Abstract
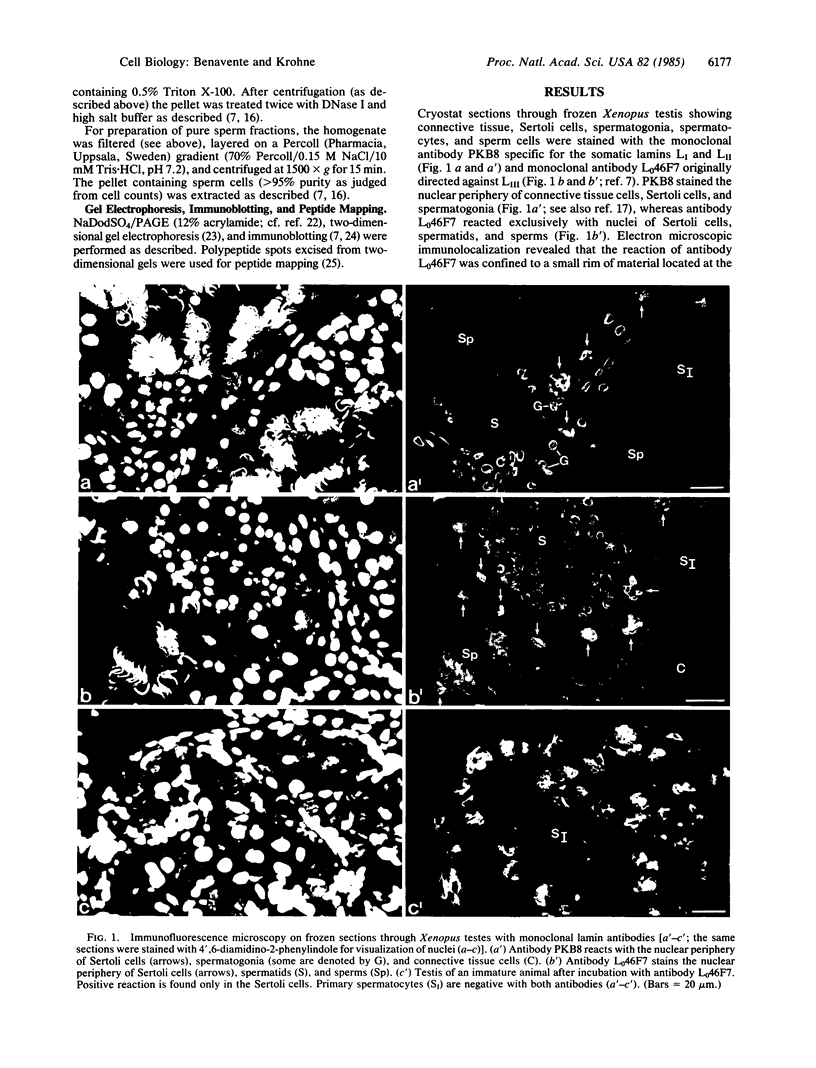
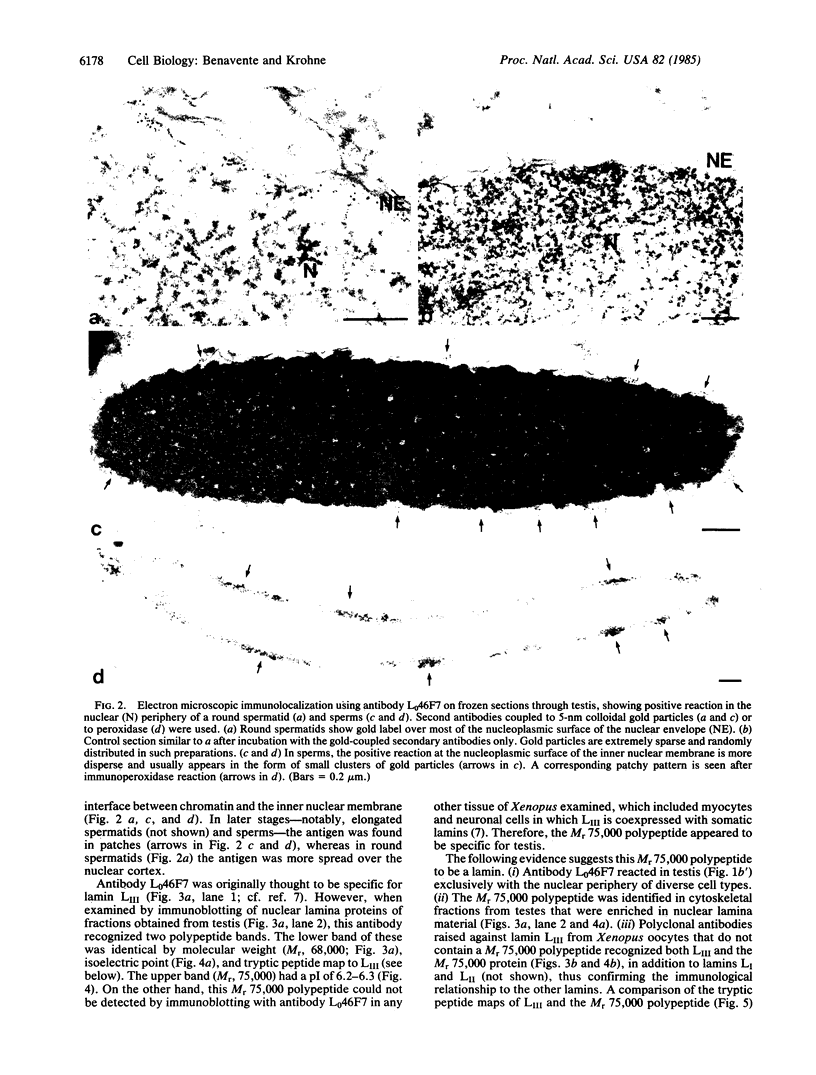
Lamins are the major constituent proteins of the nuclear lamina. In the frog, Xenopus laevis, they are the products of a multigene family whose expression can be correlated to certain routes of cell differentiation. For example, lamins LI (Mr, 72,000) and LII (Mr, 68,000) is expressed, together with LI/LII, in certain highly differentiated cell types such as neurons and muscle cells and is the only lamin present in diplotene oocytes. Here we report the identification by means of two monoclonal antibodies of a fourth lamin (LIV) of Mr 75,000, which is expressed specifically during the later stages of spermatogenesis. In the seminiferous tubules, Sertoli cells contain LI/LII and LIII whereas, among the spermatogenic cells, spermatogonia contain only LI and LII. In contrast, in spermatids and sperm cells these lamins are completely replaced by lamin LIV. Primary spermatocytes are negative with both antibodies, indicating that a switch in the expression of lamins occurs early in spermatogenesis. Lamin LIV is distributed in patches along the nuclear envelopes of elongated spermatids and sperm cells rather than in the characteristic continuous lamina pattern found in most other cells. We hypothesize that the specific expression of lamin LIV is related to the conspicuous changes of nuclear architecture and chronmatin composition that are known to take place during the late stages of sperm development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson R. P., Blobel G. Isolation of nuclear pore complexes in association with a lamina. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1007–1011. doi: 10.1073/pnas.72.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente R., Krohne G., Franke W. W. Cell type-specific expression of nuclear lamina proteins during development of Xenopus laevis. Cell. 1985 May;41(1):177–190. doi: 10.1016/0092-8674(85)90072-8. [DOI] [PubMed] [Google Scholar]
- Benavente R., Krohne G., Schmidt-Zachmann M. S., Hügle B., Franke W. W. Karyoskeletal proteins and the organization of the amphibian oocyte nucleus. J Cell Sci Suppl. 1984;1:161–186. doi: 10.1242/jcs.1984.supplement_1.11. [DOI] [PubMed] [Google Scholar]
