatial attention in rats (Burcham et al., 1997; Corwin and Reep, 1998; Reep et al., 2004). Unilateral lesions of AGm or DCS result in severe multimodal neglect of visual, auditory, and tactile stimuli presented contralaterally that is qualitatively similar to neglect in primates (Crowne and Pathria, 1982; Corwin et al., 1986; Van Vleet et al., 2002), and recent studies indicate that the integrity of DCS is essential for recovery of function from lesion-induced attentional disorders. Axon-sparing lesions of DCS produce severe multimodal neglect of stimuli presented contralesionally, whereas lesions placed more laterally and ventrally do not (Van Vleet et al., 2002). The dopamine agonist apomorphine induces acute recovery in animals with severe neglect produced by cortical lesions (Corwin et al., 1986, 1996; King and Corwin, 1990), and in these cases direct infusion of apomorphine into DCS results in dose-dependent acute recovery (Van Vleet et al., 2003). However, apomorphine does not result in recovery when administered to animals with lesions of DCS (Van Vleet et al., 2000), indicating that DCS is not only an essential component of the circuitry for neglect, but also that its integrity is necessary for recovery. Vargo and Marshall (1996a, 1996b) were the first to suggest that sprouting in the dorsolateral quadrant of the striatum, which includes the DCS, was correlated with neglect and behavioral recovery from AGm-induced neglect. They found that neglect was correlated with decreases in NMDA and kainate receptors in the ipsilesional dorsolateral striatum, and recovery was correlated with a normalization of kainate receptors and a 10% increase in NMDA receptors in this region.
Although the recovery observed by Vargo et al. could be based solely on changes at the receptor level, recent studies following lesions of motor cortex have found that loss of corticostriatal input can result in sprouting from the contralesional homotopic cortex, and functional recovery (Napieralski et al., 1996; Cheng et al., 1997; Cheng et al., 1998; McNeill et al., 1999; Meshul et al., 2000). These finding suggest the intriguing possibility that sprouting from the contralesional AGm may be the basis for the receptor changes found by Vargo and Marshall in the dorsolateral striatum. Therefore, DCS has become the focus for efforts to induce plasticity and long-term functional recovery from neglect in the rat model, and to determine the neurological basis for recovery.
Changes in the density of corticostriatal projections have been used as a measure of neuronal plasticity following cortical lesions and subsequent treatments intended to induce neuronal sprouting and functional recovery. Several studies have assessed treatment-related alterations in the ratio of contralateral to ipsilateral (contra/ipsi) axonal labeling following a cortical injection of the anterograde tracer BDA (Napieralski et al., 1996; Kartje et al., 1999; Carmichael and Chesselet, 2002; Riban and Chesselet, 2006). In normal and sham-operated animals, quantification of axonal density revealed mean contra/ipsi ratios of 0.20 – 0.26 in dorsolateral striatum following tracer injection in sensorimotor cortex (including areas AGl, FL, and HL) (Kartje et al., 1999; Carmichael and Chesselet, 2002), and a mean ratio of 0.62 in dorsomedial striatum (Kartje et al., 1999). A similar study in mice produced a mean contra/ipsi axon density ratio of 0.37 in dorsolateral striatum (Riban and Chesselet, 2006). One of the advantages of using the contra/ipsi ratio is that it controls for variation in the size and density of BDA injection sites. This is a variable which can have a significant influence on the absolute amount of labeling seen, particularly in the corticostriatal projection, which consists largely of small caliber collaterals that can be difficult to fill completely. One limitation of these studies is that changes in axon density are at best an indirect measure of changes in synaptic density which are likely to be the actual basis for functional recovery.
We wished to develop a normal baseline by which to assess sprouting in DCS in rats with neglect resulting from a unilateral lesion in AGm, followed by treatment with agents that promote sprouting and functional recovery in other systems. Our goal was to develop a method to quantify synaptic density at the light microscopic level, based on the fact that axon varicosities represent presynaptic profiles in a 1:1 manner in normal corticostriatal axons, as indicated by electron microscopic analysis (Kincaid et al., 1998).
Results
BDA injection sites in AGm
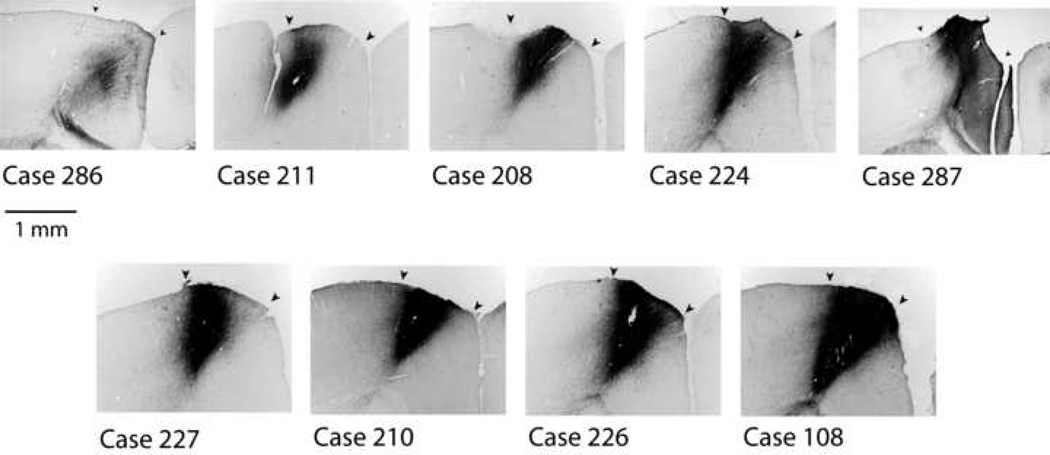
Nine injection sites met the criteria of being confined to area AGm and involving most or all of the cortical layers (Fig.1). Among these cases, a range of injection site sizes and densities was obtained. In each of these cases labeled axons and varicosities were present in the ipsilateral and contralateral striatum, thalamus and contralateral cortex.
Figure 1.
Nine cases of BDA injection sites in AGm, arranged in order of increasing size and density. The center of each injection site is shown at equivalent magnification and illumination. All injections were confined to AGm and centered at a-p +0.8. Arrowheads indicate boundaries of AGm determined from adjacent Nissl-stained sections.
