Abstract
Background & Aims
Hepatic gluconeogenesis helps maintain systemic energy homeostasis by compensating for discontinuities in nutrient supply. Liver specific deletion of cytosolic phosphoenolpyruvate carboxykinase (PEPCK-C) abolishes gluconeogenesis from mitochondrial substrates, deregulates lipid metabolism and affects TCA cycle. While, mouse liver almost exclusively expresses PEPCK-C, humans equally present a mitochondrial isozyme (PEPCK-M). Despite clear relevance to human physiology, the role of PEPCK-M and its gluconeogenic potential remain unknown. Here, we test the significance of PEPCK-M in gluconeogenesis and TCA cycle function in liver-specific PEPCK-C knockout and WT mice.
Methods
The effects of the overexpression of PEPCK-M were examined by a combination of tracer studies and molecular biology techniques. Partial PEPCK-C re-expression was used as a positive control. Metabolic fluxes were evaluated in isolated livers by NMR using 2H and 13C tracers. Gluconeogenic potential, together with metabolic profiling, were investigated in vivo and in primary hepatocytes.
Results
PEPCK-M expression partially rescued defects in lipid metabolism, gluconeogenesis and TCA cycle function impaired by PEPCK-C deletion, while ~10% re-expression of PEPCK-C normalized most parameters. When PEPCK-M was expressed in the presence of PEPCK-C, the mitochondrial isozyme amplified total gluconeogenic capacity, suggesting autonomous regulation of oxaloacetate to phosphoenolpyruvate fluxes by the individual isoforms.
Conclusions
We conclude that PEPCK-M has gluconeogenic potential per se, and cooperates with PEPCK-C to adjust gluconeogenic/TCA flux to changes in substrate or energy availability, hinting at a role in the regulation of glucose and lipid metabolism in human liver.
INTRODUCTION
Phosphoenolpyruvate carboxykinase (PEPCK) (GTP; EC 4.1.1.32) catalyzes the conversion of oxaloacetate (OAA) to phosphoenolpyruvate (PEP). Its activity is distributed both in the cytosol and mitochondria as a result of two enzymatically indistinct isozymes, PEPCK-C and PEPCK-M [1, 2], encoded by different nuclear genes (Pck1 and Pck2, respectively) [3]. PEPCK-C has been widely studied and is considered a key pathway for hepatic gluconeogenesis and overlaps with many other biosynthetic and oxidative pathways [4, 5]. Its gene transcription is up-regulated in response to hormones during fasting and is robustly down-regulated by insulin and glucose [4]. Although global ablation of the PEPCK-C gene causes hypoglycemia and perinatal lethality [6, 7], metabolic control of this enzyme over gluconeogenesis is surprisingly low [6, 8-10]. However, acute reduction of PEPCK-C in the liver of db/db mice was sufficient to improve glycemia [11], indicating this pathway as a potential therapeutic target. The uncertain role of PEPCK-C in regulating gluconeogenesis and lipid metabolism, and the recent finding that it may not be increased in humans with type 2 diabetes [12], led us to contemplate PEPCK-M as a possible contributor to the normal and pathologic liver.
The metabolic characteristics of the mitochondrial isozyme remain largely unknown because PEPCK-M accounts for 1 and 5% of the total PEPCK-activity in mouse and rat liver, respectively [2, 13], the most commonly used models to study hepatic gluconeogenesis. However, the mitochondrial isoform makes up about half of the total hepatic PEPCK activity in other mammals, including humans [14-16]. In marked contrast to rat mitochondria that produced little or no PEP, mitochondria from these other species exhibit high rates of PEP production and export from TCA cycle intermediates [17-21]. However, assessing the specific role of PEPCK-M in hepatocytes containing both isozymes is not possible since they catalyze identical chemical reactions and produce identical labeling schemes in tracer experiments. Therefore, we overexpressed PEPCK-M in the liver of hepatic-specific PEPCK-C knock-out mice (pcklox/lox+AlbCre) to produce mice that only express PEPCK-M. These mice were examined by a combination of tracer studies and molecular biology techniques to elucidate the physiological characteristics of PEPCK-M in (i) gluconeogenesis, (ii) cataplerosis and TCA cycle activity, and (iii) to determine the interplay between PEPCK-C and PEPCK-M in these metabolic processes. Our data demonstrate that PEPCK-M has a distinct but complementary metabolic role to PEPCK-C in the regulation of gluconeogenesis and TCA cycle dynamics in liver.
MATERIALS AND METHODS
Please refer to the Supplementary Materials and methods section for more detailed descriptions.
Experimental animals and adenovirus
Liver-specific PEPCK-deficient (pcklox/lox+AlbCre) and control (pcklox/lox) mice were generated as previously described [6]. At the beginning of the experiment, animals were 12–16 weeks old. All animal protocols were approved by the Ethics Committee at the University of Barcelona or the UTSWMC Institutional Animal Care and Use Committee. Recombinant E1-E3 deficient adenovirus (serotype 5) expressing full-length cDNA of mouse pck1 (AdPck1) and pck2 (AdPck2) genes were generated in our laboratory. Liver specific tropism of the adenovirus was demonstrated after iv injection of an adenovirus encoding Aequorea victoria green fluorescent protein (AdGFP)(UPV-CBATEG) (Supplementary Fig. 1A).
Liver Perfusion Experiments and NMR Analysis
Briefly, livers were isolated after a 18 hr fast and perfused without recirculation for 60 min as previously detailed [9, 10, 22]. Effluent perfusate was collected for assays of glucose production as well as isolation of glucose for NMR analysis as previously described [9, 10, 22].
Blood and liver metabolites
Hepatic glycogen and TAG content were determined as previously described [11, 23, 24]. Phosphoenolpyruvate and malate were determined by standard procedures [25]. Plasma amino acids were quantified by ESI-MS/MS analysis as previously reported [26]. Serum metabolites were measured by the Veterinarian Clinical Biochemistry Service, U.A.B. (Barcelona, Spain).
Gene expression analysis, inmunobloting, enzymatic assays, histology and immunofluorescence
Quantitative RT-PCR, western blot and PEPCK activity assays were performed in liver samples essentially as described previously [11, 23, 24]. Antibodies against PEPCK-M, Ab1 and Ab2, were purchased from Abcam (ab70359) and Everest (EB06944), respectively (peptide sequence used to generate Ab2 is 100% homologous in mouse, rat, rabbit, and human). Gene expression was quantified using an Applied Biosystems TaqMan® Low Density Array in a HT7900 Real-Time RT-PCR system. PEPCK-M was immunostained as previously described [23], using Ab1 antibody.
Substrate challenges and endurance exercise study
Tests were performed in 16-h-fasted mice. Pyruvate and glycerol challenges, were carried out by intraperitoneal injection of 2g/kg of substrate (Sigma-Aldrich). Endurance test was composed of three 20-min running periods. The speed in these periods was 12, 15 and 18 m/minute. Glucose levels were measured at the indicated time points using a Glucocard Memory-2 (Menarini).