- Benavente R., Krohne G., Stick R., Franke W. W. Electron microscopic immunolocalization of a karyoskeletal protein of molecular weight 145 000 in nucleoli and perinucleolar bodies of Xenopus laevis. Exp Cell Res. 1984 Mar;151(1):224–235. doi: 10.1016/0014-4827(84)90370-7. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Fisher P. A., Berrios M., Blobel G. Isolation and characterization of a proteinaceous subnuclear fraction composed of nuclear matrix, peripheral lamina, and nuclear pore complexes from embryos of Drosophila melanogaster. J Cell Biol. 1982 Mar;92(3):674–686. doi: 10.1083/jcb.92.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Scheer U., Krohne G., Jarasch E. D. The nuclear envelope and the architecture of the nuclear periphery. J Cell Biol. 1981 Dec;91(3 Pt 2):39s–50s. doi: 10.1083/jcb.91.3.39s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Blobel G. Nuclear lamina and the structural organization of the nuclear envelope. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):967–978. doi: 10.1101/sqb.1982.046.01.090. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blum A., Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol. 1978 Nov;79(2 Pt 1):546–566. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Comeau C., Benson M. Organization and modulation of nuclear lamina structure. J Cell Sci Suppl. 1984;1:137–160. doi: 10.1242/jcs.1984.supplement_1.10. [DOI] [PubMed] [Google Scholar]
- Ierardi L. A., Moss S. B., Bellvé A. R. Synaptonemal complexes are integral components of the isolated mouse spermatocyte nuclear matrix. J Cell Biol. 1983 Jun;96(6):1717–1726. doi: 10.1083/jcb.96.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G., Dabauvalle M. C., Franke W. W. Cell type-specific differences in protein composition of nuclear pore complex-lamina structures in oocytes and erythrocytes of Xenopus laevis. J Mol Biol. 1981 Sep 5;151(1):121–141. doi: 10.1016/0022-2836(81)90224-2. [DOI] [PubMed] [Google Scholar]
- Krohne G., Debus E., Osborn M., Weber K., Franke W. W. A monoclonal antibody against nuclear lamina proteins reveals cell type-specificity in Xenopus laevis. Exp Cell Res. 1984 Jan;150(1):47–59. doi: 10.1016/0014-4827(84)90700-6. [DOI] [PubMed] [Google Scholar]