Axonal labeling in ipsilateral and contralateral dorsocentral striatum
The topographic distribution of labeling is similar in ipsilateral and contralateral dorsocentral striatum. However, the density and area of labeling both appear to be greater ipsilaterally (Fig. 2). At high magnification one can distinguish two size classes of axons and varicosities. Large caliber axons are most often seen in fascicles on the dorsomedial border of the labeled area. Their collaterals, known to originate from cortical neurons projecting to the thalamus, brainstem, and spinal cord, exhibit large varicosities (cf. Reiner et al., 2003). Other corticostriatal axons are thinner and exhibit distinctly smaller varicosities (fig. 3).
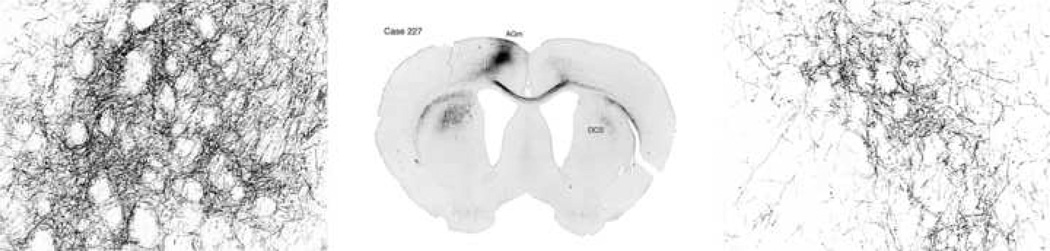
Figure 2.
Axonal labeling in ipsilateral and contralateral dorsocentral striatum in case 227. This representative case illustrates axonal labeling in DCS at low and medium magnification.
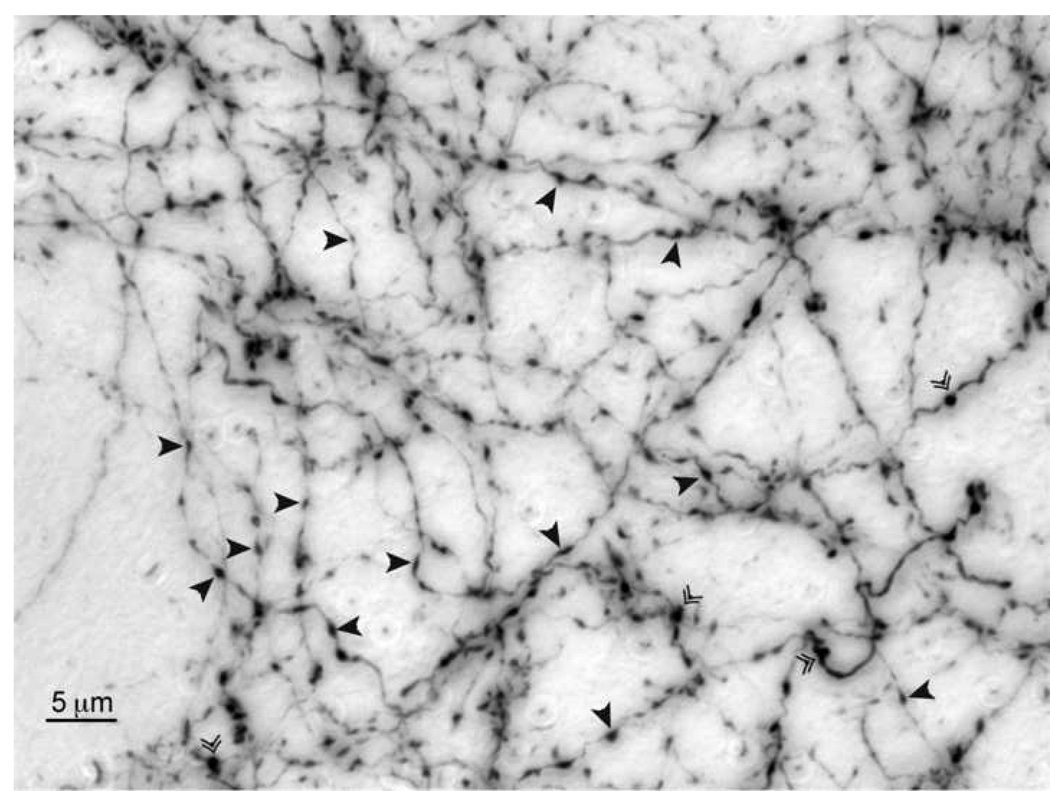
Figure 3.
Axonal varicosities in dorsocentral striatum. Two size classes of axons are visible. Large caliber axons project to the thalamus, brainstem, and spinal cord. Thinner axons are associated with wholly intratelencephalic projections. Arrowheads denote varicosities. Double arrowheads denote cut axons.