Hepatocyte studies
Hepatocytes were isolated as previously described [27] except that Liberase-TM (Roche) was used. 4 hours fasted pcklox/lox+AlbCre or overnight fasted wild type mice were used.
Statistical analysis
Results are expressed as the means ± SEM. Statistical analysis was always performed by one-way ANOVA (Newman-Keuls post-hoc test), two-way ANOVA (Bonferroni post-hoc test) and two-tailed Student's t-test, using GraphPad Prism® software. P < 0.05 was considered significant.
RESULTS
Liver-specific PEPCK-M and PEPCK-C overexpression in pcklox/lox+AlbCre
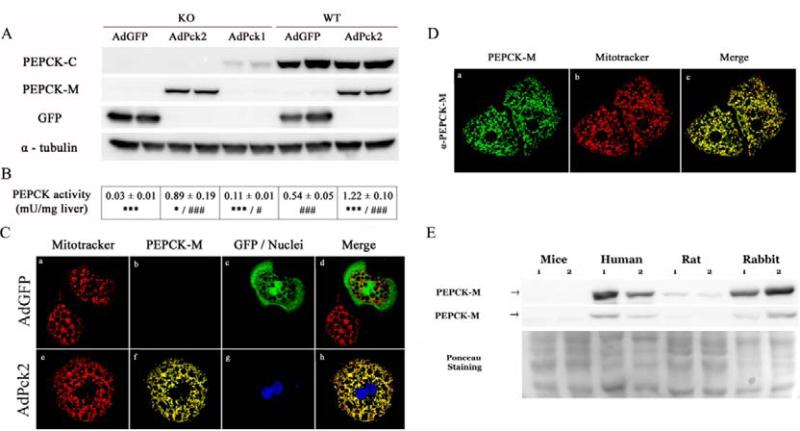
Treating pcklox/lox+AlbCre mice (PEPCK-C KO) and their wild type littermates (pcklox/lox; WT) with adenovirus expressing PEPCK-M (AdPck2) achieved a ~100-fold increase hepatic pck2 mRNA which resulted in a similar increase in PEPCK-M protein content and PEPCK activity (Fig. 1 A,B and Supplementary Fig, 1C and Supplementary Tables 3-4). PEPCK-C expressing adenovirus (AdPck1) yielded levels of PEPCK-C protein and activity that reinstated ~10% of those found in fasted wild-type mice (Fig. 1 A,B). As expected by the presence of a canonical mitochondrial targeting signal in the PEPCK-M cDNA, mitochondrial localization was confirmed using a spectral confocal microscope and co-localization with a mitochondrial marker (Fig. 1C; high resolution images in Supplementary Fig. 2), and using subcellular fractionation combined with western blotting (Supplementary Fig. 3). Immunohistochemistry demonstrated endogenous PEPCK-M protein in human hepatocytes (Fig. 1D) contrary to the barely detectable signal in mouse liver (Fig. 1C). Consistently, western blot analysis of total liver extracts from mouse, human, rat and rabbits using an antibody cross-reacting with PEPCK-M from different species, demonstrate that mouse liver is practically deficient in PEPCK-M as compared to rat and, especially human and rabbit (Fig. 1E).
Figure 1. Adenovirus mediated overexpression in the liver.
An adenoviral dose of 3×1010 p.f.u./kg was administered by intravenous injection in liver specific PEPCK-C knock-out mice and their wild type littermates. Protein expression (A) and PEPCK enzymatic activity (B) were analyzed in total liver extracts of overnight fasted animals, 7 days after treatment. Data are presented as mean ± SEM, n=4-6. *P<0.05; ***P<0.001 relative to WT AdGFP. #P<0.05; ###P<0.001 relative to KO AdGFP; One-way ANOVA, with a Newman-Keuls post-hoc test. (C) Indirect immunedetection using confocal microscopy of PEPCK-M and GFP fluorescence performed on cultured hepatocytes isolated from pcklox/lox+AlbCre mice treated with AdGFP (a-d) or AdPck2 (e-h). PEPCK-M (red) is visualized with α-PEPCK-M antibody, GFP fluorescence is shown in green and nuclei are stained in blue using YOYO®-1. (D) Immunofluorescent staining of human PEPCK-M endogenously expressed in primary culture of human hepatocytes. PEPCK-M staining is shown in green. Mitochondria are visualized using MitoTracker Red CMXRos (red). In C, note the presence of infected and uninfected hepatocytes. (E) Hepatic PEPCK-M detection in different species. Protein expression in total liver extracts of human, rat, mouse and rabbit was analyzed by western blot (40 μg of protein). Two different primary antibodies were used to detect PEPCK-M; Ab1 and Ab2 (see methods). Membranes were normalized using Ponceau staining.
Mitochondrial gluconeogenesis and TCA cycle activity in isolated livers
Liver-specific deletion of PEPCK-C (pcklox/lox+AlbCre) impairs hepatic gluconeogenesis from lactate and pyruvate and limits tricarboxylic acid (TCA) cycle function, resulting in an accumulation of lipids and cytosolic malate [6, 9]. Thus, we first evaluated the ability of PEPCK-M or PEPCK-C expression to restore these pathways in isolated perfused livers using 2H2O to evaluate the contribution of gluconeogenesis to glucose production and [U-13C3]-propionate to evaluate the contribution of the TCA to gluconeogenesis. Since PEPCK activity is necessary for the enrichment of glucose in the H6R and H6S position by 2H2O [28], or labeling with 13C in any position [29, 30] (Fig. 2 A,B), these tracer approaches reveal the impact of the treatments on metabolic fluxes such as gluconeogenesis, pyruvate cycling, PEPCK, and TCA cycle oxidation [9, 10]. PEPCK-M overexpression in KO mice increased the 2H NMR signal at the H6R and H6s resonances and in the 13C NMR C2 multiplets of glucose (Fig. 2C). These signals were undetectable in KO livers treated with AdGFP. These data reflect de novo glucose production from TCA cycle substrates and a concomitant increment in the relative fluxes through PEPCK, pyruvate cycling, and gluconeogenesis (relative to TCA cycle flux). More precisely, PEPCK-M overexpression caused a ~38% increase in total glucose production (0.40 ± 0.03 vs 0.29 ± 0.03 μmol/min/g; P ≤ 8*10−3) and a 4-fold increase in PEP contribution to gluconeogenesis (11.7 ± 0.88 % vs 2.87 ± 0.57 %; P ≤ 2*10−5) compared to KO mice treated with AdGFP) (Fig. 2D).
Figure 2. PEPCK-M and PEPCK-C overexpression increase 2H and 13C tracer incorporation into glucose in pcklox/lox+AlbCre mice.