- Krohne G., Franke W. W., Ely S., D'Arcy A., Jost E. Localization of a nuclear envelope-associated protein by indirect immunofluorescence microscopy using antibodies against a major polypeptide from rat liver fractions enriched in nuclear envelope-associated material. Cytobiologie. 1978 Oct;18(1):22–38. [PubMed] [Google Scholar]
- Krohne G., Franke W. W. Proteins of pore complex--lamina structures from nuclei and nuclear membranes. Methods Enzymol. 1983;96:597–608. doi: 10.1016/s0076-6879(83)96052-4. [DOI] [PubMed] [Google Scholar]
- Krohne G., Franke W. W., Scheer U. The major polypeptides of the nuclear pore complex. Exp Cell Res. 1978 Oct 1;116(1):85–102. doi: 10.1016/0014-4827(78)90067-8. [DOI] [PubMed] [Google Scholar]
- Krohne G., Stick R., Kleinschmidt J. A., Moll R., Franke W. W., Hausen P. Immunological localization of a major karyoskeletal protein in nucleoli of oocytes and somatic cells of Xenopus laevis. J Cell Biol. 1982 Sep;94(3):749–754. doi: 10.1083/jcb.94.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G., Avdalović N. Nuclear envelope proteins from Spisula solidissima germinal vesicles. Exp Cell Res. 1980 Nov;130(1):229–240. doi: 10.1016/0014-4827(80)90059-2. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Scheer U., Kartenbeck J., Trendelenburg M. F., Stadler J., Franke W. W. Experimental disintegration of the nuclear envelope. Evidence for pore-connecting fibrils. J Cell Biol. 1976 Apr;69(1):1–18. doi: 10.1083/jcb.69.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stick R., Hausen P. Changes in the nuclear lamina composition during early development of Xenopus laevis. Cell. 1985 May;41(1):191–200. doi: 10.1016/0092-8674(85)90073-x. [DOI] [PubMed] [Google Scholar]
- Stick R., Krohne G. Immunological localization of the major architectural protein associated with the nuclear envelope of the Xenopus laevis oocyte. Exp Cell Res. 1982 Apr;138(2):319–313. doi: 10.1016/0014-4827(82)90181-1. [DOI] [PubMed] [Google Scholar]
- Stick R., Schwarz H. Disappearance and reformation of the nuclear lamina structure during specific stages of meiosis in oocytes. Cell. 1983 Jul;33(3):949–958. doi: 10.1016/0092-8674(83)90038-7. [DOI] [PubMed] [Google Scholar]
- Stick R., Schwarz H. The disappearance of the nuclear lamina during spermatogenesis: an electron microscopic and immunofluorescence study. Cell Differ. 1982 Jun;11(4):235–243. doi: 10.1016/0045-6039(82)90071-9. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]