Synaptic density
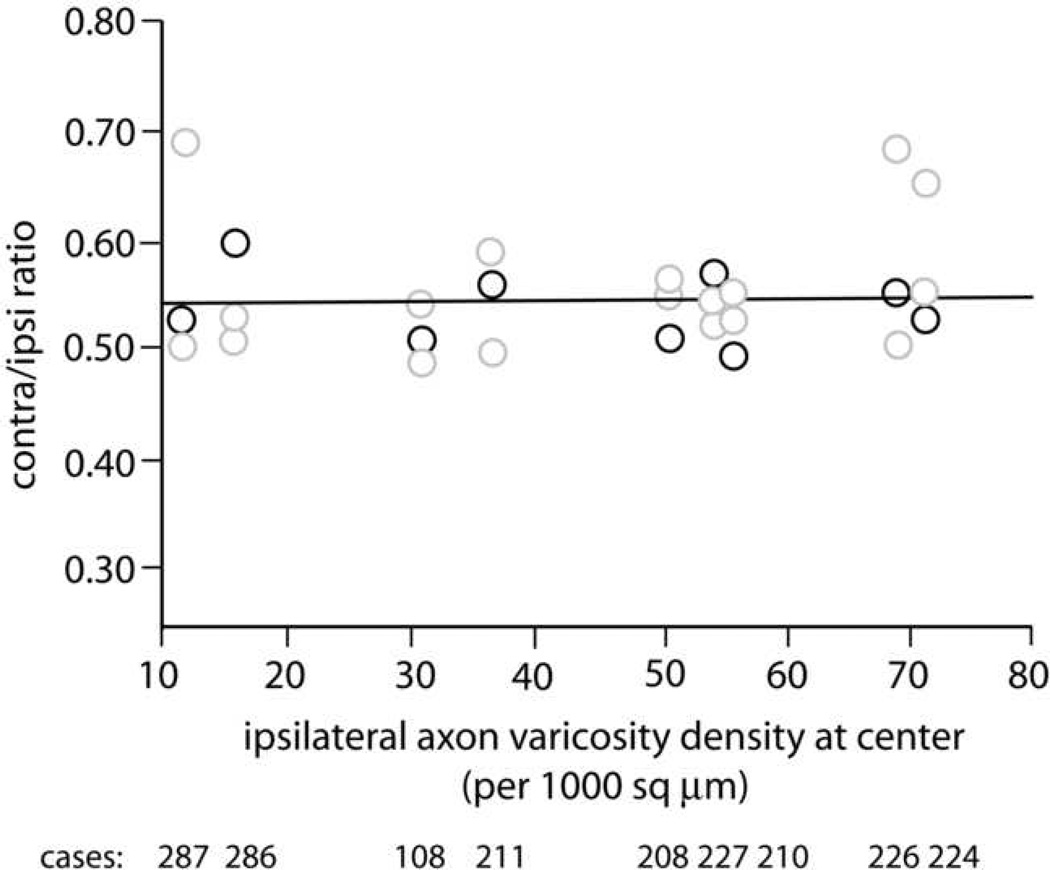
Regardless of the appearance of an injection site, many factors can influence the amount of transported label. Therefore, we chose to use ipsilateral density of striatal labeling as an index of the effective size and density of each injection site. As illustrated in Figure 4, the contra/ipsi ratio of density of axon varicosities exhibited a range of 0.48 – 0.69, with a mean value of 0.55 (SD = 0.05). The slope of 0.00025 (r2 = 0.009) indicates a very flat baseline across the greater than seven-fold range of labeling densities represented by this sample of nine brains. The number of varicosities sampled per section was roughly similar on the contralateral and ipsilateral sides (87–210 ipsilaterally, 75–187 contralaterally). This was accomplished by sampling a larger percentage of the region of interest on the contralateral side.
Figure 4.
Synaptic density assessed by unbiased sampling of axonal varicosities. Ipsilateral density at the center of the a-p range of labeling was used as an index of the effective size and density of the injection site, and is plotted on the x-axis. Black circles represent contra/ipsi ratios at these central locations, gray circles indicate densities at locations within 240 µm rostral or caudal of the central location. Mean contralateral density is half (0.55) that of the ipsilateral side. The flat slope represents a stable baseline over a greater than seven-fold range of ipsilateral densities. Case numbers are shown below the x-axis, and each is aligned with the data from that brain.
Axon density
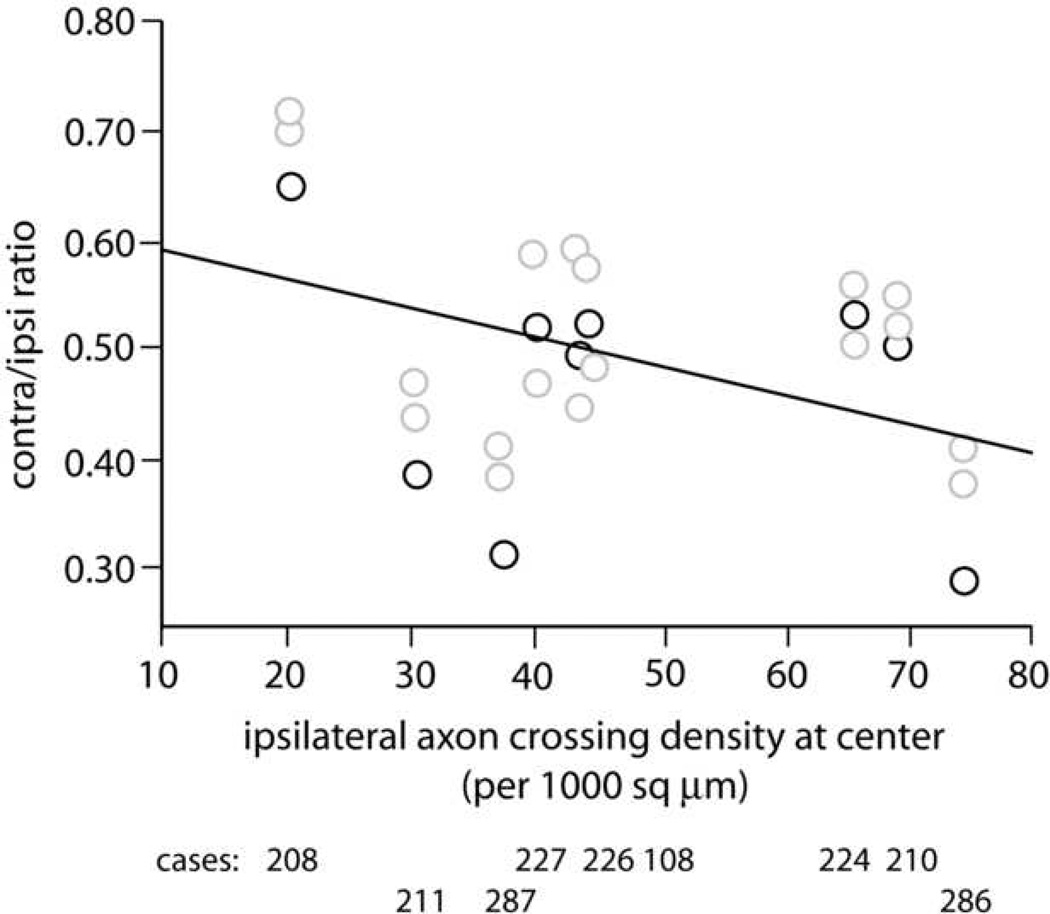
We used ipsilateral axon density as an index of injection site size and density, similar to the procedure used when analyzing axon varicosities. The mean contra/ipsi ratio of axon density is 0.50 (range 0.28 – 0.72) (Fig. 5), similar to the ratio of 0.55 for varicosities, but with greater variability (SD = 0.11). The slope (−0.002, r2 = 0.11) is somewhat greater than that for axon varicosities, but is still relatively flat. As was the case for varicosities, the ranges overlapped for counts made per section (ipsilateral, 99–310; contralateral, 68–239).