Livers of overnight fasted mice were perfused without recirculation for 60 min. Perfusate include 0.1 mM [U-13C3]propionate, and 3% v/v D2O as labeled substrates. Effluent perfusate was collected for assays of glucose production as well as isolation of glucose for NMR analysis to determine the positional enrichment of 2H and 13C. (A) Glucose enrichment in H6S and 13C occurs in the TCA cycle. Chemical exchange at the H6S happens at the level of fumarase, and [U-13C3]propionate enters via succinyl-CoA (Mit: mitochondrial matrix; Cyt: cytosol). (B) Structure of monoacetone glucose (MAG) derived from glucose. Carbon 2 is used to determine fluxes. Subindex in aliphatic hydrogens indicate the positions in the 2H spectra of MAG. (C) Representative 2H and 13C NMR spectra of MAG from experimental groups. Treatment with AdPck1 or AdPck2 increase 2H and 13C enrichment of H6s position (left box) and C2 13C multiplets (right box), almost absent in PEPCK-C null livers (KO AdGFP). Q (quartet), D12 (doublet 12), D23 (doublet 23), and S (singlet). (D) The area of the 13C multiplets of the 13C NMR spectra of MAG were used to determine relative fluxes associated with the TCA cycle. Glucose production was assayed in the effluent perfusate. Livers were weighted and freeze clamped and malate content determined. (E) By combining the relative fluxes determined from 2H and 13C data with the rate of glucose production, absolute flux rates of the metabolic pathways connecting GNG and the TCA cycle were calculate. Data are presented as mean ± SEM, n:4–5. *P<0.05; **P<0.01; ***P<0.001 vs. WT AdGFP. #P<0.05; ##P<0.01; ###P<0.001 vs KO AdGFP; Student's t-test.
To examine the efficiency of PEPCK-M, as compared to PEPCK-C, we also performed experiments in WT mice and KO mice re-expressing PEPCK-C (KO AdPck1). Glucose production by WT livers was 3-fold higher than KO + AdGFP livers (0.29 vs 0.83 μmol/min/g) and the PEPCK pathway contributed to nearly 50% of this flux (compared to less than 3% in KO +AdGFP) (Fig. 2D). Although KO + AdPck1 restored PEPCK-C protein content to only 10% of WT liver, this maneuver resulted in a normalization of all parameters examined, bringing glucose production, relative fluxes and malate levels close to those observed in wild type mice (Fig. 2D). Finally, by combining the relative fluxes determined from 2H and 13C data with the rate of glucose production, absolute flux rates of the metabolic pathways connecting gluconeogenesis and the TCA cycle were calculated (Fig. 2E). Gluconeogenesis, PEPCK, and TCA cycle flux increased in the order of WT > KO+Pck1 > KO+Pck2 > KO. These findings demonstrate that PEPCK-M can support cataplerosis and gluconeogenesis in the mouse liver, but that it is less efficient than PEPCK-C.
Glucose and lipid metabolism in treated pcklox/lox+AlbCre mice
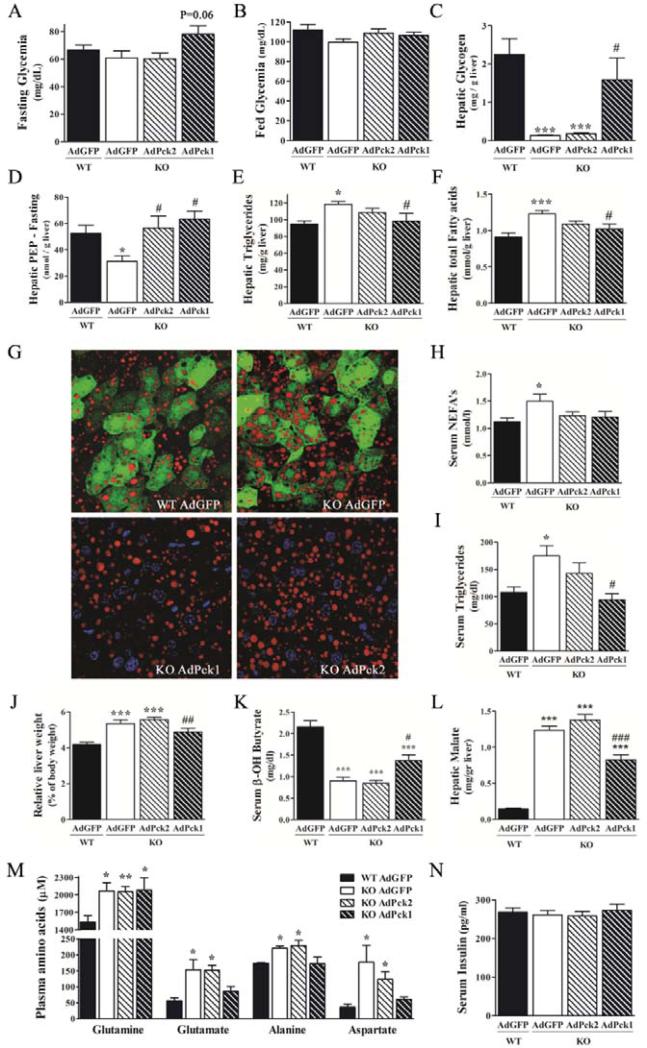
We next determined if re-expression of PEPCK-C or overexpression of PEPCK-M restores metabolic parameters altered in the KO phenotype. Liver specific PEPCK-C KO mice are euglycemic during fasting [6] despite the inability to support hepatic gluconeogenesis from lactate and pyruvate [9]. These mice adapt by inducing extrahepatic gluconeogenesis and have normal basal fasting endogenous glucose production but reduced peripheral glucose utilization in response to insulin. None of the treatments changed plasma glucose or insulin levels in overnight-fasted (Fig. 3A,N) or fed KO mice (Fig, 3B; Supplementary Table 1). However, AdPck1 treatment restored liver glycogen (Fig. 3C), whereas both AdPck1 and AdPck2 treatments normalized hepatic PEP content (Fig. 3D). In agreement with lower hepatic gluconeogenic potential, knock-out mice had elevated plasma levels of gluconeogenic amino acids, which were effectively normalized by AdPck1 but not AdPck2 treatment (Fig. 3M). In addition, mRNA content of key gluconeogenic proteins, g6pc (Glucose-6-phosphatase-catalytic subunit) and slc2a2 (GLUT2) were reduced in KO livers and neither AdPck1 nor AdPck2 administration fully corrected mRNA content (Table 1).
Figure 3. PEPCK isozymes overexpression: differential effects on glucose and lipid metabolism fasted pcklox/lox+AlbCre.
(See also supplemental information for complete data on metabolic parameters measured in fed and fasted mice including WT AdPck2). (A) Overnight fasting and (B) fed glycemia (n=7-12) (C) Fasting hepatic glycogen content (n=8) (D) Fasting hepatic phosphoenlopyruvate content (n=8) (E)(F) Hepatic triglycerides and total fatty acid content (G) Representative examples of immunofluorescence analysis of hepatic lipid content in fasted treated mice detected by nile red staining (red). GFP (green) and nuclei (blue) are also shown. Original magnification x40; n=3 (H) Serum levels of non esterified fatty acids and (I) triglycerides. (J) Relative liver weight expressed as % of body weight. (K) Serum β-hydroxybutyrate levels. (L) Hepatic malate content. (M) Serum gluconeogenic amino acids (n=5-6). (N) Serum insulin. Data are presented as mean ± SEM. n=8, otherwise indicated. *P<0.05; **P<0.01; ***P<0.001 relative to WT AdGFP. #P<0.05; ##P<0.01; ###P<0.001 relative to KO AdGFP; One-way ANOVA, with a Newman-Keuls post-hoc test.
Table 1.
Effect of treatment with PEPCK isozymes on hepatic mRNA content in overnight fasted mice (n = 8).
| WT AdGFP | KO AdGFP | KO AdPck2 | KO AdPck1 | |
|---|---|---|---|---|
| Acaca | 1.10 ± 0.19 | 0.58 ± 0.06* | 0.61 ±0.04** | 0.52 ± 0.05** |
| Acly | 1.02 ± 0.13 | 0.62 ± 0.07 | 0.59 ± 0.04 | 0.66 ± 0.18 |
| Cpt1a | 1.02 ± 0.07 | 0.92 ± 0.09 | 0.98 ± 0.09 | 0.99 ± 0.10 |
| Cpt2 | 1.01 ± 0.06 | 1.29 ± 0.05* | 0.95 ± 0.09# | 0.89 ± 0.09# |
| Fasn | 1.09 ± 0.19 | 0.46 ± 0.04** | 0.56 ±0.07* | 0.61 ± 0.10* |
| Foxo1 | 1.05 ± 0.17 | 0.68 ± 0.10 | 0.76 ± 0.07 | 1.02 ± 0.13 |
| G6pc | 1.04 ± 0.11 | 0.65 ± 0.07* | 0.68 ± 0.08* | 0.71 ± 0.07* |
| Gck | 1.08 ± 0.17 | 0.42 ± 0.07** | 0.44 ± 0.10** | 0.79 ± 0.11# |
| Gpd1 | 1.03 ± 0.10 | 0.64 ± 0.12* | 0.64 ± 0.08* | 0.75 ± 0.10 |
| II6 | 1.04 ± 0.12 | 0.55 ± 0.13** | 0.50 ± 0.04** | 0.43 ± 0.10** |
| Ppara | 1.00 ± 0.06 | 0.62 ± 0.12** | 0.69 ± 0.08** | 0.93 ± 0.12 |
| Ppargc1a | 1.03 ± 0.09 | 2.18 ± 0.25** | 2.05 ±0.27** | 1.12 ± 0.15## |
| Pparg | 1.04 ± 0.12 | 0.74 ± 0.11 | 1.00 ± 0.14 | 0.84 ± 0.09 |
| Pygl | 1.01 ± 0.04 | 0.39 ± 0.04*** | 0.44 ± 0.04*** | 0.61 ± 0.05***/## |
| Scd1 | 1.02 ± 0.07 | 0.71 ± 0.08* | 0.48 ± 0.12** | 0.54 ± 0.09** |
| Slc2a2 | 1.03 ± 0.11 | 0.33 ± 0.04*** | 0.43 ± 0.05*** | 0.59 ± 0.08***/# |
| Sod1 | 1.01 ± 0.04 | 0.72 ± 0.08* | 0.68 ± 0.05* | 0.91 ± 0.08# |
| Sod2 | 1.04 ± 0.12 | 0.88 ± 0.07 | 0.90 ± 0.06 | 0.94 ± 0.11 |
| Ucp2 | 1.11 ± 0.18 | 3.05 ± 0.57** | 2.36 ± 0.27* | 1.82 ± 0.36 |
Data are means ± SEM.
p <0.05
p <0.01
p <0.001 relative to WT AdGFP.
p <0.05
p <0.01 relative to KO AdGFP group.
One-way ANOVA, with a Newman-Keuls past hoc test.
Deletion of hepatic PEPCK-C results in reversible fatty infiltration of the liver during fasting [6, 9] with no evidence of increased inflammatory markers (Table 1). Therefore, we next determined the impact of PEPCK-C/M re-expression on defects in lipid metabolism. Consistent with metabolic flux data, partial restoration of PEPCK-C activity (KO AdPck1) proved more efficient at reducing circulating lipids and hepatic triglycerides and neutral lipid than PEPCK-M overexpression (Fig. 3E-I). In fact, only AdPck1 administration (10% normal PEPCK-C) normalized relative liver weight (Fig. 3J) and lipid accumulation as assessed using Nile Red staining in histological sections (Fig. 3G). Paradoxically, lipid accumulation in KO mice liver did not enhance ppara (PPAR-α) transcription (Table 1) and ketone bodies were reduced (Fig. 3K). The mRNA content of enzymes related to β-oxidation such as ppargc1a (PGC1α), ucp2 and cpt2, but not cpt1a (Table 1) were increased. AdPck1, in contrast to AdPck2, normalized mRNA and β-hydroxybutyrate levels. Similarly, only AdPck1 restored hepatic malate content in fasted mice (Fig. 3L), whereas AdPck2 treatment significantly reduced malate content in fed liver (Supplementary Table 1). Interestingly, mRNA content of genes responsible for lipogenesis, such as acaca, gck and fasn were diminished both in overnight fasted (Table 1) and fed KO mice (Supplementary Table 3). Partial restoration of PEPCK-C activity or overexpression of PEPCK-M were insufficient to normalize lipogenic gene expression. These data indicate that lipid and TCA cycle metabolism, but not lipogenesis, is restored in vivo by incomplete re-expression of PEPCK-C, whereas PEPCK-M over-expression conferred a partial restoration.
Impact of PEPCK isozymes on glucose production in vivo
We next sought to evaluate the physiological contribution of PEPCK-M in protecting against severe exercise induced hypoglycemia described in pcklox/lox+AlbCre mice [6] (Fig. 4A). As expected, KO AdGFP mice could not maintain normal glycemia in any stage of the endurance test (60 minutes at increasing speed - 900m) and rapidly reached exhaustion and hypoglycemia. In contrast, AdPck2 treated mice were able to sustain resting glycemia after the initial period of the endurance test (20 min; 12 m/m), and tolerated longer running time (Supplementary Fig. 4A). However, upon increasing exercise intensity AdPkc2 mice eventually failed to maintain euglycemia. All AdPck1 treated mice finished the test with exercise tolerance indistinguishable from wild type mice, showing reduced post-test hepatic malate content (Supplementary Fig. 4B).
Figure 4. Overexpression of PEPCK isozymes: impact on glucose metabolism in vivo and glucose production in primary mouse hepatocytes.