Figure 5.
Axon density assessed by unbiased sampling. Similar to the axon varicosity data in Figure 4, ipsilateral axon density at the center of the a-p range of labeling was used as an index of the effective size and density of the injection site, and is plotted on the x-axis. The mean contralateral/ipsilateral ratio of axon density by this measure is 0.50.
Relationship of synaptic density to axon density
The density of varicosities and axons are both roughly double on the ipsilateral vs. contralateral side, producing a mean contra/ipsi ratio of ~0.5 in each case. However, because there is variation from brain to brain, we wished to determine if there was a consistent relationship between the observed density of axon labeling and that of varicosities. Over the range of densities in this sample, absolute synaptic density is not correlated with absolute axon density. However, there is a significant correlation between the ratio measures (r2 = −0.58; p< 0.001).
Discussion
Our findings raise several interrelated issues. First, what is the cellular basis for the ~0.5 contra/ipsi ratio for density of axons and their varicosities, and how consistent are these patterns across cortical areas? There are many different cell types found in cerebral cortex, and cortical areas differ with respect to thickness and density of cortical layers. Is the cellular composition of the corticostriatal projection relatively constant in spite of these differences, such that we would expect the 0.5 ratio to generalize across cortical areas? Second, what are the implications of these considerations for studies of plasticity following strokes or other types of brain damage? Are some cell types more likely than others to engage in plastic responses to brain injury?
Sources of the corticostriatal projection from AGm
Our data indicate that contralateral labeling density is about half that seen ipsilaterally, whether we measure axons or varicosities. Considering that our cortical injections produce transport from hundreds of neurons in AGm, it is useful to examine the different cell types that contribute to the corticostriatal projection system, in order to attempt to explain the source of the ~0.5 contra/ipsi ratio.
The work of Wilson and colleagues (Wilson, 1987; Cowan and Wilson, 1994; Kincaid and Wilson, 1996; Kincaid et al., 1998; Zheng and Wilson, 2002) has defined two main classes of corticostriatal projections from AGm. Neurons of the first class are located in layers Vb and VI, and have large caliber axons that travel in the ipsilateral internal capsule and project to thalamus, brainstem and spinal cord. These axons give off collaterals in ipsilateral striatum which usually arborize in a focal manner and make terminations in the patch compartment. Neurons of the second class are located in layer Va and layer III. They have smaller caliber axons that tend to form extended arborizations that terminate largely in the matrix compartment. However, for both classes the exact proportion of focal vs. extended arborizations is unknown (Zheng and Wilson, 2002).
The ipsilateral-only pattern of striatal collateralization seen in large caliber axons from AGm appears to be a general feature of layer Vb/VI neurons, as it is also seen in the large caliber projections from prelimbic cortex (Levesque and Parent, 1998), area SI (Reiner et al., 2003) and area SII (Levesque et al. 1996). Following a striatal injection of retrograde tracer that labels neurons in ipsilateral AGm, a very small number of neurons are labeled in layer Vb and VI compared to the number seen in layers Va and III (Wilson, 1987; Cheatwood et al., 2003), about 12% of the total ipsilateral projection (estimated from Fig. 2 of Wilson (1987) by enlarging it and counting the cells). This fact, together with the focal nature of most large caliber collaterals, strongly suggests that brainstem-projecting axon collaterals constitute a relatively minor contribution to ipsilateral striatal labeling.
A variety of data suggests that most layer Va/III neurons do not project equivalently to ipsilateral and contralateral striatum. If they did, striatal injections of retrograde tracer should produce equal numbers of labeled cells in ipsilateral and contralateral AGm (ignoring the ~12% ipsilateral contribution from layer Vb/VI neurons). However, Wilson (1987) found that such injections produced many more labeled cells in ipsilateral than contralateral AGm (see his Fig. 2). In the same year, McGeorge and Faull (1987) reported quantitative data showing that injection of a retrograde tracer into the striatum produces approximately 55% as many cells labeled in contralateral vs. ipsilateral cortex (calculated from data in their Table 1).
Table 1.
Neuron types contributing to the corticostriatal projection from AGm, based upon retrograde labeling (see text).
| Neuron type | # neurons | % neurons |
|---|---|---|
| Ipsi Va/III | 70 | 51 |
| Contra Va/III | 37 | 27 |
| Bilateral Va/III | 18 | 13 |
| Ipsi Vb/VI | 12 | 9 |
| Totals | 137 | 100 |
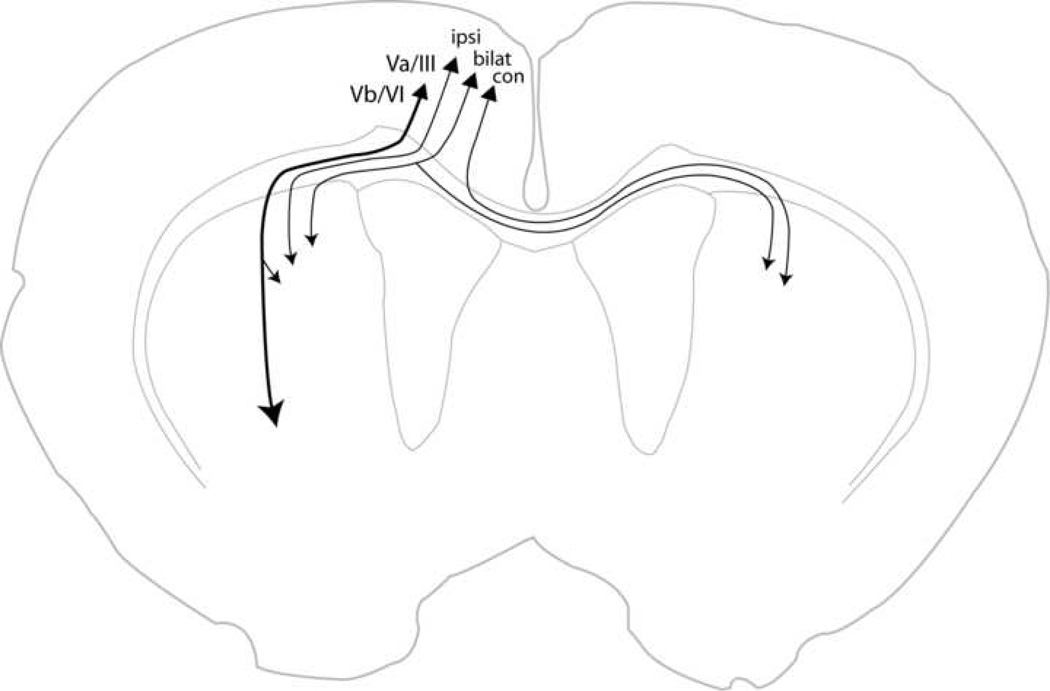
Figure 6 summarizes the four types of neurons that contribute to corticostriatal projections. Layer Va/III neurons are known to project to ipsilateral or contralateral striatum, or bilaterally, based upon single neuron reconstructions from prelimbic cortex (Levesque and Parent, 1998), AGm, cingulate, and lateral agranular cortex (Wilson, 1987; Cowan and Wilson, 1994; Kincaid et al., 1998; Zheng and Wilson, 2002). Therefore, following an injection of retrograde tracer into striatum, labeling in the contralateral cortex includes two neuron types - those projecting to contralateral striatum and those projecting bilaterally (Wilson, 1987; Cowan and Wilson, 1994; Kincaid et al., 1998; Levesque and Parent, 1998; Zheng and Wilson, 2002). Thus, for an injection of retrograde tracer in right striatum the 0.55 ratio of McGeorge and Faull represents: (contra Va/IIIl + bilat Va/IIIl) / (ipsi Va/IIIr + bilat Va/IIIr + ipsi Vb/VIr), where the subscripts indicate the side of cortex on which each cell type is labeled, the symbols contra, ipsi and bilat indicate the striatal target, the symbol Va/III represents neurons residing in layers Va and III, and the symbol Vb/VI represents neurons residing in layers Vb and VI. Two items should be noted: first, the 0.55 ratio involves no labeling of contralateral projecting neurons on the side of the injection site; second, we assume that the distribution of cell types is symmetrical in left and right homotopic areas of cortex.
Figure 6.
Schematic diagram of the four neuron types that contribute to corticostriatal projections. Neurons in layer Vb/VI have large caliber axons that project to thalamus, brainstem, and spinal cord, giving off collaterals in striatum. Neurons in layer Va/III project to ipsilateral striatum, contralateral striatum, or both.
Approximately 18% of cortical neurons labeled ipsilateral to a striatal injection project bilaterally (calculated from data in Table 1 of McGeorge and Faull, 1987), having branching axons that terminate in ipsilateral striatum, contralateral striatum, and contralateral cortex (McGeorge and Faull, 1987; Wilson, 1987). As noted above, ~12% of ipsilateral labeled cortical neurons are located in layer Vb/VI. Thus, the remaining ~70% are located in Va/III and project ipsilaterally.
Based on the above findings, we can ask: for 100 neurons in ipsilateral cortex distributed as indicated above, 12 Vb/VI, 18 Va/III bilat, 70 Va/III ipsi, what number of each cell type in contralateral cortex would produce the contra/ipsi ratio of 0.55 observed by McGeorge and Faull? Because we assume that the cell types are represented equally on both sides, we know that there will be 18 Va/III bilat cells on the contralateral side. Therefore, 37 Va/III contra cells must be present on the contralateral side to yield a contra/ipsi ratio of 0.55. However, this means that there must also be 37 Va/III (unlabeled) contra cells on the ipsilateral side. Therefore, for the total population of 137 cells on the ipsilateral side, the overall proportions of the four cell types contributing to the corticostriatal projection from AGm must be as shown in Table 1.
How might these data derived from retrograde labeling pertain to our observed ~0.55 contra/ipsi ratio of axon varicosities? Consider an injection of BDA into AGm that produces transport from 100 of the four neuron types, in the proportions given in the right hand column of Table 1. In order to realistically represent the numbers of varicosities produced by each of these neurons, it is necessary to realize that they are not equivalent in this regard. For example, collaterals of layer Vb/VI neurons tend to have more focal arborizations, thus fewer varicosities, than the extended arborizations typical of layer Va/III neurons, although the proportion of focal vs. extended arborizations for each neuron type is unknown (Kincaid et al., 1998; Zheng and Wilson, 2002). The density of varicosities per unit length does appear to be similar in both cases, resulting in very different total numbers of varicosities: 22 for a focal arborization vs. 934 for an extended, in one example (Kincaid et al., 1998). We now make the following working assumptions: 1) Most layer Vb/VI neurons (~70%) have focal arborizations; 2) most layer Va/III neurons (~70%) have extended arborizations; 3) focal arborizations produce ~22 varicosities whereas extended arborizations produce ~934. When these values are incorporated into the above calculations, we arrive at the distribution of varicosities shown in Table 2, and a contra/ipsi ratio of 0.53. Therefore, this model represents a plausible explanation of the source of our observed contra/ipsi ratios of axons and their varicosities.
Table 2.
Neuron types contributing to the observed contra/ipsi ratio of 0.54 for axon varicosities, assuming 100 neurons total and differing proportions of focal vs. extended arborizations for each neuron type (see text).
| Neuron type | # neurons | # ipsi varicosities | # contra varicosities |
|---|---|---|---|
| Ipsi Va/III | 51 | 33,680 | 0 |
| Contra Va/III | 23 | 0 | 15,189 |
| Bilateral Va/III | 13 | 8,585 | 8,585 |
| Ipsi Vb/VI | 9 | 2,660 | 0 |
| Totals | 100 | 44,925 | 23,774 |
Do these findings on the organization of the corticostriatal projection from AGm generalize to other cortical areas? Even if the ~0.5 contra/ipsi ratio of axons and varicosities is similar across areas, the cellular makeup of the projection system could differ. McGeorge and Faull (1987) found that the projections from primary motor cortex and primary somatic sensory cortex are similar in their contra/ipsi ratios, so the simplest hypothesis is that they are also similar with regard to the proportions of cell types that make up their corticostriatal projections, but this remains to be examined directly. The axon arborizations of relatively few single corticostriatal neurons have been observed and quantified, but it does appear that many cortical areas are likely to possess all four of the neuron types depicted in Figure 6.
Relationship between axons and varicosities
We found no relationship between the absolute densities of axons and varicosities, but their ratio measures were correlated. This suggests that whatever variables influence the relationship between axon density and varicosity density, they do so equivalently on the ipsilateral and contralateral sides within each brain. One such variable would be the proportions of the four types of corticostriatal projection neurons that take up and transport label in a given case. In addition, there may be variation among individual animals in this relationship.