(A) Endurance test. Overnight fasted mice (n=5-6) performed exercise on a motordriven treadmill (see also Supplementary methods). Exercise was divided in three 20 min running periods with increasing intensities (12, 15 and 18 m/min). (A) Blood glucose levels were determined at indicated times. Arrows represent statistical trend for glycemia with respect to resting glycemia (End=60 min or exhaustion). (B) IPPTT: overnight fasted male pcklox/lox+AlbCre mice and their wild type littermates (n=4) were given an i.p. injection of pyruvate (2 g /kg). Blood glucose levels were measured at the indicated times. (Δ) WTAdGFP (▲) KOAdGFP (●) KOAdPck2 (■) KOAdPck1. (C) IPPTT: effect of AdPck2 (○) treatment vs AdGFP (∆) in glucose production from pyruvate in wild type mice (n=6). Primary hepatocytes were isolated from KO mice (D, E and H) or wild type mice (F and G) and transduced with AdPck1, AdPck2, AdGFP or combinations, as indicated. Cultured Cells were incubated for 6 hours in glucose-free medium containing indicated substrates and drugs or vehicle. (D) Glucose production from lactate, pyruvate, lactate/pyruvate or glycerol in KO hepatocytes. (E) Effect of aminooxyacetate (AOA) in glucose production from lactate in KO hepatocytes. (F) Glucose production from lactate, pyruvate or lactate/pyruvate in WT hepatocytes. (G) Effect of aminooxyacetate (AOA) in glucose production from lactate in WT hepatocytes. (H) Combination of PEPCK-C and PEPCK-M on glucose production from lactate/pyruvate in KO hepatocytes (Total MOI per well: 32). Hepatocyte data are representative of at least 3 independent experiments with at least 3 independent wells in each. Data are presented as mean ± SEM. *P<0.05; **P<0.01; ***P<0.001 relative to WT AdGFP (or AdGFP in hepatocytes). #P<0.05; ##P<0.01; ### P<0.001 relative to KO AdGFP group (or basal media / vehicle, within the same group, in hepatocytes), otherwise indicated. One-way ANOVA, with a Newman-Keuls post-hoc test. For glycemic response to exercise a t-student test was used.
To assess the impact of PEPCK-M on gluconeogenesis in vivo, we performed a pyruvate challenge. As shown in Fig. 4B, KO mice demonstrated a blunted glycemic response to an exogenous bolus of pyruvate, but not to the PEPCK independent substrate, glycerol (Supplementary Fig. 4C). Analysis of 13C incorporation into plasma glucose by GC-MS (Supplementary Fig. 4D) during a 13C-labeled pyruvate challenge confirmed that exogenous 13C-pyruvate carbons were the major contributors to plasma glucose in wild type mice and demonstrated that pcklox/lox+AlbCre retained the ability to produce glucose from pyruvate in extrahepatic tissues as previously reported [8, 9], albeit with lower efficiency, demonstrating a key role of hepatic gluconeogenesis in this test. As expected, AdPck1 treatment ameliorated the glycemic response to pyruvate, but AdPck2 treatment conferred no significant improvement (Fig. 4B). However, WT liver treated with AdPck2 produced a significant increase in the response to a pyruvate challenge as indicated by a higher AUC (Fig. 4C). These data indicate that the contribution of PEPCK-M to gluconeogenesis might be more effective in the presence of PEPCK-C activity or vice versa.
Gluconeogenesis in primary hepatocytes from treated KO and WT mice
The gluconeogenic potential of PEPCK-M was further elucidated in primary hepatocytes isolated from liver specific PEPCK-C KO mice and infected in vitro with AdPck1, AdPck2 or AdGFP (Fig. 4D, E and F). Cumulative glucose production (6 hours) from control treated hepatocytes (KO AdGFP) was barely detectable. In agreement with tracer experiments described above, PEPCK-M expression increased glucose production from pyruvate, lactate or pyruvate/lactate (1:10) and this increase was even greater when 10% of normal PEPCK-C was re-expressed (Fig. 4D). Glucose production from glycerol was close to WT levels in all treatment groups.
Strikingly, AdPck2 also increased glucose production in hepatocytes isolated from overnight-fasted WT mice, suggesting independent gluconeogenic potential (Fig. 4F). The additive effect of PEPCK-M and PEPCK-C was recapitulated in KO hepatocytes with a combined overexpression of both isozymes (Fig. 4H).
Among the metabolic differences between PEPCK-C and PEPCK-M pathways is that PEPCK-C requires mitochondrial export of oxaloacetate as aspartate to produce glucose from lactate, in a pathway that is dependent on aspartate aminotransferase activity. Consistently, WT (Fig. 4G) and KO hepatocytes re-expressing PEPCK-C (Fig. 4E) had reduced gluconeogenesis from lactate when treated with the transaminase inhibitor aminooxyacetate (AOA). In contrast, AOA treatment increased glucose production in KO hepatocytes expressing PEPCK-M (Fig. 4E), and the suppression of gluconeogenesis by AOA in WT hepatocytes was blunted when PEPCK-M was overexpressed (Fig. 4G), illustrating the independence of PEPCK-M driven gluconeogenesis on the aspartate shuttle.
DISCUSSION
The mitochondrial isoform of PEPCK is an often forgotten component in the regulation of gluconeogenesis and mitochondrial metabolism. The lack of attention this enzyme receives is perpetuated by its low expression in common rodent models of disease. Yet, humans possess equal amounts of the cytosolic and mitochondrial isoforms of PEPCK, making it critically important to understand how these two enzymes function together. We investigated the capacity of PEPCK-M to normalize hepatic gluconeogenesis and mitochondrial cataplerosis in a conditional knockout of PEPCK-C using adenovirus to express PEPCK-M at a level similar to the normal PEPCK-C level. PEPCK-M re-established the metabolic activities lost in the PEPCK-C KO, but its overall capacity was lower than that of PEPCK-C expressed at even 10% normal levels. Despite the lower efficiency of PEPCK-M when expressed alone, this isoform potentiated gluconeogenesis in PEPCK-C expressing hepatocytes, suggesting that their activities can independently alter fluxes through these pathways.
Our findings demonstrate that PEPCK-M overexpression in livers lacking PEPCK-C partially reestablishes glucose production and the contribution of PEP to gluconeogenesis, hepatic TCA cycle flux, cataplerosis and pyruvate cycling. These results expand on previous attempts to the study of the role of PEPCK-M [17-21, 31-33], and demonstrate that PEPCK-M can contribute to hepatic glucose production from PEP. The most significant consequence of PEPCK-C loss is the disruption of TCA cycle dynamics [9, 10] and elevated intermediate pool sizes, particularly with regard to malate which is elevated 10-fold in liver KO mice [6]. Differences between the in vivo, ex vivo and in vitro capacity of PEPCK-M to enhance glucose production correlated with its capacity to reduce malate content. Our data also agree with a direct relationship between PEPCK-M function and TCA cycle flux reported in non-gluconeogenic tissue; Stark et al. showed reduced insulin secretion in β-cells after pck2 knock-down [34] due to reduced anaplerosis and decreased pyruvate cycling. Additionally, they linked PEPCK-M activity in pancreas to the TCA cycle flux through its capacity to recycle GTP generated in the succinyl-CoA synthase (G-SCS) reaction. It is noteworthy that similar levels of G-SCS expression are found across various species, including rodents [35].
A ~10 % add-back of PEPCK-C was sufficient to normalize hepatic metabolism. As we previously reported, liver specific PEPCK-C KO mice showed reduced hepatic TCA cycle flux [9], resulting in a marked accumulation of hepatic lipids, malate and circulating gluconeogenic amino acids [6]. Re-expression of PEPCK-C completely rescued TCA cycle flux, glucose production and malate content, consistent with our previous study in pcklox+neo/del mice showing that only 10% PEPCK-C protein levels maintain gluconeogenic flux [10]. Interestingly, PEPCK-M expression ameliorated the lipid profile, although no clear effects were observed on Nile red stained sections, a qualitative assessment. No evidence of increased mobilization of fatty acids in the AdPck2 group was found in contrast to AdPck1 mediated increased ketone body production and reduced liver weight.
Fasting glycemia was slightly increased in AdPck1 treated knock-out mice. This observation suggests that metabolic adaptations that maintain euglycemia in KO mice remain after AdPck1 mediated rescue of hepatic gluconeogenesis. Part of this adaptation was evidenced by reduced glucose utilization in KO mice during a hyperglycemic clamp [8], which may be transiently sustained after re-expression, as we found decreased mRNA for genes related to glucose utilization, such as GLUT2 and GK, and several lipogenic enzymes. This adaptation might also help reconcile findings of low metabolic control of PEPCK over gluconeogenesis [10] with the ability of acute knock-down to rescue glycemia in diabetic mouse models [11, 23].
In contrast, TCA cycle flux and total glucose output in AdPck2 treated livers, although proportional to the increased contribution of PEP to gluconeogenesis, were far from reaching the levels found in WT animals. The limited capacity of AdPck2 treatment to bolster glucose homeostasis was exemplified by its effectiveness to sustain euglycemia only in the early phase of an endurance test.
We don't completely understand the factors that make PEPCK-M inefficient at stimulating TCA flux in the KO liver, though limited mitochondrial export of PEP might be one of them. Hepatic PEP was normalized in AdPck2 treated livers, however the fraction of each pool (mitochondrial and cytosolic) was not examined. Alternatively, cytosolic redox potential might play a role by limiting the flux of PEP to the triose-phosphate pool. Even though the cytosolic redox potential was not evaluated in this work, we found increased mitochondrial reducing potential in the liver of PEPCK-C KO mice, consistent with increased malate content [9]. Additionally, the changes in redox state upon PEPCK inhibition using 3-mercaptopicolinic acid in vivo [36] and in perfused liver [37] have been previously examined. These studies found that PEPCK inhibition resulted in metabolic derangements closely mimicking those found in liver-specific PEPCK-C KO mice, including increased NADH/NAD+ ratio in the cytosol and mitochondria. Moreover, since, we assayed gluconeogenic flux in the presence of glycerol, increased reducing potential is expected from the glycerol-3-phosphate dehydrogenase step. Therefore, it is unlikely that unfavorable redox state in the cytosol limits PEPCK-M flux in vivo. However, primary KO hepatocytes transfected with AdPck2 had substantial glucose production rates (about half of those from PEPCK-C transfected hepatocytes), suggesting that additional factors might contribute to the limited impact of PEPCK-M on gluconeogenesis in the KO liver. For instance, fetal rat liver, which is deficient in PEPCK-C but contains twice the adult levels of the mitochondrial enzyme, is unable to synthesize glucose from 4 carbon intermediates, in contrast to fetal guinea pig liver which expresses both isoforms [38]. However, maximal glucose production in fetal and newborn rabbit hepatocytes is measured after birth, when PEPCK-C levels increase substantially [39]. The enhanced response of PEPCK-M treated animals to a pyruvate challenge in the presence of PEPCK-C (WT AdPck2) suggests that during fasting, hepatic PEPCK-C enables PEPCK-M function, or that perhaps PEPCK-M potentiates PEPCK-C activity. Experiments in cultured hepatocytes provided further evidence that the two isozymes may coordinate gluconeogenesis from different substrates and physiological states. For instance, PEPCK-M expression in WT hepatocytes facilitated PEPCK flux most effectively with lactate as a substrate. However, in the absence of PEPCK-C, PEPCK-M did not demonstrate a substrate preference, probably due to the disruption of TCA cycle intermediate pool sizes intrinsic to KO hepatocytes. To test the importance of mitochondrial substrate and redox shuttling for the function of the two isozymes, we performed experiments with AOA, an inhibitor of the aspartate shuttle [40]. Suppression of the aspartate shuttle stimulated gluconeogenesis from lactate in knock-out hepatocytes expressing PEPCK-M, but inhibited it in hepatocytes re-expressing PEPCK-C. Thus, PEPCK-M may function to support gluconeogenesis across a broader range of cellular redox and mitochondrial substrate shuttling capacity than PEPCK-C alone. This metabolic association between the two isozymes is at least partly supported by the aspartate shuttle, as illustrated by the inability of AOA to suppress gluconeogenesis in wild-type hepatocytes treated with AdPck2 (i.e. expressing both isozymes).
The manner in which the two isozymes are coordinately regulated to afford a flexible response to nutrients and hormones remains unclear. PEPCK-M expression is essentially constitutive [31] and expressed homogenously across the hepatic acinus [41]. In contrast, PEPCK-C is most richly express in the periportal region of the hepatic acinus and its hormonal and nutritional regulation is well documented. Hence, gluconeogenesis from PEPCK-M is likely a quasi-continuous process. Teleologically, the combined properties of PEPCK-M to be constitutive expressed and to be most efficient for gluconeogenesis from lactate may support a role in recycling lactate from glycolytic erythrocytes even when PEPCK-C is suppressed by physiological milieu. Lactate can also be a precursor for gluconeogenesis from PEPCK-C, so it is unlikely that substrate selectivity entirely explains the role of each of the isozymes. However, gluconeogenesis from pyruvate and alanine (supplied by a meal or proteolysis), is most efficiently handled by PEPCK-C which can be coordinated with a host of other hormonally regulated pathways. It has been also suggested that PEPCK-C might operate in the reverse direction from PEP to oxaloacetate by using glycolysis-generated PEP imported from the cytosol to replenish the citrate cycle [42, 43]. However, our data on fed wild-type mice overexpressing PEPCK-M did not show phenotypic alterations or changes on hepatic glycolytic or lipogenic proteins (Supplementary Table 4).
In conclusion, the present work demonstrates that PEPCK-M requires the presence of PEPCK-C to substantially affect gluconeogenesis and the TCA cycle when expressed in the mouse liver. Thus, although PEPCK-M has gluconeogenic capacity per se, its function may be particularly important in species where hepatic PEPCK-M and PEPCK-C are both highly expressed, such as in humans. The synergistic nature of these two enzymes (PEPCK-M works better in the presence of PEPCK-C) indicates an important metabolic complementation that exists in humans but is apparently not required in mice. Further, although the role of PEPCK-M in gluconeogenesis is conceptually hindered by the lack of nutritional or hormonal regulation of the gene, recent data indicates that lysine acetylation may be involved in the regulation of PEPCK-M in humans [44], in a manner similar to the demonstrated regulation of PEPCK-C activity [44-46]. These data compel further consideration of species specific factors that contribute to gluconeogenic regulation in humans, particularly with regard to therapeutic strategies aimed at reducing the impact of dysregulated gluconeogenesis on hyperglycemia.