Evaluating plasticity
The status of corticostriatal projections has been used as an index of plasticity in several studies, following experimental cortical infarcts or lesions (Chen and Hillman, 1990; Napieralski et al., 1996; Cheng et al., 1997; Cheng et al., 1998; Kartje et al., 1999; McNeill et al., 1999; Uryu et al., 2001; Carmichael and Chesselet, 2002; Riban and Chesselet, 2006). The present study contributes to these efforts by specifying specific criteria for the quantitative evaluation of axonal varicosities, the most sensitive anatomical index of corticostriatal synapses at the light microscopic level.
Our findings indicate that BDA-labeled axonal varicosities provide a suitable basis for evaluating changes in corticostriatal synaptic density after experimental treatments. We also found that to achieve the greatest quantitative resolution it is important to focus the analysis at the coronal level corresponding to greatest contralateral labeling density. The extent of this region is dependent upon the size of the injection site. Within the range of injection sites used in the present study, which produced a greater than six-fold range in striatal density, the contra/ipsi baseline is stable when assessed in this central region. therefore, based upon the existence of a stable baseline over an extended range of densities, we suggest that in experimental scenarios analyzing this region, a conservative threshold for evidence of synaptogenesis after injections of BDA in AGm would be mean contra/ipsi varicosity density ratios > 0.78 (Max + 2 SD). Presumably, this conclusion may apply not only to DCS but also to other regions of the dorsal striatum, but naturally baseline data would be needed for each region.
In the event of plasticity following a lesion, what would be the likely source of a change in the contra/ipsi ratio? A unilateral cortical lesion produces ipsilesional striatal denervation involving ipsi Va/III, bilat Va/III and Vb/VI neurons, and contralesional striatal denervation involving bilat Va/III and contra Va/III neurons. Similarly, any sprouting from contralesional cortex into contralesional striatum would potentially include ipsi Va/III, bilat Va/III and Vb/VI neurons, whereas sprouting into ipsilesional striatum would involve bilat Va/III and contra Va/III neurons. A previous study of corticostriatal plasticity found evidence of changed axonal density in projections from contralesional sensorimotor cortex to ipsilesional dorsolateral striatum but non-significant changes in the projection from the same cortical area to contralesional dorsolateral striatum (Kartje et al., 1999). The present data suggest that the ~2× greater denervation on the ipsilesional side may have triggered sprouting whereas the lesser denervation contralesionally did not.
It appears that contra/ipsi ratios for axon density and axon varicosities differ according to the cortical projection in question. Studies of normal axonal density in striatum after injections of BDA in rat sensorimotor cortex have found contra/ipsi ratios of 0.20–0.62 (Kartje et al., 1999; Carmichael and Chesselet, 2002), whereas in the present study we found a mean of 0.50 for AGm. This emphasizes the importance of establishing a normal baseline in each particular case.
Axon density ratios exhibit greater variation than axonal varicosity density ratios (SD = 0.11 vs. 0.05). The contra/ipsi ratio of axon density is correlated with the contra/ipsi ratio of varicosity density, but assessment of changes in axon varicosity density represent a more direct measure of synaptic plasticity. These results suggest that assessments of changes in the ratio of axon density after experimental treatments (cf. Kartje et al., 1999; Carmichael and Chesselet, 2002; Riban and Chesselet, 2006) do reflect changes in synaptic density. However, axon varicosities are likely to be the most sensitive anatomical parameter by which to assess plasticity at the light microscopic level.
Experimental Procedures
Axonal tracer injection and brain processing
All animal procedures were carried out in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals, and with IACUC approval at the University of Florida. In nine normal Long Evans hooded rats, a single injection of the anterograde axonal tracer BDA (10K mol.wt. biotinylated dextran amine, Molecular Probes, Inc.) was made into the left cortical area AGm (a-p +0.8. m-l +1.0 relative to bregma), through glass pipettes having a tip diameter of 30–40 µm, using nitrogen gas pulses of 20–40 psi and 3–10 msec duration delivered to the pipette via a Picospritzer II (General Valve), resulting in the delivery of controlled small droplets of tracer (~100 nl). From 1–3 pulses were delivered at 30 sec intervals at 2 depths. We strove to achieve a range of injection site densities to permit assessment of the stability of normal contralateral/ipsilateral labeling ratios. Ten days postsurgery, rats were sacrificed and the brains fixed by transcardial perfusion with saline followed by 4% paraformaldehyde. Frozen coronal sections were cut at 40 µm thickness. Two series, each having the sections spaced 240 µm apart, were processed for BDA using avidin-biotin horseradish peroxidase histochemistry.
Quantification of labeling
We used unbiased sampling to measure two parameters in the dorsocentral striatum: axon varicosity density and axon density. This was done using an M5 imaging system (Imaging Research, Inc., St. Catharines, Ontario, Canada) interfaced to a Zeiss Axiophot 2 microscope equipped with a motorized scanning stage and video display.
Varicosity density
For each brain, three sections were analyzed. First, the section with highest labeling density in contralateral DCS was chosen for analysis. This was an unambiguous decision, easily agreed upon by two observers. Because the cortical injections were all made in the same location, each of these sections chosen for analysis was located at approximately a-p +0.4. A second and third series of sections located within ~240 µm on either side of the region of greatest density were analyzed as well.
Care was taken to match the location within DCS that was sampled ipsilateral and contralateral to the BDA injection site. The region of maximal contralateral labeling was outlined first, excluding visible white matter fascicles. This ranged from 30,909 – 38,642 µm2 (corresponding to a volume range of 1,236,360 – 1,545,680 µm3, given our section thickness of 40 µm). Next, sampling boxes were distributed within this area by the program, and varicosities were counted within each box at a primary microscope magnification of 80×, corresponding to a video screen magnification of 1200×. A comparable area was outlined ipsilaterally, taking care to match the location and area (within 10%) to that sampled contralaterally. Sampling frequency ranged from 8–43% depending on the specimen, and a sampling box size of ~11,340 µm3 (~18 × 18 × 35 µm) was used, with guard volumes of 1 µm at each end of the focusing range. Actual counts were converted to estimated total counts, and these were divided by the area of interest to obtain an estimate of varicosity density.