Supplementary Material
ACKNOWLEDGMENTS
A.M.L. has received a fellowship from Ministerio de Educación y Ciencia (FPI). This study was supported by a grant from the Ministerio de Educación y Ciencia (BFU200907506), and grants by the U.S. National Institutes of Health (RO1DK078184, P01DK058398), and the American Diabetes Association (7-09-BS-24). We thank Novellasdemunt L. and Peña-Rico M.A. (University of Barcelona) for discussion and support, the Research Support Services from the Biology Unit of Bellvitge (University of Barcelona), Mehdibeigi R. and Riordan M. (UT-Southwestern Medical Center) for their technical assistance. We are also indebted to Dr M. Magnuson (Vanderbilt University) for providing liver-specific PEPCK-C knockout mice. J.C.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
AUTHOR CONTRIBUTIONS A.M.L. designed experiments, performed research, analyzed data and wrote the manuscript. J.D., N.E.S., S.S., T.H. and X.F. performed research and helped analyzed data. J.B. reviewed/edited manuscript and contributed to discussion. S.C.B designed experiments, analyzed data, and reviewed/edited manuscript and contributed to discussion. J.C.P. designed experiments, performed research, analyzed data and wrote the manuscript.
Authors declare no conflict of interest.
REFERENCES
- 1.Chang HC, Lane MD. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966;241:2413–2420. [PubMed] [Google Scholar]
- 2.Nordlie RC, Lardy HA. Mammalian liver phosphoneolpyruvate carboxykinase activities. The Journal of biological chemistry. 1963;238:2259–2263. [PubMed] [Google Scholar]
- 3.Hanson RW, Garber AJ. Phosphoenolpyruvate carboxykinase. I. Its role in gluconeogenesis. The American Journal of Clinical Nutrition. 1972;25:1010–1021. doi: 10.1093/ajcn/25.10.1010. [DOI] [PubMed] [Google Scholar]
- 4.Hanson RW, Garber AJ, Reshef L, Ballard FJ. Phosphoenolpyruvate carboxykinase. II. Hormonal controls. The American Journal of Clinical Nutrition. 1973;26:55–63. doi: 10.1093/ajcn/26.1.55. [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Kalhan SC, Hanson RW. What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem. 2009;284:27025–27029. doi: 10.1074/jbc.R109.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.She P, Shiota M, Shelton KD, Chalkley R, Postic C, Magnuson MA. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Molecular and cellular biology. 2000;20:6508–6517. doi: 10.1128/mcb.20.17.6508-6517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hakimi P, Johnson MT, Yang J, Lepage DF, Conlon RA, Kalhan SC, et al. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr Metab (Lond) 2005;2:33. doi: 10.1186/1743-7075-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.She P, Burgess SC, Shiota M, Flakoll P, Donahue EP, Malloy CR, et al. Mechanisms by which liver-specific PEPCK knockout mice preserve euglycemia during starvation. Diabetes. 2003;52:1649–1654. doi: 10.2337/diabetes.52.7.1649. [DOI] [PubMed] [Google Scholar]
- 9.Burgess SC, Hausler N, Merritt M, Jeffrey FM, Storey C, Milde A, et al. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. The Journal of biological chemistry. 2004;279:48941–48949. doi: 10.1074/jbc.M407120200. [DOI] [PubMed] [Google Scholar]
- 10.Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell metabolism. 2007;5:313–320. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gómez-Valadés AG, Méndez-Lucas A, Vidal-Alabró A, Blasco FX, Chillón M, Bartrons R, et al. Pck1 gene silencing in the liver improves glycemia control, insulin sensitivity and dislipidemia in db/db mice. Diabetes. 2008 doi: 10.2337/db07-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuel VT, Beddow SA, Iwasaki T, Zhang XM, Chu X, Still CD, et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proc Natl Acad Sci U S A. 2009;106:12121–12126. doi: 10.1073/pnas.0812547106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiese TJ, Lambeth DO, Ray PD. The intracellular distribution and activities of phosphoenolpyruvate carboxykinase isozymes in various tissues of several mammals and birds. Comparative biochemistry and physiologyB, Comparative biochemistry. 1991;100:297–302. doi: 10.1016/0305-0491(91)90378-q. [DOI] [PubMed] [Google Scholar]
- 14.Wieland O, Evertz-Prusse E, Stukowski B. Distribution of pyruvate carboxylase and phosphoenol-pyruvate carboxikinase in human liver. FEBS Lett. 1968;2:26–28. doi: 10.1016/0014-5793(68)80091-2. [DOI] [PubMed] [Google Scholar]
- 15.Diesterhaft M, Shrago E, Sallach HJ. Human liver phosphoenolpyruvate carboxykinase: Evidence for a separate mitochondrial and cytosol enzyme. Biochem Med. 1971;5:297–303. [Google Scholar]
- 16.Vidnes J, Sovik O. Gluconeogenesis in infancy and childhood. III. Deficiency of the extramitochondrial form of hepatic phosphoenolpyruvate carboxykinase in a case of persistent neonatal hypoglycaemia. Acta Paediatr Scand. 1976;65:307–312. doi: 10.1111/j.1651-2227.1976.tb04890.x. [DOI] [PubMed] [Google Scholar]
- 17.Gamble JL, Jr., Mazur JA. Intramitochondrial metabolism of phosphoenolpyruvate. J Biol Chem. 1967;242:67–72. [PubMed] [Google Scholar]
- 18.Garber AJ, Ballard FJ. Phosphoenolpyruvate synthesis and release by mitochondria from guinea pig liver. The Journal of biological chemistry. 1969;244:4696–4703. [PubMed] [Google Scholar]
- 19.Jomain-Baum M, Hanson RW. The effect of fatty acids on the synthesis of P-enolpyruvate by human liver mitochondria. FEBS Lett. 1973;29:145–148. doi: 10.1016/0014-5793(73)80546-0. [DOI] [PubMed] [Google Scholar]
- 20.Garber AJ, Hanson RW. The interrelationships of the various pathways forming gluconeogenic precursors in guinea pig liver mitochondria. J Biol Chem. 1971;246:589–598. [PubMed] [Google Scholar]
- 21.Gevers W. The regulation of phosphoenolpyruvate synthesis in pigeon liver. Biochem J. 1967;103:141–152. doi: 10.1042/bj1030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hausler N, Browning J, Merritt M, Storey C, Milde A, Jeffrey FM, et al. Effects of insulin and cytosolic redox state on glucose production pathways in the isolated perfused mouse liver measured by integrated 2H and 13C NMR. Biochem J. 2006;394:465–473. doi: 10.1042/BJ20051174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Valades AG, Vidal-Alabro A, Molas M, Boada J, Bermudez J, Bartrons R, et al. Overcoming diabetes-induced hyperglycemia through inhibition of hepatic phosphoenolpyruvate carboxykinase (GTP) with RNAi. Mol Ther. 2006;13:401–410. doi: 10.1016/j.ymthe.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Vidal-Alabro A, Gomez-Valades AG, Mendez-Lucas A, Llorens J, Bartrons R, Bermudez J, et al. Liver Glucokinase(A456V) Induces Potent Hypoglycemia without Dyslipidemia through a Paradoxical Induction of the Catalytic Subunit of Glucose-6-Phosphatase. Int J Endocrinol. 2011;2011:707928. doi: 10.1155/2011/707928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergmeyer HU. Methods of enzymatic analysis. 2d English ed. Verlag Chemie; Academic Press; Weinheim, New York: 1974. [Google Scholar]
- 26.Piraud M, Vianey-Saban C, Petritis K, Elfakir C, Steghens JP, Morla A, et al. ESI-MS/MS analysis of underivatised amino acids: a new tool for the diagnosis of inherited disorders of amino acid metabolism. Fragmentation study of 79 molecules of biological interest in positive and negative ionisation mode. Rapid Commun Mass Spectrom. 2003;17:1297–1311. doi: 10.1002/rcm.1054. [DOI] [PubMed] [Google Scholar]
- 27.Klingmuller U, Bauer A, Bohl S, Nickel PJ, Breitkopf K, Dooley S, et al. Primary mouse hepatocytes for systems biology approaches: a standardized in vitro system for modelling of signal transduction pathways. Syst Biol (Stevenage) 2006;153:433–447. doi: 10.1049/ip-syb:20050067. [DOI] [PubMed] [Google Scholar]
- 28.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Use of 2H2O for estimating rates of gluconeogenesis. Application to the fasted state. J Clin Invest. 1995;95:172–178. doi: 10.1172/JCI117635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landau BR, Schumann WC, Chandramouli V, Magnusson I, Kumaran K, Wahren J. 14C- labeled propionate metabolism in vivo and estimates of hepatic gluconeogenesis relative to Krebs cycle flux. Am J Physiol. 1993;265:E636–647. doi: 10.1152/ajpendo.1993.265.4.E636. [DOI] [PubMed] [Google Scholar]
- 30.Jones JG, Solomon MA, Sherry AD, Jeffrey FM, Malloy CR. 13C NMR measurements of human gluconeogenic fluxes after ingestion of [U-13C]propionate, phenylacetate, and acetaminophen. Am J Physiol. 1998;275:E843–852. doi: 10.1152/ajpendo.1998.275.5.E843. [DOI] [PubMed] [Google Scholar]
- 31.Arinze IJ, Garber AJ, Hanson RW. The regulation of gluconeogenesis in mammalian liver. The role of mitochondrial phosphoenolpyruvate carboxykinase. The Journal of biological chemistry. 1973;248:2266–2274. [PubMed] [Google Scholar]
- 32.Somberg EW, Mehlman MA. Regulation of gluconeogenesis and lipogenesis. The regulation of mitochondrial pyruvate metabolism in guinea-pig liver synthesizing precursors for gluconeogenesis. The Biochemical journal. 1969;112:435–447. doi: 10.1042/bj1120435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng YS, Brooks M, Elson C, Shrago E. Contribution of the cytosol and mitochondrial pathways to phosphoenolpyruvate formation during gluconeogenesis. J Nutr. 1973;103:1489–1495. doi: 10.1093/jn/103.10.1489. [DOI] [PubMed] [Google Scholar]
- 34.Stark R, Pasquel F, Turcu A, Pongratz RL, Roden M, Cline GW, et al. Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. J Biol Chem. 2009;284:26578–26590. doi: 10.1074/jbc.M109.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambeth DO, Tews KN, Adkins S, Frohlich D, Milavetz BI. Expression of two succinyl- CoA synthetases with different nucleotide specificities in mammalian tissues. J Biol Chem. 2004;279:36621–36624. doi: 10.1074/jbc.M406884200. [DOI] [PubMed] [Google Scholar]
- 36.Blackshear PJ, Holloway PA, Aberti KG. The effects of inhibition of gluconeogenesis on ketogenesis in starved and diabetic rats. Biochem J. 1975;148:353–362. doi: 10.1042/bj1480353b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Kombu RS, Kasumov T, Zhu SH, Cendrowski AV, David F, et al. Metabolomic and mass isotopomer analysis of liver gluconeogenesis and citric acid cycle. I. Interrelation between gluconeogenesis and cataplerosis; formation of methoxamates from aminooxyacetate and ketoacids. J Biol Chem. 2008;283:21978–21987. doi: 10.1074/jbc.M803454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longshaw ID, Bowen NL, Pogson CI. The pathway of gluconeogenesis in the cortex of guinea-pig kidney. Use of aminooxyacetate as a transaminase inhibitor. Eur J Biochem. 1972;25:366–371. doi: 10.1111/j.1432-1033.1972.tb01705.x. [DOI] [PubMed] [Google Scholar]
- 39.El Manoubi L, Callikan S, Duee PH, Ferre P, Girard J. Development of gluconeogenesis in isolated hepatocytes from the rabbit. Am J Physiol. 1983;244:E24–30. doi: 10.1152/ajpendo.1983.244.1.E24. [DOI] [PubMed] [Google Scholar]
- 40.Rognstad R, Clark DG. Effects of aminooxyacetate on the metabolism of isolated liver cells. Arch Biochem Biophys. 1974;161:638–646. doi: 10.1016/0003-9861(74)90348-8. [DOI] [PubMed] [Google Scholar]
- 41.Agius L, Tosh D. Acinar zonation of cytosolic but not organelle-bound activities of phosphoenolpyruvate carboxykinase and aspartate aminotransferase in guinea-pig liver. Biochem J. 1990;271:387–391. doi: 10.1042/bj2710387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Modaressi S, Brechtel K, Christ B, Jungermann K. Human mitochondrial phosphoenolpyruvate carboxykinase 2 gene. Structure, chromosomal localization and tissue-specific expression. Biochem J. 1998;333(Pt 2):359–366. doi: 10.1042/bj3330359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garber AJ, Salganicoff L. Regulation of oxalacetate metabolism in liver mitochondria. Evidence for nicotinamide adenine dinucleotide-malate dehydrogenase equilibrium and the role of phosphoenolpyruvate carboxykinase in the control of oxalacetate metabolism in intact guinea pig and rat liver mitochondria. The Journal of biological chemistry. 1973;248:1520–1529. [PubMed] [Google Scholar]
- 44.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J, Kong X, Martins-Santos ME, Aleman G, Chaco E, Liu GE, et al. Activation of SIRT1 by resveratrol represses transcription of the gene for the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) by deacetylating hepatic nuclear factor 4alpha. J Biol Chem. 2009;284:27042–27053. doi: 10.1074/jbc.M109.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin YY, Lu JY, Zhang J, Walter W, Dang W, Wan J, et al. Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell. 2009;136:1073–1084. doi: 10.1016/j.cell.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.