A specific set of criteria was used to exclude potential false positive counts, and two observers performed all counts together. Each candidate varicosity must first meet three criteria to be counted: 1) an axon must exist and touch on both sides of the candidate varicosity, 2) the candidate varicosity must distinctly appear approximately two times wider than the parent axon, and 3) the candidate varicosity should be relatively round in shape and either moderately darker in labeling intensity or the same as its axon of origin. Often, shadows of crossing axons produced a “bump” at the point of intersection, so care was taken to exclude possible intersection “bumps” as well. Generally, these false positives were recognized because no single axon could clearly be identified to be attached to the “bump”. Additionally, varicosities were distinguished from illusions of varicosities created by the cut ends of axons. Cut axons on the edge of tissues created bumps that could be distinguished by their white shadows, perfectly round shape, unusually darker intensity, and location on the edge of the microscope focal plane. The orientations and configurations of axons also would create illusions of potential varicosities, so there was also a thorough examination of the focal planes involving the possible varicosity. Finally, any potential counts that both observers disagreed upon would be included or excluded in an alternating manner while those that were ambiguous to both observers were automatically excluded. The contra/ipsi ratio was computed and used as a measure of relative synaptic density.
Axon density
Assessment of axon density was done on the same three sections per brain used for varicosity analysis, chosen as described above, and using a similar method to that described above. However, instead of counting varicosities inside of each sampling box, we counted the number of axons that crossed the top or right hand boundary of the box (the green “include” lines). The contra/ipsi ratio was used as a measure of relative axon density.
Acknowledgements
Supported by NIMH grant MH60399 and NINDS grant 40960. We appreciate the expert technical assistance of Maggie Stoll, Lori Lazar, Elyse Morin, Harumi Kamishina, and Steve Wagner.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burcham K, Corwin JV, Stoll M, Reep RL. Disconnection of medial agranular and posterior parietal cortex produces multimodal neglect in rats. Behav. Brain Res. 1997;86:41–47. doi: 10.1016/s0166-4328(96)02241-3. [DOI] [PubMed] [Google Scholar]
- Carman JB, Cowan WM, Powell TPS, Webster KE. A bilateral cortico-striate projection. J. Neurol. Neurosurg. Psychiat. 1965;28:71–77. doi: 10.1136/jnnp.28.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Chesselet M-F. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J. Neurosci. 2002;22:6062–6070. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatwood JL, Reep RL, Corwin JV. The associative striatum: cortical and thalamic projections to the dorsocentral striatum. Brain Res. 2003;968:1–14. doi: 10.1016/s0006-8993(02)04212-9. [DOI] [PubMed] [Google Scholar]
- Chen S, Hillman DE. Robust synaptic plasticity of striatal cells following partial deafferentation. Brain Res. 1990;520:103–114. doi: 10.1016/0006-8993(90)91695-d. [DOI] [PubMed] [Google Scholar]
- Cheng H-W, Rafols JA, Goshgarian HG, Anavi Y, Tong J, McNeill TH. Differential spine loss and regrowth of striatal neurons following multiple forms of deafferentation: a Golgi study. Exp. Neurol. 1997;147:287–298. doi: 10.1006/exnr.1997.6618. [DOI] [PubMed] [Google Scholar]
- Cheng H-W, Tong J, McNeill TH. Lesion-induced sprouting in the deafferented striatum of adult rat. Neurosci. Lett. 1998;242:69–72. doi: 10.1016/s0304-3940(98)00050-0. [DOI] [PubMed] [Google Scholar]
- Corwin JV, Reep RL. Rodent posterior parietal cortex as a component of a cortical network mediating directed spatial attention. Psychobiology. 1998;26:87–102. [Google Scholar]
- Corwin JV, Kanter S, Watson RT, Heilman KM, Valenstein E, Hashimoto A. Apomorphine has a therapeutic effect on neglect produced by unilateral dorsomedial prefrontal cortex lesions in rats. Exp. Neurol. 1986;94:683–698. doi: 10.1016/0014-4886(86)90247-5. [DOI] [PubMed] [Google Scholar]
- Corwin JV, Burcham KJ, Hix GI. Apomorphine produces an acute dose-dependent therapeutic effect on neglect produced by unilateral destruction of the posterior parietal cortex in rats. Behav. Brain Res. 1996;79:41–49. doi: 10.1016/0166-4328(95)00260-x. [DOI] [PubMed] [Google Scholar]
- Cospito JA, Kultas-Ilinsky K. Synaptic organization of motor corticostriatal projections in the rat. Exp. Neurol. 1981;72:257–266. doi: 10.1016/0014-4886(81)90221-1. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J. Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- Crowne DP, Pathria MN. Some attentional effects of unilateral frontal lesions in the rat. Behav. Brain Res. 1982;6:25–39. doi: 10.1016/0166-4328(82)90079-1. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Ziegler BTS. The crossed cortico-caudate projection in the rhesus monkey. Neurosci. Lett. 1979;15:29–32. doi: 10.1016/0304-3940(79)91524-6. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Shiota C, Levine MS, Hull CD, Buchwald NA. Interhemispheric organization of corticocaudate projections in the cat: a retrograde double labelling study. Neurosci. Lett. 1984;48:369–373. doi: 10.1016/0304-3940(84)90066-1. [DOI] [PubMed] [Google Scholar]
- Kartje GL, Schulz MK, Lopez-Yunez A, Schnell L, Schwab ME. Corticostriatal plasticity is restricted by myelin-associated neurite growth inhibitors in the adult rat. Ann. Neurol. 1999;45:778–786. doi: 10.1002/1531-8249(199906)45:6<778::aid-ana12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TPS. The cortico-striate projection in the monkey. Brain. 1970;93:525–546. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Wilson CJ. Corticostriatal innervation of the patch and matrix in the rat neostriatum. J. Comp. Neurol. 1996;374:578–592. doi: 10.1002/(SICI)1096-9861(19961028)374:4<578::AID-CNE7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Zheng T, Wilson CJ. Connectivity and convergence of single corticostriatal axons. J. Neurosci. 1998;18:4722–4731. doi: 10.1523/JNEUROSCI.18-12-04722.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King V, Corwin JV. Neglect following unilateral ablation of the caudal but not the rostral portion of medial agranular cortex of the rat and the therapeutic effect of apomorphine. Behav. Brain Res. 1990;37:169–184. doi: 10.1016/0166-4328(90)90092-s. [DOI] [PubMed] [Google Scholar]
- Levesque M, Parent A. Axonal arborization of corticostriatal and corticothalamic fibers arising from prelimbic cortex in the rat. Cerebral Cortex. 1998;8:602–613. doi: 10.1093/cercor/8.7.602. [DOI] [PubMed] [Google Scholar]
- Levesque M, Gagnon S, Parent A, Deschenes M. Axonal arborizations of corticostriatal and corticothalamic fibers arising from the second somatosensory area in the rat. Cerebral Cortex. 1996;6:759–770. doi: 10.1093/cercor/6.6.759. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RLM. The organization and collateralization of corticostriate neurones in the motor and sensory cortex of the rat brain. Brain Res. 1987;423:318–324. doi: 10.1016/0006-8993(87)90855-9. [DOI] [PubMed] [Google Scholar]
- McNeill TH, Mori N, Cheng H-W. Differential regulation of the growth-associated proteins, GAP-43 and SCG-10, in response to unilateral cortical ablation in adult rats. Neurosci. 1999;90:1349–1360. doi: 10.1016/s0306-4522(98)00482-5. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Cogen JP, Cheng HW. Alterations in rat striatal glutamate synapses following a lesion of the cortico-and/or nigrostriatal pathway. Exp. Neurol. 2000;165:191–206. doi: 10.1006/exnr.2000.7467. [DOI] [PubMed] [Google Scholar]
- Napieralski JA, Butler AK, Chesselet M-F. Anatomical and functional evidence for lesion-specific sprouting of corticostriatal input in the adult rat. J. Comp. Neurol. 1996;373:484–497. doi: 10.1002/(SICI)1096-9861(19960930)373:4<484::AID-CNE2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV. Topographic organization of the striatal and thalamic connections of rat medial agranular cortex. Brain Res. 1999;841:43–52. doi: 10.1016/s0006-8993(99)01779-5. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, Hashimoto A, Watson RT. Efferent connections of the rostral portion of medial agranular cortex in rats. Brain Res. Bull. 1987;19:203–221. doi: 10.1016/0361-9230(87)90086-4. [DOI] [PubMed] [Google Scholar]
- Reep RL, Cheatwood JL, Corwin JV. The associative striatum: organization of cortical projections to the dorsocentral striatum in rats. J. Comp. Neurol. 2003;467:271–292. doi: 10.1002/cne.10868. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, Cheatwood JL, Van Vleet TM, Heilman K, Watson RT. A rodent model for investigating the neurobiology of contralateral neglect. Cog. Behav. Neurology. 2004;17:191–194. [PubMed] [Google Scholar]
- Reiner A, Jiao Y, Del Mar N, Laverghetta AV, Lei WL. Differential morphology of pyramidal tract-type and intratelencephalically projecting-type corticostriatal neurons and their intrastriatal terminals in rats. J. Comp. Neurol. 2003;457:420–440. doi: 10.1002/cne.10541. [DOI] [PubMed] [Google Scholar]
- Riban V, Chesselet M-F. Region-specific sprouting of crossed corticofugal fibers after unilateral cortical lesions in adult mice. Exp. Neurol. 2006;197:451–457. doi: 10.1016/j.expneurol.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Royce GJ. Laminar origin of cortical neurons which project upon the caudate nucleus: a horseradish peroxidase investigation in the cat. J. Comp. Neurol. 1982;205:8–29. doi: 10.1002/cne.902050103. [DOI] [PubMed] [Google Scholar]
- Uryu K, Mackenzie L, Chesselet M-F. Ultrastructural evidence for differential axonal sprouting in the striatum after thermocoagulatory and aspiration lesions of the cerebral cortex in adult rats. Neuroscience. 2001;105:307–316. doi: 10.1016/s0306-4522(01)00203-2. [DOI] [PubMed] [Google Scholar]
- Van Vleet TM, Burcham KJ, Corwin JV, Reep RL. Unilateral destruction of the medial agranular cortical projection zone in the dorsocentral striatum produces severe neglect in rats. Psychobiology. 2000;28:57–66. [Google Scholar]
- Van Vleet TM, Guerrettaz K, Heldt SA, Corwin JV, Reep RL. Unilateral destruction of the dorsocentral striatum in rats produces neglect but not extinction to bilateral simultaneous stimulation. Behav. Brain Res. 2002;136:375–387. doi: 10.1016/s0166-4328(02)00296-6. [DOI] [PubMed] [Google Scholar]
- Van Vleet TM, Heldt SA, Corwin JV, Reep RL. Infusion of apomorphine into the dorsocentral striatum produces acute recovery from neglect induced by unilateral medial agranular cortex lesions in rats. Behav. Brain Res. 2003;143:147–157. doi: 10.1016/s0166-4328(03)00040-8. [DOI] [PubMed] [Google Scholar]
- Vargo JM, Marshall JF. Frontal cortex ablation reversibly decreases striatal zif/268 and junB expression: Temporal correspondence with sensory neglect and its spontaneous recovery. Synapse. 1996a;22:291–303. doi: 10.1002/(SICI)1098-2396(199604)22:4<291::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Vargo JM, Marshall JF. Unilateral frontal cortex ablation producing neglect causes time-dependent changes in striatal glutamate receptors. Behav. Brain Res. 1996b;77:189–199. doi: 10.1016/0166-4328(95)00229-4. [DOI] [PubMed] [Google Scholar]
- Webster KE. Cortico-striate interrelations in the albino rat. J. Anat. 1961;95:532–544. [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. Postsynaptic potentials evoked in spiny neostriatal projection neurons by stimulation of ipsilateral and contralateral neocortex. Brain Res. 1986;367:201–213. doi: 10.1016/0006-8993(86)91593-3. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Morphology and synaptic connections of crossed corticostriatal neurons in the rat. J. Comp. Neurol. 1987;263:567–580. doi: 10.1002/cne.902630408. [DOI] [PubMed] [Google Scholar]
- Zheng T, Wilson CJ. Corticostriatal combinatorics: the implications of corticostriatal axonal arborizations. J. Neurophysiol. 2002;87:1007–1017. doi: 10.1152/jn.00519.2001. [DOI] [PubMed] [Google Scholar]